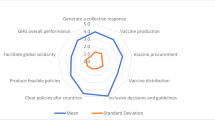
Abstract
Background
Infectious disease outbreaks pose a significant threat to public health, and achieving herd immunity highlights the importance of addressing conflicts of interest (COI) in vaccine development and policy-making. This policy brief aims to present policy options that address COI regarding vaccines in infectious disease outbreaks, based on good governance for health approach.
Methods
Our study used a scoping review methodology. We conducted a systematic search, which led to identifying 43 eligible articles. A qualitative approach (i.e., content analysis) was employed for data analysis, using “ATLAS.ti 9” software. The primary results underwent a process of cleaning, categorisation, and subsequent discussion in three sessions with the research team.
Results
Relationships between theindustry and “government/policymakers” as well as "academic institutions/researchers" are prominent origins of COI regarding the vaccine in infectious disease outbreaks. To address this issue, we present nine policy options that target both the root cause of the problem and the adoption of good governance for health approach.
Conclusions
The key principles of good governance for health, including, “Transparency”, “The Rule of Law”, “Effectiveness”, “Efficiency”, “Participation”, “Consensus Orientation”, “Equality”, “Responsibility”, “Responsiveness” and “Accountability” must be taken into account when formulating policy options to address COI regarding the vaccine in infectious disease outbreaks. The effectiveness of the policy options outlined in this policy brief should be assessed in practical contexts, as this evaluation may uncover the need for revisions.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Outbreaks significantly threaten public health and health policy-making [1]. The COVID-19 pandemic, one of the most critical crises in the last 50 years, has claimed millions of lives; As of July 5, 2023, the World Health Organization (WHO) COVID-19 dashboard reports a total of 6,948,764 deaths [2]. Vaccination and achieving herd immunity are crucial in combating infectious disease outbreaks [3, 4]. Within the medical field, discussions on vaccination have consistently been contentious, with various groups accusing one another of conflicts of interest (COI) [5, 6]. It is worth acknowledging that the necessity of achieving herd immunity, coupled with the substantial financial contracts involved in vaccine production, can create an environment susceptible to the influence of COI on vaccine development, guidelines, and policy-making [7]. Building Public trust serves as a fundamental pillar for successful vaccination campaigns. However, the low utilisation of vaccines such as measles, tetanus, and COVID-19 in various countries [8,9,10,11,12,13]. indicates a decline in public trust towards scientific research and the guidelines provided by health organisations, which raises serious concerns [14, 15]. A significant factor contributing to this decreased public trust is the presence of COI within the vaccines field, leading to corruption in vaccine production and development and related policies [7, 16,17,18,19,20,21,22,23]. As a result, one of the primary arguments by anti-vaccine groups revolves around the perceived COI of experts, researchers, and policymakers due to their financial connections with the industry [7]. Other factors contribute to the concern surrounding the potential detrimental effects of industry relationships on public health decisions. For instance, in the swine flu pandemic in 2009, there were debates about the adequacy of evidence supporting the declaration of a pandemic by the WHO and their prediction of infection in nearly two billion individuals [24]. Notably, vaccine production companies, as the primary stakeholders, reaped substantial benefits from the situation [25].
COI arise when public officials face a conflict between their public duties and private interests, where their private-capacity interests can potentially exert undue influence on fulfilling their official responsibilities [26]. Corruption, as defined by the World Bank, is the misuse of public office for personal and private interests [27]. Therefore, while COI can potentially lead to corruption, it is essential to acknowledge that corruption consistently stems from COI [28].
Research has demonstrated that existing policies about identifying and mitigating COI in developing and evaluating vaccines [28,29,30] and policies addressing the COI of decision-making bodies are insufficient and require bolstering [24, 31,32,33,34,35,36,37,38].
In Iran, notable policy measures specifically targeting COI regarding the vaccine during the COVID-19 pandemic were not formulated by the National Headquarters for COVID-19 and the Ministry of Health and Medical Education. Specific individuals who held positions within these organisations were also involved in vaccine development teams, including the “Barakat vaccine”, which sparked considerable discussions surrounding COI [39].
It is crucial to prioritise public health over industry interests, address COI in vaccine research and evaluation, and develop vaccine instructions and policies. These efforts prevent corruption, foster public trust, and enhance public health outcomes. Therefore, based on a scoping review, this policy brief aims to provide policy options for effectively addressing COI regarding the vaccine in infectious disease outbreaks. It takes the good governance for health approach, incorporating principles such as “Consensus Building’, “Participation”, “Transparency”, “Equality”, “The Rule of Law”, “Effectiveness and Efficacy”, “Responsibility”, and “Accountability” [40].
Methods
This study utilises the scoping review method, which is suitable for addressing “what” and “why” questions. As described in the definitions provided for scoping review, this approach is specifically designed to swiftly examine critical and fundamental concepts within a particular research field while identifying key sources and evidence. In this study, we employed the protocolFootnote 1 developed by O'Malley and Arksey to carry out the scoping review [41].
