Abstract
Background
The growing demand for electrophysiology (EP) treatment in China presents a challenge for current EP care delivery systems. This study constructed a discrete event simulation (DES) model of an inpatient EP care delivery process, simulating a generalized inpatient journey of EP patients from admission to discharge in the cardiology department of a tertiary hospital in China. The model shows how many more patients the system can serve under different resource constraints by optimizing various phases of the care delivery process.
Methods
Model inputs were based on and validated using real-world data, simulating the scheduling of limited resources among competing demands from different patient types. The patient stay consists of three stages, namely: the pre-operative stay, the EP procedure, and the post-operative stay. The model outcome was the total number of discharges during the simulation period. The scenario analysis presented in this paper covers two capacity-limiting scenarios (CLS): (1) fully occupied ward beds and (2) fully occupied electrophysiology laboratories (EP labs). Within each CLS, we investigated potential throughput when the length of stay or operative time was reduced by 10%, 20%, and 30%. The reductions were applied to patients with atrial fibrillation, the most common indication accounting for almost 30% of patients.
Results
Model validation showed simulation results approximated actual data (137.2 discharges calculated vs. 137 observed). With fully occupied wards, reducing pre- and/or post-operative stay time resulted in a 1–7% increased throughput. With fully occupied EP labs, reduced operative time increased throughput by 3–12%.
Conclusions
Model validation and scenario analyses demonstrated that the DES model reliably reflects the EP care delivery process. Simulations identified which phases of the process should be optimized under different resource constraints, and the expected increases in patients served.
Similar content being viewed by others
Background
Cardiac arrhythmias encompass a wide range of heart rhythm and heart rate disorders [1]. Clinical presentation can range from asymptomatic to life-threatening, such as sudden cardiac arrest [1]. Arrhythmias can be managed with antiarrhythmic medication or various electrophysiological (EP) procedures, such as ablation or implantation of pacemakers or defibrillators [2]. The most common type of arrhythmia is atrial fibrillation (AF) [3], which is linked to higher risks of stroke, heart failure, dementia, and death [4,5,6,7,8,9]. This significantly impacts healthcare costs owing to hospitalizations and loss of productivity [10].
The prevalence of AF in China is estimated to be 1.6% and increases with age [11]. This poses a formidable challenge to China’s healthcare system owing to its aging population [12]. While the ablation treatment rate among Chinese AF patients is unclear, it is reasonable to assume that over 176,000 ablation procedures may be needed based on the US ablation rate of 0.79% in 2005 [13].
Furthermore, overcrowding at tertiary public hospitals is a major problem in China [14]. While economic growth slows, rapid expansion of hospital operations and infrastructure is not a viable option to meet the demand for EP treatments [15]. Thus, decision-makers must utilize limited medical resources to deliver healthcare services more efficiently [15] in order to meet this growing demand.
To this end, China’s National Health Commission has issued guidelines to improve public hospital operational management and healthcare delivery [16]. The Commission not only recommends optimizing resource allocation and processes but also emphasizes data collection and analysis, as well as improving the quality of decision-making [16]. Public hospitals are urged to collect and manage operational data for analysis, establish an analytical decision-making framework, and implement analysis results [16].
Optimizing EP service delivery efficiency is a complex, multidimensional problem involving factors ranging from patient flow management to novel technology adoption. Discrete event simulation (DES) is an operational research tool that helps decision-makers assess different management strategies, enabling them to evaluate not only the performance of current healthcare delivery systems but also that of hypothetical scenarios [17]. This allows them to choose between different approaches of healthcare delivery to prioritize and pursue without undesirably impacting current systems [17].
This study established a generalized DES model of an inpatient EP treatment process, which can be applied across tertiary hospitals in China. The model examines how cardiology departments under different resource constraints can serve more EP patients by improving the efficiency of each phase of the healthcare delivery process, including pre-operative preparation, operative time, or post-operative recovery. This enables hospital decision-makers to clarify which phases of the EP care delivery process to prioritize in different situations in order to better meet the demand for EP treatment.
