Abstract
Background
Fatal drug overdoses and serious injection-related infections are rising in the US. Multiple concurrent infections in people who inject drugs (PWID) exacerbate poor health outcomes, but little is known about how the synergy among infections compounds clinical outcomes and costs. Injection drug use (IDU) converges multiple epidemics into a syndemic in the US, including opioid use and HIV. Estimated rates of new injection-related infections in the US are limited due to widely varying estimates of the number of PWID in the US, and in the absence of clinical trials and nationally representative longitudinal observational studies of PWID, simulation models provide important insights to policymakers for informed decisions.
Methods
We developed and validated a MultimorbiditY model to Reduce Infections Associated with Drug use (MYRIAD). This microsimulation model of drug use and associated infections (HIV, hepatitis C virus [HCV], and severe bacterial infections) uses inputs derived from published data to estimate national level trends in the US. We used Latin hypercube sampling to calibrate model output against published data from 2015 to 2019 for fatal opioid overdose rates. We internally validated the model for HIV and HCV incidence and bacterial infection hospitalization rates among PWID. We identified best fitting parameter sets that met pre-established goodness-of-fit targets using the Pearson’s chi-square test. We externally validated the model by comparing model output to published fatal opioid overdose rates from 2020.
Results
Out of 100 sample parameter sets for opioid use, the model produced 3 sets with well-fitting results to key calibration targets for fatal opioid overdose rates with Pearson’s chi-square test ranging from 1.56E-5 to 2.65E-5, and 2 sets that met validation targets. The model produced well-fitting results within validation targets for HIV and HCV incidence and serious bacterial infection hospitalization rates. From 2015 to 2019, the model estimated 120,000 injection-related overdose deaths, 17,000 new HIV infections, and 144,000 new HCV infections among PWID.
Conclusions
This multimorbidity microsimulation model, populated with data from national surveillance data and published literature, accurately replicated fatal opioid overdose, incidence of HIV and HCV, and serious bacterial infections hospitalization rates. The MYRIAD model of IDU could be an important tool to assess clinical and economic outcomes related to IDU behavior and infections with serious morbidity and mortality for PWID.
Similar content being viewed by others
Background
People who inject drugs (PWID) experience many complications from the substances used and from injection drug use (IDU). These complications can often occur together in PWID and include substance use disorder (e.g., opioid use disorder [OUD]), drug overdoses, and serious injection-related infections such as HIV, hepatitis C virus (HCV), and severe bacterial infections like endocarditis. These complications also escalate morbidity and mortality among PWID, particularly for those without access to harm reduction measures such as sterile syringes and other equipment. The increasing use of synthetic opioids, such as fentanyl, are driving fatal drug overdoses in the US [1]. PWID experience higher infection rates and poorer outcomes from infections (HIV, HCV, bacterial infections), creating a syndemic between drug use and these infections [2,3,4,5]. PWID may also experience infections concurrently (e.g., HIV/HCV co-infection), and one infection can exacerbate poor outcomes for another. For example, people living with HIV have an increased risk of both HCV-related fibrosis and cirrhosis as well as invasive bacterial infections such as endocarditis, particularly at lower CD4 counts [6, 7].
Despite high morbidity and mortality rates, nationally representative data from observational cohort studies on HIV, HCV, and bacterial infections among PWID and randomized control trials on interventions to reduce these infections among PWID are lacking. Policymakers often need information on long-term outcomes and cost-effective interventions to make important health decisions. In such cases, decision-analytic models can enrich the evaluation of clinical and public health interventions beyond the time horizons of clinical trials and observational studies. This importance is clear when studies focusing on PWID are limited, when trials have excluded PWID, or when important outcomes of public health interventions occur among people not engaged in care and thus are missed in trials and cohort studies [8]. However, previously published models of IDU do not consider multiple infections that PWID often experience concurrently nor account for the compounding clinical and economic effects of co-infections [9,10,11,12]. A multimorbidity model that accounts for multiple drug- and infection-related complications in PWID would improve estimations of the clinical and economic impact of the syndemic of drug use and related infections as well as more clearly demonstrate how interventions could impact multiple complications simultaneously.