The research team searched international (Web of Science, Scopus, PubMed, and EMBASE) and national databases (MagIran and SID) to identify relevant studies from the inception of these databases until August 2022. We also mined Google Scholar to increase the chance of finding potentially relevant studies on the topic under scrutiny. Additionally, we searched Grey literature and thesis. The search was conducted in English and Persian languages. The reference list of the selected articles was hand-searched to increase the chance of not missing potential articles. We provide the search strategy for each database in (Appendix 1). Epidemic, pandemic, vaccine, conflicts of interest, outbreak, fraud, and corruption keywords were used to search the international databases. Additionally, the Persian counterparts of these keywords were employed to search national databases. All stages of the search process were conducted independently by two researchers.
Inclusion criteria for this study encompassed research articles written in English and/or Persian that provided examples or solutions addressing COI regarding the vaccine in the context of infectious disease outbreaks. Conversely, studies that lacked relevance to the research topic or offered incomplete information were excluded based on the exclusion criteria.
The articles underwent screening based on the PRISMA framework [42]. Initially, duplicate articles were eliminated. Subsequently, two independent researchers screened the titles and abstracts of all articles based on the predefined inclusion and exclusion criteria. In line with the anticipated objectives of this article, any studies considered irrelevant were excluded from further analysis. In the following stage, articles that were considered relevant based on their title and abstract, as well as those for which the determination of relevance solely based on title and abstract was not feasible, were selected for full-text retrieval. Subsequently, two individuals reviewed these articles, and those that closely corresponded with the objectives of our study were included for further analysis. In instances of disagreement, consensus was achieved through discussions, and if needed, a third reviewer was consulted to facilitate resolution. Initially, the search strategy yielded a total of 1,635 articles, of which 80 were duplicates.
Consequently, the title and abstract of 1,555 articles and the full text of 223 articles were assessed, resulting in the inclusion of 43 eligible articles (Appendix 2). Then, we subjected the included articles to quality appraisal, using a specific assessment checklist for each category: “The JBI Critical Appraisal Checklist for Text and Opinion Papers” for evaluating theoretical-analytical, commentary, editorial, and news articles [43] “qualitative CASP checklist” for evaluating qualitative studies [44]; and, the “CASP checklist for review” for evaluating review articles [45]. Due to the limited literature on the topic, excellent and average-quality articles were included. Following the extraction of primary data, eligible articles were assessed to address the following inquiries:
-
1.
What was the primary objective and methodology employed in the study?
-
2.
What bottlenecks of COI regarding the vaccine in infectious disease outbreaks were discussed in this study?
-
3.
What solutions were implemented to address COI regarding the vaccine in infectious disease outbreaks?
-
4.
What were the advantages, disadvantages, and practical considerations associated with the mentioned strategies for addressing COI regarding the vaccine in infectious disease outbreaks?
Data analysis was conducted using a qualitative approach (i.e., content analysis) utilising ATLAS.ti9 software. Themes and sub-themes were extracted from the data and underwent a review, refinement, and classification process. Through this process, bottlenecks and inferred policy options were identified. In three sessions with the research team, we followed these steps: Similar bottlenecks of COI and inferred policy options were grouped. Next, based on the identified origins of COI regarding the vaccine in infectious disease outbreaks, nine policy options were categorised into three distinct categories.
Results
Based on the scoping review conducted by the research team, the prominent cases of COI regarding the vaccines in infectious disease outbreaks were found to be originated from “relationships with the industry”. This category can be further divided into two subcategories:
-
1.
Relationships between the industry and government/policymakers.
-
2.
Relationships between the industry and academic institutions/researchers.
After reviewing articles and available evidence, ten bottlenecks of COI regarding the vaccine in infectious disease outbreaks were identified. These bottlenecks were categorised into two groups based on their origin, as outlined in (Table 1). Inferred Policy options derived from the literature review to address these bottlenecks are presented in (Table 2).
Based on the research team sessions, the policy options for addressing COI regarding the vaccine in infectious disease outbreaks, as categorised by origin and approach to strengthening good governance for health, are as follows:
-
Common policy options:
-
1.
Promoting the disclosure and transparency of COI for involved individuals and organisations along with the standardisation of disclosure procedures;
-
2.
Restricting or excluding those with COI from participating in technical and policy committees;
-
3.
Providing training to decision-makers and researchers, facilitating dialogue, and promoting a shared discourse on COI;
-
4.
Revising and enhancing current anti-corruption laws and policies and establishing legal sanctions for violations;
-
1.
-
Policy options to address COI arising from the relationships between the industry and government /policymakers:
-
5.
Strengthening monitoring, evaluation, and accountability mechanisms;
-
6.
Empowering the community and news media for advocacy;
-
7.
Establishing an intersectoral and multisectoral decision-making body comprising diverse stakeholders;
-
5.
-
Policy options to address COI arising from the relationships between the industry and academic institutions/ researchers:
-
8.
Developing Policies on data protection, publication, and retraction of vaccine research;
-
9.
Establishing standardised procedures and protocols for conducting studies and developing vaccine guidelines;
-
8.
Description of policy options
Common policy options
1. Promoting the disclosure and transparency of COI for involved individuals and organisations along with the standardisation of disclosure procedures
The true importance of public disclosure lies in its capacity to foster accountability within relevant committees, thereby empowering the public to assess the alignment of a committee's actions regarding COI with its stated intentions (60). Any actual or potential COI must be disclosed, made accessible to the public, and promptly updated if any changes arise. Promoting public transparency enables the continuation of productive and beneficial endeavours and serves as a deterrent against improper relationships. It is a cautionary message from the public to those with COI [60].