Methods
Model setting and perspective
We constructed a model to simulate the inpatient journey of individual EP patients from admission to discharge in the cardiology department of a tertiary hospital in China, using the “simmer” package [18] in R [19]. To build a generalized model applicable to different hospitals in China, clinicians from two large tertiary hospitals were consulted. We established a preliminary design for the model based on the inpatient management process of the Cardiology Department of the First Affiliated Hospital of Zhejiang University (FAHZU). We further examined the patient care delivery flow at Shanghai General Hospital’s Cardiology Department, fine-tuning the preliminary design. The final model abstracted and generalized a common inpatient care pathway without focusing on implementation details specific to an individual hospital.
These two cardiology departments have a number of catheter laboratories, some of which are dedicated electrophysiology laboratories (EP labs) that are prioritized for EP procedures. They can only be used for other cardiology procedures after all EP procedures scheduled for that day are completed.
The primary study population is EP patients who pass through the EP labs (i.e., the target EP patients, TEP patients). The model explicitly tracks the pre-operative phase, operation, and post-operative stay of these patients. The other two patient types include catheter lab EP patients (CLEP patients) who receive their procedure in the catheter labs and non-EP cardiology patients (NEP patients). Both CLEP and NEP patients compete with TEP patients for hospitalization resources, but not for resources used in EP lab operations. Therefore, only the hospitalization stays of CLEP and NEP patients were included in the model.
Model structure
Arrival process
The model generates three patient types: TEP, CLEP, and NEP. Unlike patients receiving care in outpatient facilities or emergency rooms, inpatient visits for elective procedures do not arrive randomly at the hospital. Patients in this model are scheduled to arrive at the same time daily (one hour before working hours). The daily arrivals of each type of patient was randomly generated.
Resources
The model considers three resource types: cardiology ward beds, electrophysiologists, and EP labs. All three types of patients share bed resources, but only TEP patients require electrophysiologists and EP labs. The schedules for electrophysiologists and EP labs are managed by hospital staff.
Inpatient process
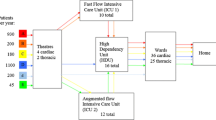
The process for TEP patients is shown in Fig. 1. Upon arrival, the TEP patient is assigned to one of several EP procedures and admitted if a ward bed is available. The patient’s stay consists of three phases: pre-operative stay, EP procedure, and post-operative stay. Once the patient’s pre-operative phase is complete, they are assigned to an electrophysiologist and enter the queue for EP lab procedures the next day. If the EP labs are not open the next day, the patient waits until the subsequent workday. Once the patient’s assigned electrophysiologist and an EP lab become available during working hours, the patient enters the EP lab to undergo the procedure. Once a procedure is started, it will continue until completion, regardless of the pre-defined working hours. However, no new procedures are allowed after working hours. Upon procedure completion, the electrophysiologist leaves the EP lab and the patient returns to the ward. The catheter lab becomes available after cleanup and preparation for the next procedure. After post-operative recovery, the patient is discharged.
For CLEP and NEP patients, the process is simpler. Patients are admitted if ward beds are available and discharged after their length of stay is reached.
Model inputs
Model inputs were based on actual data collected between May 1–June 30, 2022 from FAHZU’s Cardiology Department. Admission and discharge dates were recorded for TEP, CLEP, and NEP patients. Inputs related to the number of admissions per day and length of stay were based on patients admitted between May 1–June 25, as both admission and discharge dates were available for these patients. In addition, for TEP patients, procedure dates, procedure types, procedure start and end times, and assigned electrophysiologists were also collected.
Simulation
The daily arrival of TEP, CLEP, and NEP patients is modeled according to a negative binomial distribution. The procedure type for TEP patients is randomly drawn according to the proportions of the different EP procedures. Their electrophysiologist is randomly assigned based on the day of the week of their procedure and the average allocation of patients between different electrophysiologists on that day.
Processing times of TEP patients are determined by procedure type. The length of stay and days of pre-operative stay are randomly generated from a truncated lognormal distribution and rounded to integers. The post-operative stay is the difference between the length of stay and the pre-operative stay. The operative time, in minutes, is also modeled by a truncated lognormal distribution.
Following ISPOR guidelines, the simulation first runs for ten days with CLEP and NEP patients as a “warm up” period [20]. The TEP patient flow is generated from day 11. The simulation is then run for an additional 61 days, corresponding to the observation period of the actual data. Each scenario was simulated 1000 times.
Validation
The simulated total number of discharged TEP patients per replication and the simulated daily discharges were compared against actual data. Means and standard deviations are reported. The simulated and real daily discharges were also compared using the Mann-Whitney U test.