While transparency is essential in preserving public trust, it alone is insufficient. In situations where the underlying context is poorly understood, disclosures can become colourful descriptions of numerous and varied business relationships [68]. In addition, disclosures are often missed, incomplete, inconsistent, and unavailable [34]. Overemphasising disclosure may also lead researchers and policymakers to develop a false sense of moral immunity, absolving them of their responsibility to address COI. The disclosure of COI fails to provide transparent information about the intensity and impact of those conflicts on the public. Consequently, it fails to disclose the underlying interests upon which the decisions and policies are based [60]. This failure becomes particularly concerning when minutes of meetings and the perspectives of each person involved are unclear, making it difficult for people to assess the extent to which COI may affect an individual's views. Therefore, it is crucial to have apparent, measurable, clarified, and standardised primary interests accompanied by precise definitions for COI [32].
Additionally, an independent committee should continuously evaluate and validate the standards related to COI [60]. In situations like infectious disease outbreaks, where vaccine purchase and production contracts are hastily concluded due to emergency conditions, bypassing predetermined laws and formalities, or before thorough research on vaccine effectiveness and safety is completed, government decisions must be appropriately documented and clarified. This documentation and clarification ensure that the decision-makers’ interests do not inappropriately influence the outcome [36]; Moreover, in cases where concerns arise about the impact of COI on decisions (perceived interests), official organisations should promptly and transparently report to the public without resorting to defensive statements, in order to maintain public trust [69, 70].
2. Restricting or excluding those with COI from participating in technical and policy committees
According to guidelines from organisations like EMA (European Medicines Agency), OECD (Organization for Economic Co-operation and Development), and WHO, effective management of COI involves identifying and mitigating risks rather than systematically excluding stakeholders with COI. Meanwhile, in some situations, COI may limit the involvement of experts, either individually or organizationally, in particular decisions or actions. However, if these professionals can contribute in other beneficial ways unaffected by their COI, there may be no need to exclude them from the entire project [55].
Expert committees should establish clear criteria for defining high-risk interests or relationships, select members and voting rights based on consistent and transparent standards Preference should be given to those without COI or individuals willing to set aside their interests [60]. In cases where there is no alternative but to involve individuals with COI (e.g., due to their specialised expertise), engaging their services in an independent advisory committee is possible by developing innovative strategies [24, 60, 69].
3. Providing training to decision-makers and researchers, facilitating dialogue, and promoting a shared discourse on COI
Due to ongoing debates regarding the approach to address COI and the normalisation of the relationship between industry, academia, and governance, it is not unexpected that policymakers and academics may still require further clarification on which types of relationships give rise to COI. Insufficient understanding of the ethical basis of COI appears to be prevalent [63]. Therefore, fostering dialogue and familiarising policymakers and academics with the existing COI literature is essential, presenting them with concrete cases exemplifying the negative consequences that can arise.
4. Revising and enhancing current anti-corruption laws and policies and establishing legal sanctions for violations
Revisions of laws should ensure the active participation of civil society, the rule of law, effective management, transparency, and accountable governance [46]. Additionally, it is crucial to ensure the enforcement of laws to prevent corruption among vaccine manufacturers by imposing legal sanctions. Possible measures include facility inspections, product seizure, trade limitations (e.g., legal suspension of business activity), professional development training (e.g., regulatory and business ethics training courses), and import bans [57].
Policy options to address COI arising from the relationships between the industry and government/policymakers
5. Strengthening monitoring, evaluation, and accountability mechanisms
Few countries [71], like the Republic of Korea, Singapore, China, and Egypt have National Regulatory Authorities (NRAs) classified by the WHO as advanced or functional and integrated regulatory systems (Maturity level 3 or 4 based on the WHO Global Benchmarking Tool (GBT)) [72] that protect the majority of the population, particularly against substandard and counterfeit medical products.
To strengthen supervision, developing and implementing national and local strategies and programs to combat corruption, including in emergencies, is necessary by converting knowledge into concise and targeted solutions. International guidelines can be utilised for this purpose [46].
Establishing a committee to assess quality and conduct audits from the early stages of infectious disease outbreaks is crucial. This committee enables the evaluation and addressing COI. The impact of COI on research and governance functions should be assessed at both individual and organisational levels through a transparent COI management program overseen by the quality control and audit committee [55]. Public institutions can employ corruption risk assessment to identify vulnerabilities in their operations and develop practical and cost-effective strategies to mitigate them. Additionally, it is recommended to establish a dedicated committee to monitor emergency funds and vaccine deployment. This committee should encompass monitoring capabilities for emergency funds, vaccine procurement and distribution, and related processes in real-time to promptly identify any risks [46].
Local innovative mechanisms like the African Vaccine Regulatory Forum could contribute to addressing emergencies such as COVID-19 [59]. Also, new technologies such as blockchain can enhance data transparency and vaccine supply chain management. Establishing tracking systems for secure vaccine storage and distribution is advisable to strengthen monitoring. This system includes safeguarding vaccines in undisclosed locations and closely monitoring their transportation, thereby minimising corruption and diversion risks [46].
6. Empowering the community and news media for advocacy
To empower community advocacy, establishing a transparent central system accessible to the public is essential [66]. However, sharing a list of COI cases with the public is ineffectual and undermines trust in policymakers. Instead, it is crucial to address identified COI and provide clear explanations for the presence of individuals with COIs in decision-making committees. Furthermore, public awareness should be increased regarding the distinction between COI and corruption, emphasising communication benefits between researchers, industry, and the government. Additionally, creating a platform for civil society participation, utilising mobile software and hotlines can enable reporting and decision-making on vaccines. Developing a secure, effective, and gender-sensitive reporting system, such as anonymous proxies, is recommended [46].
During crises, the media is vital in revealing realities through investigative journalism and critical reporting on government policies. This role helps prevent COI cases that may divert crisis management from public health to political-economic fields, potentially resulting in corruption [64]. Establishing a public registration and publishing platform to disclose the COI of researchers and policymakers reminds journalists that COI can also influence news sources. This initiative can encourage journalists to expose COI cases [65]. Strengthening independent media and non-governmental organisations is necessary to prevent media COI and ensure impartial coverage of COI-related news.
7. Establishing an intersectoral and multisectoral decision-making body comprising diverse stakeholders
Emphasising the establishment of a collaborative decision-making body inclusive of various stakeholders, such as academics, the pharmaceutical industry, the health sector, and legislative institutions, is crucial. This approach helps prevent undue influence from any single stakeholder and mitigates the negative impact of COI [6]. In addition to the decision-making institution, all decisions should be based on evidence and made in meetings attended by experts from the Ministry of Health, people’s representatives, and other relevant stakeholders. Transparency and accessibility of the decision-making process should be prioritised, with no room for ambiguity. Furthermore, a mechanism should be in place to update decisions based on emerging evidence, ensuring clarity and transparency [53].
Policy options to address COI arising from the relationships between the industry and academic institutions/researchers
8. Developing policies on data protection, publication, and retraction of vaccine research
Journals that publish basic vaccine research and clinical trials on vaccine efficacy and safety should emphasise the mandatory provision of raw data and enhance the scrutiny of research data, research protocols, and statistical conclusions. Also, they should carefully review the authors’ COI. Furthermore, the data management systems utilised in the studies relied on conventional data sources, lacking the ability to guarantee data immutability throughout the data generation and analysis stages. To address this issue, the adoption of blockchain technology can be considered. This innovative technology prevents data manipulation and enables transparent tracking of data modifications. Consequently, publications, journals, and stakeholders should require researchers to utilise blockchain-based systems and establish the necessary infrastructure [29].
To ensure confidentiality and prevent scientific misconduct and fraud, research should be grounded in good practice and ethical principles [67]. However, it is essential to note that while emphasising ethical principles preserves professional values, these principles do not possess legal and executive guarantees, and ensuring adherence by all researchers is challenging.
Another measure to address COI in the publication of vaccine-related research is to adopt practices similar to the bioRxiv preprint server and other relevant platforms. These servers display a yellow warning banner atop all new COVID-19 studies, serving as a reminder that these articles are preliminary reports and have not undergone peer review [49]. Another critical aspect to discuss in this context is the retraction of articles. The current mechanisms primarily focus on reporting potential fraudulent cases and retracting incomplete articles. However, there is a notable lack of transparency in writing the reports, identifying recipients, and ensuring proper follow-up to notify affected groups about the retracted article. To address this gap, it is crucial to establish a robust system that includes a centralised body capable of reporting suspected cases of fraud in vaccine research and effectively informing organisations and individuals affected by retracted articles. This system should be built upon a well-connected network of contacts and nodes, with prompt identification of network members. Any articles retracted due to fraud should be publicly announced to ensure transparency and accountability [50].
9. Establishing standardised procedures and protocols for conducting research and developing vaccine guidelines
The development of guidelines should rely on valid handbooks to prevent potential harm to patients and the healthcare system caused by misinformation and misunderstandings. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach is a robust tool that can systematically be employed as a standard for developing vaccine guidelines. This approach involves three main stages: assessing the quality of evidence, comparing favourable and unfavourable effects, and determining the strength of the recommendation [35]. Noteworthy, following the instructions for developing vaccine-related guidelines should be mandatory and legally required for publication and approval. Simply recommending adherence to these guidelines would not carry sufficient weight. Guideline evaluators should utilise tools like AGREE and RIGHT to assess the quality of reporting and methodology in vaccine guidelines. Revising COI sections within the guidelines is crucial, enhancing their clarity.
Moreover, journal editors and guideline evaluators must establish a platform that facilitates open and impartial discussions, enabling the publication of research that examines both the support and challenges surrounding the safety and efficacy of vaccines. This platform should function independently and cross-departmentally, ensuring minimal COI and promoting an environment of free and unbiased scientific discourse [6]. Establishing an evaluation committee and involving multiple evaluators can be an effective strategy to achieve this objective. Vaccine research contracts and guideline development should also incorporate a COI declaration and establish a process to update and address potential COI. All research group members must disclose any interests that could lead to COI using a standardised form. Furthermore, the study’s final report should transparently publish information about financial sources, affiliations, and any possible COI, along with the results [37].
Discussion
This policy brief, based on a scoping review study, highlights the bottlenecks and COI cases regarding vaccines in infectious disease outbreaks that demand the attention of policymakers and health sector managers. As previously mentioned, ten bottlenecks rooted in the “relationships with the industry” were identified and categorised as “relationships between the industry and government/ policymakers” and “relationships between the industry and academic institutions/ researchers”.