Scenario analyses
There are two significant capacity-limiting scenarios (CLS) for hospital cardiology departments in China. First, cardiology departments often must manage inpatient beds as a shared resource. Therefore, in this CLS, the inpatient ward is fully occupied before the EP labs reach their capacity. Second, EP lab capacity may be overwhelmed by demand. The second CLS usually occurs in hospitals with insufficient EP labs compared to ward beds.
Based on data from FAHZU, we performed scenario analyses to first investigate the patient throughput capacities under both CLSs and then the potential throughput when different phases of the inpatient process were optimized. Since optimizing all procedure types simultaneously in the real world is difficult, AF ablations (for paroxysmal and persistent AF) were selected for scenario analysis, as these were the most common procedures accounting for almost 30% of TEP patients. Patient throughput was measured as the total number of discharged TEP patients. For each CLS, we report the mean and standard deviation of the results from 1000 simulations.
First, we examined the scenarios with fully occupied cardiology ward beds. Because input from FAHZU had higher bed utilization than that of EP labs, changing the number of resources was not necessary. We increased the number of TEP patients arriving daily so that they would fully occupy any available beds remaining after the other two types of patients were admitted. This was considered the base case scenario under the condition of a fully occupied cardiology ward. Under this scenario, we reduced total length of stay of paroxysmal and persistent AF ablation patients by 10%, 20%, and 30% as the test cases. For each level of reduction, we calculated the expected difference in days and applied this reduction to different phases: the pre-operative stay (the minimum pre-operative stay remained 1 day); the post-operative stay; and both the pre-operative and post-operative stays reduced proportionally (the minimum pre-operative stay remained 1 day). For comprehensiveness, we also examined a scenario where operative times of paroxysmal and persistent AF ablation were reduced by 10%, 20%, and 30%, although this was expected to result in minimal change while EP labs were not at capacity.
We then analyzed scenarios with fully occupied EP labs. In addition to adjusting the patient flow as described above, the number of ward beds and EP lab resources were adjusted. This was the base case scenario under the condition of fully occupied EP labs. For comparison, we generated scenarios with reduced length of stay and reduced operative time in the same manner as above.
Results
Model inputs
FAHZU’s cardiology department has 87 ward beds, two EP labs, and eight electrophysiologists. Two EP labs are available every workday (Monday to Friday) from 8:00 AM to 10:00 PM. Two electrophysiologists are on duty during the working hours of each workday. Average patient allocation between the two electrophysiologists on duty each day is shown in Table 1.
The number of daily arrivals by patient type is shown in Table 2. Inputs relating to processing times for each phase in the TEP patient’s journey are summarized in Tables.
Table 3, stratified by EP procedure type. Average length of stay for CLEP and NEP patients was 5.427 (SD 4.261) and 3.758 (SD 2.718), respectively.
Model validation
The simulated total number of TEP patients discharged per replication was 137.167 (SD 17.856), compared to 137 in the actual data. The distribution of the simulated results is shown in Fig. 2A.
The simulated daily number of discharges was 2.249 (SD 2.455), compared with the actual figure of 2.245 (SD 2.364). Distribution of the simulated results, compared with that of actual results, is shown in Fig. 3. The Mann-Whitney U test showed no significant difference between the locations of the two distributions (p = 0.747).
Scenario analysis
In the base case scenario with fully occupied cardiology wards, the total number of discharges per replication was 220.612 (SD 44.110), the distribution of which is shown in Fig. 2B. The results of this group of scenarios are presented in Table 4; Fig. 4. Paroxysmal and persistent AF ablation patients, whose processing times are adjusted in these scenarios, comprise approximately 30% of all patients. Reducing their length of stay increased the total number of discharges by 1–7%, regardless of which phase the reduction was applied to. Reducing operative time did not have any apparent effect.
To simulate conditions with fully occupied EP labs, the number of ward beds was increased to 110 and the number of EP labs was decreased to one. The results of these scenarios are shown in Table 5; Fig. 5. Average total discharges was 271.634 (SD 10.379) in the base case scenario. The distribution is shown in Fig. 2C. Reducing operative time increased the total number of discharges by 3–12%. A reduction in the length of stay did not affect the total number of discharges.
Discussion
In this study, we developed and validated a generalizable DES model based on inpatient EP care delivery processes from two large tertiary hospitals in China. We used the model to simulate hospitalization stays of EP patients in scenarios with fully occupied ward beds and EP labs, and evaluated the effects of accelerating different phases of the delivery process under the two conditions. The model can support hospital decision-makers to identify which phase of the EP care delivery process to prioritize under different resource constraints in order to best satisfy demand for EP treatment. Decision-makers can then consider different methods of EP care delivery to target the relevant phase.
Under the condition of a fully occupied ward, reducing operative time had little to no effect, as this was not the bottleneck. On the other hand, reducing length of stay by 10–30% in paroxysmal and persistent AF ablation patients, which account for approximately 30% of all patients, increased total discharges by 1–7%. There was no difference whether the reduction was applied in the pre-operative or post-operative phase. However, it is imperative that reduction in hospitalization time does not compromise the quality of care. This goal can be achieved by integrating evidence-supported new technology or practices. For instance, the prevailing practice in China involves a pre-operative transesophageal echocardiogram (TEE), typically necessitating a hospital stay of 2 to 3 days prior to the procedure [21]. Intracardiac echocardiography (ICE) has been demonstrated as a safe and effective alternative to TEE, conducted during the procedure, thereby requiring only a 0–1 day pre-operative stay [21]. Pertaining to the post-operative phase, proactive prevention of complications contributes to reducing the length of stay while maintaining the quality of care [22]. This can be realized through careful management of patients with pre-existing medical conditions [23] and the accumulation of physician experience [24]. However, in the context of large tertiary hospitals in China, as in our study, the complication rate is generally well managed, leaving limited room for further improvement. In addition, the post-operative stay in China exceeds that of the US, where the standard practice leans towards an overnight stay [25], or even outpatient procedures in some instances [22]. This disparity may be attributed to the US’s adoption of diagnostic-related groups (DRGs) [26]. It is expected that the length of stay in China may decrease as the DRG payment system gains traction in the Chinese healthcare landscape [27].
When EP labs were fully occupied, reducing operative time by 10–30% in AF ablation patients led to a 3–12% increase in total discharges This improvement is made possible by several technological innovations that can reduce AF ablation operative times. The key to AF ablation is achieving pulmonary vein isolation [28]. Conventional point-to-point radiofrequency (RF) ablation involves creating multiple lesions, which may leave gaps in between [29] and drive long operative times [30]. Better navigation techniques, such as remote magnetic navigation-guided RF ablation, may reduce operative times [31]. The Q-FFICIENCY trial showed that very high-power, short-duration ablation using contact force-sensing RF catheters can reduce operative times by almost 50% [32], which is higher than the 30% reduction used in our analyses. According to the model, this reduction level would result in 330 total discharges (21% increase) using the specifications described previously.
In simulations of fully utilized EP labs, reducing operative time led to a more significant increase in discharges compared to reducing the length of stay in scenarios with fully occupied ward beds. This discrepancy may stem from the fact that the existing length of stay is already brief, allowing limited room for reduction, while there is more potential for improving EP lab efficiency. Our real-world data revealed that, over a two-month period, on 25 out of 40 working days, the working hours in the EP labs exceeded 8 h, with 29 days surpassing 7 h. This underscores the high utilization rate of the EP labs on a substantial number of days. However, it’s essential to note that reducing operative time does not necessarily ensure improved throughput, given the inherent variability in operational processes, including fluctuating patient flows. This variability in real-world settings emphasizes the need for adaptable and versatile modeling approaches. Our model aims not only to analyze the specific hospital from which the data originated but also to demonstrate its broader applicability as a decision-making tool for hospital managers. Recognizing the variability in hospital conditions, we tested two scenarios reflecting significant operational challenges, particularly relevant in China. These scenarios illustrate how our model can adapt to different settings, providing insights into potential operational improvements. The high utilization of the EP labs observed in our data exemplifies one of these challenges, highlighting the necessity for contextually informed strategies to enhance hospital efficiency.
While US practices and technologies provide useful insights as described above, it is crucial to tailor these approaches to align with China’s unique healthcare landscape. For instance, the adaptation from the DRG system to the diagnosis-intervention packet (DIP) system in China arose from challenges faced during a DRG pilot program [33]. The DIP system offered a less technically demanding and more scalable approach [33]. Since 2020, China has been piloting a dual-track arrangement with both DRGs and DIP, acknowledging the diverse regional capacities of local health systems [33]. This initiative underlines the importance of contextualizing global practices to improve resource allocation and potentially reduce hospital stay lengths without compromising care quality, aligning with the overarching objective of enhancing efficiency in healthcare delivery.