COI stemming from the “relationships between industry and academic institutions/researchers” can result in vested interest, leading to inadequate attention to the public interest and biased technical recommendations. These COIs can further influence research and guidelines, leading to ineffective outbreak management and resource wastage, ultimately risking lives. Similarly, COI arising from the “relationships between the industry and government/ policymakers” regarding budget allocations for vaccine import or domestic production, licensing, and post-market services can lead to inappropriate budget allocation, resource wastage, incorrect licensing decisions, delayed vaccination, and insufficient monitoring of vaccine safety, efficacy, and supply chain quality.
The consequences of COI, regardless of its origin, include a trust crisis and weakened health governance foundations. This weakness, in turn, contributes to vaccine hesitancy, hindering immunisation efforts and impeding the control of infectious diseases. The COVID-19 pandemic has demonstrated the compromise of public trust in scientists, governments, the healthcare system, and other related organisations, posing a significant challenge to accepting public health measures and vaccination [73]. Trust in public health interventions, including vaccines, relies on various factors and is closely linked to the institutions’ credibility in their development, approval, and administration [74,75,76].
In part, the challenge of addressing vaccine hesitancy can be attributed to the limited measures taken to align the interests of professionals, vaccine manufacturers, governments, and the public interest [77]. Vaccine manufacturers have not taken explicit and practical actions to build trust within societies. As a result, it is crucial to collectively work towards reevaluating the norms governing vaccine discovery, research evaluation, and vaccination decision-making; upholding the integrity and accuracy of vaccine research and policy-making demands significant effort and a transformative shift in communicating with the industry.
In our scoping review, we have identified several documents encompassing international experiences that are either fully or partially relevant to addressing COI regarding the vaccine in infectious disease outbreaks. These experiences have been remarkable and worthy of highlighting. Two of these experiences are related to The Innovative Medicines Initiative’s Accelerated Development of VAccine benefit-risk Collaboration in Europe (ADVANCE) consortium. The first experience is the “Guidance for the governance of public–private collaborations in vaccine post-marketing settings in Europe”. This guidance was developed after the 2009 influenza pandemic based on the need for appropriate infrastructures to strengthen public–private collaborations (PPCs), improve stakeholder interactions, and enhance the collection and analysis of safety and effectiveness data [55]. The ADVANCE consortium also released a “Code of Conduct for collaborative vaccine studies”. The development of this code of conduct was guided by three core values: best science, strengthening public health, and transparency. It involved a thorough review of existing guidance and relevant published articles. The ADVANCE Code of Conduct includes ten topics, including COI [30]. Another significant document is a supplementary document about the vaccine, which contains detailed descriptions of the experiences and processes of 15 well-established National Immunization Technical Advisory Committees from all world regions. These committees are crucial in providing national governments with information for evidence-based decisions regarding vaccine and immunisation policy [33]. These documents highlight the importance of addressing COI regarding the vaccine, establishing effective governance, promoting transparency, and making evidence-based decisions in vaccine-related collaborations and policy development during infectious disease outbreaks.
In Iran, there is a growing focus on COI within the health system, as evidenced by official documents and papers addressing COI (for example Islamic Parliament Research Center (IPRC) report on COI in the health sector) [78]. However, there is a lack of official documentation and academic papers dedicated explicitly to COI regarding the vaccine in infectious disease outbreaks. Our research primarily yielded news articles and expert critiques on this topic. During the COVID-19 pandemic in Iran, cases of COI emerged in various areas, including allocating funds to domestic vaccine producers, vaccine importation, and vaccine-related research. These COIs raised concerns about potential biases, fairness, and transparency in decision-making processes. However, despite these COIs, Iran has not implemented a comprehensive policy strategy to address them effectively. This lack of a robust policy approach to managing COIs may have impeded efforts to ensure fair and unbiased decision-making throughout the pandemic response [54, 62, 79].
Iranian Policymakers need to acknowledge the importance of managing COI regarding the vaccine in infectious disease outbreaks and prioritise developing and implementing appropriate policies and strategies.
Finally, it is essential to acknowledge the limitations of this study. Firstly, a scoping review methodology in this policy brief may narrow the available data coverage, limiting the overall understanding breadth. Additionally, it should be noted that while the optional consultation with stakeholders and experts for additional insights is recommended in the O'Malley and Arksey protocol, we have chosen not to pursue this step in our project. Consequently, there is a possibility of failing to fully capture the diverse perspectives of stakeholders involved in the decision-making process.
Lastly, including articles with average quality due to the limited availability of literature on the topic can introduce uncertainty or inconsistency in the findings and recommendations presented in the policy brief.
Conclusion
Regarding the necessity of establishing relationships with the industry, it is crucial to take appropriate measures to address COI while fully recognising the COI that may arise from such relationships. In this regard, following sessions conducted by the research team and expert consultations and with consideration given to the principles underpinning good governance for health, the proposed policy options in this policy brief are summarised and prioritised as follows:
-
1.
Promoting disclosure and transparency of COI, standardisation of disclosure procedures, and subsequently, limitations on the involvement of members with COI are directly aligned with the key principles of good governance for health, including “Transparency”, “The Rule of Law”, “Effectiveness and Efficiency”, “Responsibility”, and “Accountability”.