A previous study by Kowalski et al. investigated the economic value of reducing AF ablation operative times using DES [34]. However, this study differed from ours conducted in China. Kowalski et al. did not include a hospitalization process [34], which may have been a choice in the model design or because AF ablations in their study were day surgeries. In addition, ablation procedures were arranged in block schedules such that two ablations were performed in an EP lab each day, with the possibility of one additional procedure [34], placing a hard limit on the number of procedures per day. In our model, accounting for competition of ward beds was important because of the almost 100% utilization of hospital beds in large tertiary hospitals in China [35]. Furthermore, EP procedures in China do not follow a block schedule. Procedures can start as soon as the previous procedure is completed and the EP lab is ready. Therefore, there is more potential to increase the number of procedures by reducing operative time.
Although the DES technique is increasingly used in healthcare research, applying it as a generalized decision-making tool is challenging. First, a DES model based on the detailed practices of one hospital may not be suitable for other settings. In addition, employing a DES requires a large amount of data, and more detailed data increases cost and time [36]. Adapting a DES model to a new setting requires new data [37], and new data is again required if the system undergoes a change [38]. Furthermore, the simulated results of specific actions may differ from actual implementation owing to human variation [39].
In complex systems such as healthcare, a decision-making tool may be more helpful to clarify generic activity patterns instead of focusing excessively on specific processes of one particular site [38]. Therefore, we summarized and abstracted the processes at two different hospitals to build a generalized DES model of the inpatient EP care delivery process. Lowering the complexity of the model would also greatly reduce the effort required to collect data and facilitate model updates if system changes are made. This model does not prescribe specific actions but instead identifies the bottleneck of the care delivery process. Knowing which phase of the process to prioritize, the decision-maker can consider several different options to improve the healthcare delivery process and use the model to understand the expected results of these options. Further, they can also select the most worthwhile option by considering the time and effort involved compared to the expected increase in total discharges.
This study has several limitations. First, the generalizability of this model may be constrained as it was based on data from two tertiary hospitals collected over a span of two months. This model might not fully reflect the care delivery processes in other hospitals within China or in other countries. The input data may not account for potential seasonal or procedural variations. Additional research is needed to validate the applicability of the approach in more diverse settings. In addition, our approach involved testing scenarios where hospitalization time was reduced by a specified percentage. However, depending on the technology or practice applied to improve the workflow, not all patients may benefit; identifying the proportion of affected patients would yield a more robust analysis. Due to the absence of such data currently, we opted to test scenarios with varying degrees of hospitalization stay reduction to characterize the uncertainty surrounding this aspect. Furthermore, we did not include scenarios with reduced variability, although this may also impact total discharges. However, we tested several scenarios, and the changes were relatively minor. Moreover, reducing variability without changing the mean in practice is difficult, and therefore these scenarios were not included in the results. Finally, the distributions of the simulated number of discharges in some scenarios are quite wide, implying that even if the simulations are accurate, the implementation results would still be uncertain.
Conclusion
This study developed a generalized DES model to simulate an EP patient’s hospitalization and treatment at a tertiary hospital in China. Using this model, hospital decision-makers dealing with different resource constraints can identify which phase of the care delivery process to optimize in order to better meet demand for EP treatment. Simulations showed that if ward beds are fully occupied, reducing the length of stay of AF ablation patients by 10–30% resulted in a 1–7% increase in the total number of patients discharged. On the other hand, under the condition of fully occupied EP labs, reducing the operative time of AF ablation patients by 10–30% increased discharges by 3–12%.
Data availability
Data used in this study are available for research only. Data requests will be reviewed by the review committee of the First Affiliated Hospital of Zhejiang University.