-
2.
Raising awareness among policymakers and researchers, fostering dialogue, and promoting shared discourse on COI are directly aligned with the key principles of good governance for health, including “Consensus Orientation” “Participation”, “Effectiveness and Efficiency”.
-
3.
Enhancing the monitoring, evaluation, and accountability of policymakers and establishing appropriate penalties for any violations of the law are directly aligned with the key components of good governance for health, including “Participation”, “Equality”, “The Rule of Law”, “Responsibility”, “Accountability”, “Transparency” and “Effectiveness and Efficiency”.
It is essential to recognise that the findings and recommendations outlined in this policy brief may have context-specific implications and may not be directly applicable to different settings or regions. Therefore, we recommend conducting policy dialogues with stakeholders to discuss the policy options’ advantages, disadvantages, and practical considerations. Moreover, evaluating the effectiveness of these policy options in real-world scenarios is essential, as this evaluation may reveal the need for potential revisions to ensure practicality and efficacy.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Notes
The protocol consists of six stages: identification of research questions, identification of relevant articles through searches in reputable scientific databases, review of grey literature, theses, and relevant review articles, selection of relevant articles from retrieved primary studies, extraction of data into tables and charts, compilation, summarization, and dissemination of findings, and optional consultation with stakeholders and experts for additional insights.
Abbreviations
- COI:
-
Conflict of interest
- SID:
-
Scientific Information Database
- EMA:
-
European Medicines Agency
- OECD:
-
Organisation for Economic Co-operation and Development
References
Davison RM. The transformative potential of disruptions: A viewpoint. Int J Inf Manage. 2020;55:102149.
WHO COVID-19 Dashboard. Geneva: World Health Organization; 2020. Available online: https://covid19.who.int/. Last cited: 5 July 2023.
Available from: https://www.who.int/news-room/facts-in-pictures/detail/immunization. Updated Dec 5 2019.
Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis. 2011;52(7):911–6.
Bragazzi NL, Watad A, Amital H, Shoenfeld Y. Debate on vaccines and autoimmunity: do not attack the author, yet discuss it methodologically. Vaccine. 2017;35(42):5522–6.
Elisha E, Guetzkow J, Shir-Raz Y, Ronel N. Retraction of scientific papers: The case of vaccine research. Crit Public Health. 2022;32(4):533–42.
Bechini A, Zanella B, Bonito B, Paoli S, Di Pisa G, Moscadelli A, Ancillotti L, Bonanni P, Boccalini S. Quality and safety of vaccines manufacturing: an online survey on attitudes and perceptions of Italian internet users. Vaccines. 2021;9(9):1015.
Bechini A, Bonanni P, Zanella B, Di Pisa G, Moscadelli A, Paoli S, Ancillotti L, Bonito B, Boccalini S. Vaccine production process: how much does the general population know about this topic? A web-based survey. Vaccines. 2021;9(6):564.
Pew Research Center, December 2020, “Intent to Get a COVID-19 Vaccine Rises to 60% as confidence in research and development process increases”. Available from: https://www.pewresearch.org/science/wp-content/uploads/sites/16/2020/12/PS_2020.12.03_covid19-vaccine-intent_report.pdf.
Giambi C, Fabiani M, D’Ancona F, Ferrara L, Fiacchini D, Gallo T, Martinelli D, Pascucci MG, Prato R, Filia A. Parental vaccine hesitancy in Italy–results from a national survey. Vaccine. 2018;36(6):779–87.
Poland CM, Ratishvili T, Poland GA. Focus: Vaccines: Distorted Human Decision-Making as a Critical Aspect of Pandemic Planning and Preparedness. Yale J Biol Med. 2022;95(2):281.
Reinert P. Little history of antivaccination leagues. Médecine thérapeutique/Pédiatrie. 2016;19(4):282–5.
Savage M. One in three ‘unlikely to take Covid vaccine’. The Guardian. 2020;6. Available from: https://www.theguardian.com/world/2020/dec/06/one-in-three-unlikely-to-take-covid-vaccine.
Duffy B. Corona virus uncertainties: vaccines, symptoms and contested claims. London: The Policy Institute, Kings College; 2020.
Goldenberg, Maya J. Vaccine Hesitancy: public trust, expertise, and the war on science. university of Pittsburgh press, 2021. JSTOR. https://doi.org/10.2307/j.ctv1ghv4s4. Accessed 21 Sept 2023.
Braithwaite J. Corporate crime in the pharmaceutical industry (Routledge Revivals): Taylor & Francis; 2013. ISBN 9780203597712.
Doshi P. Influenza: marketing vaccine by marketing disease. Bmj. 2013;346:3037.
Dukes G, Braithwaite J, Moloney JP. Pharmaceuticals, corporate crime and public Health. 2014. https://doi.org/10.4337/9781783471102.
Elliott C. White coat, black hat: Adventures on the dark side of medicine. Beacon Press; 2011. ISBN: 978-080706144-2
Ferner RE. The influence of big pharma. BMJ. 2005;330(7496):855–6. https://doi.org/10.1136/bmj.330.7496.855.
Gotzsche P. Deadly medicines and organised crime: how big pharma has corrupted healthcare. CRC Press; 2019. ISBN 9780429084034
Habakus LK, Holland M. Vaccine epidemic: how corporate greed, biased science, and coercive government threaten our human rights, our health, and our children. Skyhorse Pub; 2011.
Posner G. Pharma: greed, lies, and the poisoning of America. Avid Reader Press / Simon & Schuster; 2020.
Cohen D, Carter P. WHO and the pandemic flu “conspiracies.” BMJ. 2010;340:c2912.
Fahmy D. Drugmakers, Doctors Rake in Billions Battling h1N1 Flu: Swine Flu is Bad for Victims, But Good for Businesses That cater to expanding Market. ABC News Retrieved. 2009;14:2009.
World Bank. The Organisation for Economic Cooperation and Development (OECD), the United Nations Office on Drugs and Crime (UNODC). Preventing and managing conflicts of interest in the public sector. Good practices guide.2020. Available from: https://www.unodc.org/documents/corruption/Publications/2020/Preventing-and-Managing-Conflicts-of-Interest-in-the-Public-Sector-Good-Practices-Guide.pdf.
Tesfaye AW, Alemneh HT. Analysis of a stochastic model of corruption transmission dynamics with temporary immunity. Heliyon. 2023;9(1):e12752.
Catchick P. Conflict of interest: Gateway to corruption. In: 2014 ACFE European Fraud Conference. 2014.
Boetto E, Golinelli D, Carullo G, Fantini MP. Frauds in scientific research and how to possibly overcome them. J Med Ethics. 2021;47(12):e19–e19.
Kurz X, Bauchau V, Mahy P, Glismann S, van der Aa LM, Simondon F. The ADVANCE Code of Conduct for collaborative vaccine studies. Vaccine. 2017;35(15):1844–55.
Abbasi K. Covid-19: politicisation, “corruption”, and suppression of science. BMJ. 2020;371:m4425.
Carlowe J. WHO expert had conflict of interest, Danish newspaper alleges. BMJ. 2010;340(7738):119.
Gessner BD, Duclos P, DeRoeck D, Nelson EAS. Informing decision makers: experience and process of 15 National Immunization Technical Advisory Groups. Vaccine. 2010;28:A1–5.
Lexchin J. COVID-19 vaccine task force and conflicts of interest. Healthc Policy. 2022;17(3):20–27. https://doi.org/10.12927/hcpol.2022.26732.
Silva ML, Perrier L, Paget JW, Mosnier A, Buthion V, Cohen JM, Späth HM. Influenza vaccination policy-making processes in France and The Netherlands: Framework and determinants. Health Policy. 2016;120(3):293–305.
Thacker PD. Conflicts of interest among the UK government’s covid-19 advisers. BMJ. 2020;371:4716.
Wang Z, Liu H, Li Y, Luo X, Yang N, Lv M, Zhou Q, Li Q, Wang L, Zhao J. COVID-19 vaccine guidelines was numerous in quantity but many lack transparent reporting of methodological practices. J Clin Epidemiol. 2022;144:163–72.
Zhao J, Demir F, Ghosh PK, Earley A, Kim M. Reforming the countermeasures injury compensation program for COVID-19 and beyond: An economic perspective. J Law Biosci. 2022;9(1):lsac008.
Available from: https://iran-bssc.ir/research-fields/conflict-of-interests/COI-analysis-invirtual-space/15159/.
Jafari F, HajiNabi K, Jahangiri K, Riahi L. Good governance in the health system: A qualitative study. 2019.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and metaAnalyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097. https://doi.org/10.1371/journal.pmed1000097.
Aromataris E, Munn Z (Editors). JBI manual for evidence synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-01.
CASP C. CASP qualitative checklist. Critical Appraisal Skills Programme. 2018.
Programme. CASP Systematic Review Checklist https://casp-uk.net/casp-tools-checklists/.
United Nations Office on Drugs and Crime(UNODC). COVID-19 vaccines and corruption risks: preventing corruption in the manufacture, allocation and distribution of vaccines. Available from: https://www.unodc.org/documents/corruption/COVID-19/Policy_paper_on_COVID-19_vaccines_and_corruption_risks.pdf.
Schönhöfer PS, Schulte-Sasse H. Swine flu pandemic in retrospect. Course of events, failures, lessons. Tagliche Praxis. 2012;53:647–54.
Available from: https://www.setaresobh.ir/fa/main/detail/78155/7.
Dinis-Oliveira RJ. COVID-19 research: pandemic versus “paperdemic”, integrity, values and risks of the “speed science.” Forensic Sci Res. 2020;5(2):174–87.
Montgomery K, Oliver AL. Conceptualizing fraudulent studies as viruses: New models for handling retractions. Minerva. 2017;55(1):49–64.
Olopade C, Tagle M Olopade O. Clinical Research in International Settings. 2018. https://doi.org/10.1016/B978-0-12-849905-4.00008-3.
Smith JC, Appleton M, MacDonald NE. Building confidence in vaccines. Hot topics in infection and immunity in children IX. 2013. p. 81–98.
Available from: hamshahrionline.ir/x7bVy.
Available from: https://daylinews.ir/?p=74563.
Torcel-Pagnon L, Bauchau V, Mahy P, Htar MTT, van der Sande M, Mahé C, Krause TG, Charrat A, Simondon F, Kurz X. Guidance for the governance of public-private collaborations in vaccine post-marketing settings in Europe. Vaccine. 2019;37(25):3278–89.