Abbreviations
- DES:
-
discrete event simulation
- EP:
-
electrophysiology
- CLS:
-
capacity-limiting scenario
- AF:
-
atrial fibrillation
- FAHZU:
-
First Affiliated Hospital of Zhejiang University
- TEP patients:
-
target electrophysiology patients
- CLEP patients:
-
catheter lab electrophysiology patients
- NEP:
-
non- electrophysiology patients
- PVC:
-
premature ventricular complex
- SVT:
-
supraventricular tachycardia
- AFlutter:
-
atrial flutter
- PAC:
-
premature atrial contraction
- AT:
-
atrial tachycardia
- GP:
-
ganglionated plexi
- PFO:
-
patent foramen ovale
- ICD:
-
implantable cardioverter defibrillator
- PM:
-
pacemaker
- CRT:
-
cardiac resynchronization therapy
References
Desai DS, Hajouli S. Arrhythmias. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 Oct 17]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK558923/.
Kastor JA, Saba MM. Cardiac Arrhythmias. In: eLS [Internet]. John Wiley & Sons, Ltd; 2009 [cited 2022 Sep 26]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0002112.pub2.
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to revise the 2001 guidelines for the management of patients with Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257–354.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8.
Hannon N, Sheehan O, Kelly L, Marnane M, Merwick A, Moore A, et al. Stroke Associated with Atrial Fibrillation– Incidence and early outcomes in the North Dublin Population Stroke Study. Cerebrovasc Dis Basel Switz. 2009;29(1):43–9.
Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5.
Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7(4):433–7.
Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49(9):986–92.
Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52.
Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD, Sinner MF, et al. Symptoms and functional status of patients with Atrial Fibrillation: state-of-the-art and Future Research opportunities. Circulation. 2012;125(23):2933–43.
Shi S, Tang Y, Zhao Q, Yan H, Yu B, Zheng Q, et al. Prevalence and risk of atrial fibrillation in China: a national cross-sectional epidemiological study. Lancet Reg Health West Pac. 2022;23:100439.
Fang EF, Scheibye-Knudsen M, Jahn HJ, Li J, Ling L, Guo H, et al. A research agenda for aging in China in the 21st century. Ageing Res Rev. 2015;24(Pt B):197–205.
Kneeland PP, Fang MC. Trends in catheter ablation for atrial fibrillation in the United States. J Hosp Med. 2009;4(7):E1–5.
Liu Y, Zhong L, Yuan S, van de Klundert J. Why patients prefer high-level healthcare facilities: a qualitative study using focus groups in rural and urban China. BMJ Glob Health. 2018;3(5):e000854.
Chen Z, Chen X, Gan X, Bai K, Baležentis T, Cui L. Technical efficiency of Regional Public hospitals in China based on the three-stage DEA. Int J Environ Res Public Health. 2020;17(24):E9383.
China National Health Commission.,. Interpretation of guidlines to reinforce public hospital operational management [Internet]. 2020 [cited 2022 Oct 17]. Available from: http://www.gov.cn/zhengce/2020-12/26/content_5573494.htm.
Jun JB, Jacobson SH, Swisher JR. Application of discrete-event simulation in health care clinics: a survey. J Oper Res Soc. 1999;50(2):109–23.
Ucar I, Smeets B, Azcorra A. simmer: Discrete-Event Simulation for R. J Stat Softw [Internet]. 2019 [cited 2023 Mar 7];90(2). Available from: http://www.jstatsoft.org/v90/i02/.
R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing.; 2021. Available from: https://www.R-project.org/.
Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Möller J, et al. Modeling using discrete event simulation: a report of the ISPOR-SMDM modeling Good Research practices Task Force–4. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2012;15(6):821–7.
Wang Y, Zhao Y, Zhou K, Zei PC, Wang Y, Cheng H, et al. Intracardiac echocardiography is a safe and effective alternative to transesophageal echocardiography for left atrial appendage thrombus evaluation at the time of atrial fibrillation ablation: the ICE-TEE study. Pacing Clin Electrophysiol PACE. 2023;46(1):3–10.
Tripathi B, Arora S, Kumar V, Abdelrahman M, Lahewala S, Dave M, et al. Temporal trends of in-hospital complications associated with catheter ablation of atrial fibrillation in the United States: an update from Nationwide Inpatient Sample database (2011–2014). J Cardiovasc Electrophysiol. 2018;29(5):715–24.
Mathew ST, Po SS. Atrial Fibrillation catheter ablation: overcoming complications and improving success. J Innov Card Rhythm Manag. 2017;8(10):2874–85.
Wu L, Narasimhan B, Ho KS, Zheng Y, Shah AN, Kantharia BK. Safety and complications of catheter ablation for atrial fibrillation: predictors of complications from an updated analysis the National Inpatient database. J Cardiovasc Electrophysiol. 2021;32(4):1024–34.
Akula N, Mariam D, Luthra W, Edward P, Katz FJ, Levi DA. Safety of same day discharge after Atrial Fibrillation ablation. J Atr Fibrillation. 2020;12(5):2150.
Felder S. The variance of length of Stay and the optimal DRG outlier payments. Int J Health Care Finance Econ. 2009;9(3):279–89.
Ma Y, Wang W. The impact of diagnosis related group payment on the performance of public hospitals. Am J Transl Res. 2021;13(6):6796–801.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962.
Haegeli LM, Calkins H. Catheter ablation of atrial fibrillation: an update. Eur Heart J. 2014;35(36):2454–9.
Reddy VY, Dukkipati SR, Neuzil P, Natale A, Albenque JP, Kautzner J, et al. Randomized, controlled trial of the Safety and Effectiveness of a contact force-sensing irrigated catheter for ablation of Paroxysmal Atrial Fibrillation: results of the TactiCath Contact Force ablation catheter study for Atrial Fibrillation (TOCCASTAR) Study. Circulation. 2015;132(10):907–15.
Elisabeth Noten AM, Kis Z, Akca F, Bhagwandien R, Wijchers S, Yap SC, et al. Robotic navigation shows superior improvement in efficiency for atrial fibrillation ablation. J Atr Fibrillation. 2019;11(5):2108.
Richard Tilz R, Sano M, Vogler J, Fink T, Saraei R, Sciacca V, et al. Very high-power short-duration temperature-controlled ablation versus conventional power-controlled ablation for pulmonary vein isolation: the fast and furious - AF study. Int J Cardiol Heart Vasc. 2021;35:100847.
He AJ. Scaling-up through piloting: dual-track provider payment reforms in China’s health system. Health Policy Plan. 2023;38(2):218–27.
Kowalski M, DeVille JB, Svinarich JT, Dan D, Wickliffe A, Kantipudi C, et al. Using Discrete Event Simulation to Model the Economic Value of Shorter Procedure Times on EP Lab Efficiency in the VALUE PVI study. J Invasive Cardiol. 2016;28(5):176–82.
China National Health Commission.,. Statistical Report on China’s Health Care Development in 2019 (2019 年我国卫生健康事业发展统计公报) [Internet]. 2020 [cited 2022 Sep 20]. Available from: http://www.nhc.gov.cn/guihuaxxs/s10748/202006/ebfe31f24cc145b198dd730603ec4442.shtml.
Hamrock E, Paige K, Parks J, Scheulen J, Levin S. Discrete event simulation for healthcare organizations: a tool for decision making. J Healthc Manag Am Coll Healthc Exec. 2013;58(2):110–24. discussion 124–125.
Zhang X. Application of discrete event simulation in health care: a systematic review. BMC Health Serv Res. 2018;18:687.
Proudlove NC, Black S, Fletcher A. OR and the challenge to improve the NHS: modelling for insight and improvement in in-patient flows. J Oper Res Soc. 2007;58(2):145–58.
Cai H, Jia J. Using Discrete Event Simulation (DES) to support performance-driven Healthcare Design. HERD. 2019;12(3):89–106.
Acknowledgements
Not applicable.
Funding
This study was funded by Johnson & Johnson Medical (Shanghai) Ltd. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for preparation.
Author information
Authors and Affiliations
Contributions
WL contributed to study design, data collection, analysis and interpretation of data, model validation, and manuscript writing and revision. SW contributed to study design, analysis and interpretation of data, model design, and manuscript writing and revision. LZ, FY, YZ, XX, FZ, ZF, and LS contributed to data collection, analysis and interpretation of data, and manuscript writing and revision. YH contributed to study design, analysis and interpretation of data, model design, and manuscript writing and revision. All authors have approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University (reference number IIT20220102B) on March 25th, 2022. The Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University has waived the requirement for informed consent due to retrospective nature of the study. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
SW and YH have received research support from Johnson & Johnson Medical (Shanghai) Ltd. The remaining authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, W., Zhang, L., Wu, S. et al. Optimizing the management of electrophysiology labs in Chinese hospitals using a discrete event simulation tool. BMC Health Serv Res 24, 67 (2024). https://doi.org/10.1186/s12913-024-10548-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-024-10548-5