Spicher VM. The Federal Vaccination Commission in Switzerland: an officially appointed independent commission ensuring evidence-based recommendations and transparent procedures. Vaccine. 2010;28:A48–53.
Bramstedt KA. Unicorn poo and blessed waters: COVID-19 quackery and FDA warning letters. Ther Innov Regul Sci. 2021;55:239–44.
Chauhan H, Gupta D, Gupta S, Singh A, Aljahdali HM, Goyal N, Noya ID, Kadry S. Blockchain enabled transparent and anti-counterfeiting supply of COVID-19 vaccine vials. Vaccines. 2021;9(11):1239.
Newton PN, Bond KC, Adeyeye M, Antignac M, Ashenef A, Awab GR, Bannenberg WJ, Bower J, Breman J, Brock A. COVID-19 and risks to the supply and quality of tests, drugs, and vaccines. Lancet Glob Health. 2020;8(6):e754–5.
Grundy Q. Commentary - From Transparency to Accountability: finding ways to make expert advice trustworthy. Healthc Policy. 2022;17(3):28–33. https://doi.org/10.12927/hcpol.2022.26731.
Bijl D, Schellekens H. The sponsored pandemic of the Mexican flu? Int J Risk Saf Med. 2011;23(2):73–9.
Available from: https://iran-bssc.ir/?p=14142.
Wheelock A, Ives J. Vaccine confidence, public understanding and probity: time for a shift in focus? J Med Ethics. 2022;48(4):250–5.
Gesser-Edelsburg A, Hijazi R, Cohen R. It takes two to tango: How the COVID-19 vaccination campaign in Israel was framed by the health ministry vs. the television news. Front Public Health. 2022;10:887579.
Holland K, Sweet M, Blood RW, Fogarty A. A legacy of the swine flu global pandemic: Journalists, expert sources, and conflicts of interest. Journalism. 2014;15(1):53–71.
McClymont E, Brophy J, Dubey V, Kwong J, Meyer S, Crowcroft N, Halperin S, MacDonald S, Simmons K, Top K. Is ‘conflict of interest’a Misnomer? Managing interests in immunization research and evaluation. Hum Vaccin Immunother. 2022;18(1):1879580.
Alba Bermúdez JM, Proaño Maldonado LM. La ética en la publicación científica en tiempos de COVID-19: The Ethics in the Publication in Times of COVID-19. Revfil [Internet]. 31 de octubre de 2021 [citado 21 de septiembre de 2023];38(99):225–40. https://doi.org/10.5281/zenodo.5644537. Available from: https://produccioncientificaluz.org/index.php/filosofia/article/view/37053.
NRCo C. COVID‑19 Vaccine task force registry of interests. 2021.
Godlee F. Conflicts of interest and pandemic flu. BMJ. 2010;340:c2947. https://doi.org/10.1136/bmj.c2947.
Gokhale SS. Monitoring the perception of Covid-19 vaccine using topic models. In: 2020 IEEE intl conf on parallel & distributed processing with applications, big data & cloud computing, sustainable computing & communications, social computing & networking (ISPA/BDCloud/SocialCom/SustainCom). IEEE; 2020. p. 867–74. https://doi.org/10.1109/ispa-bdcloud-socialcom-sustaincom51426.2020.00134.
Organization WH. List of National Regulatory Authorities (NRAs) Operating at Maturity Level 3 (ML3) and Maturity Level 4 (ML4)(as Benchmarked against WHO Global Benchmarking Tool (GBT)). 2021.
Organization WH. WHO Global Benchmarking Tool (GBT) for evaluation of national regulatory systems of medical products: revision VI. 2021.
Algan Y, Cohen D, Davoine E, Foucault M, Stantcheva S. Trust in scientists in times of pandemic: Panel evidence from 12 countries. Proc Natl Acad Sci. 2021;118(40):e2108576118.
Attwell K, Leask J, Meyer SB, Rokkas P, Ward P. Vaccine rejecting parents’ engagement with expert systems that inform vaccination programs. J Bioeth Inq. 2017;14(1):65–76.
Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–9.
Monitor WG. How does the world feel about science and health. London: Wellcome Trust. https://wellcome.ac.uk/reports/wellcome-global-monitor2018.
UK Vaccine Network accessed. Available from: https://www.gov.uk/government/groups/uk-vaccines-network.
Available from: https://rc.majlis.ir/fa/report/show/1668525
Available from: https://www.javann.ir/004Ysq.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NS and SMM conceived the study and designed its methodology. All authors participated in conducting the study. NS and SMM conducted the qualitative data analysis. NS, SMM, HGH, MHM, MHMA and HM contributed to the drafting of the manuscript. NS and SMM revised the draft and developed the final version. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Shahid Sadoughi University of Medical Sciences under the ethical code noIR.1401.111.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix 1.
Search strategy.
Additional file 2: Appendix 2.
PRISMA flow diagram.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Soleimani, N., Ghoshouni, H., Mostafavi, H. et al. Addressing conflicts of interest regarding the vaccine in infectious disease outbreaks based on good governance for health approach: a policy brief. BMC Health Serv Res 23, 1028 (2023). https://doi.org/10.1186/s12913-023-10020-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-023-10020-w