Decisionmakers also require confidence in model-generated results to reasonably reflect the clinical environment of diseases to consider the results when making medical decisions or health care resource allocation. However, uncertainty around key model inputs and structure can put that confidence at risk. To increase confidence in the model results, best practices recommend transparency in the model’s construction, validation of the model’s ability to reproduce observed real-world outcomes, and reporting and evaluation of parameter uncertainty through sensitivity analyses [13,14,15]. Our objective was to create a comprehensive decision-analytic model that simulates the dynamics of multiple infections in PWIDs. In this paper, we sought to describe in detail the structure and validation of a MultimorbiditY model to Reduce Infections Associated with Drug use (MYRIAD) that reflect national estimates in the US to be used for future policy analyses.
Methods
Model description
Overview
MYRIAD is a microsimulation model of drug use and serious infections related to specific injection behaviors (e.g., needle sharing or reuse, especially without effective HIV pre-exposure prophylaxis, or introduction of skin or oral flora via injection) that is programmed in the R computer language (version 4.2.1) [16]. The model includes 4 health conditions: drug use behavior, serious bacterial infections, HIV, and HCV (Fig. 1). Each health condition contains many possible health states that a person can be in for that condition each month. The model creates a simulated person and assigns it an age, gender, and different health states within each of the 4 health conditions based in probabilities. No one enters the model with an active bacterial infection. The model will then increase the person’s age each month and may change the health state within each health condition for that month. This change is based on probabilities from data derived from the published literature (Table 1). Additional details on input derivations are in Supplement A. All simulated persons have a probability of death each month that is based on their age, gender, and health state they are in within each of the 4 health conditions. The model will increase the person’s age and assess changes in their health states until that person is determined to die in a particular month or until that person reaches the end of pre-set duration. The model tracks individual clinical outcomes from model start until death or when the pre-set duration is reached. We repeat the process to create a hypothetical cohort in line with recent estimates of active IDU prevalence in the US; active IDU was defined as having injected drugs within the past year [17].
Schema for the Multimorbidity Model to Reduce Infections Associated with Drug Use (MYRIAD). Notes: HCV: hepatitis C virus; OUD: opioid use disorder; CNS: central nervous system. All persons in the model start in an active injection drug use state within the “low-risk,” “medium-risk,” and “OUD” states
Mortality
After the model determines people’s final health state within each health condition for the month, it estimates the probability of death for that month. People are assigned a background mortality based on age and sex, derived using cause-specific mortality from the National Center for Health Statistics and removing mortality from substance use, IDU-related bacterial infections, HIV, and HCV since they were estimated elsewhere in the model [18]. Additional mortality rates are added onto the background mortality for each person based on their drug use behavior, type of bacterial infection, CD4 state, and liver fibrosis state (Table 1) [19,20,21,22,23].
Drug use behavior
People are assigned one of four health states regarding drug use (focusing on opioid use): “Low-risk” (i.e., prescription opioid misuse without an OUD diagnosis), “Medium-risk” (i.e., illicit opioid use such as heroin without OUD), “OUD” (OUD with prescription or illicit opioids), and “Remission.” People can transition between different states each month as described in Fig. 1. Once people reach the “OUD” state, they can only transition to the “Remission” state and vice versa (indicating relapse). No one starts out in the “Remission” state. Each opioid use state confers additional monthly mortality risk due to drug overdose. Data on the monthly transition between states and additional mortality (in excess to background mortality) within each state were derived from model calibration and the published literature (Table 1) [24]. Apart from drug use and drug-related disorders (i.e., OUD), which informs the risk of fatal drug overdose in the model, we also account for the route of drug administration. People are also assigned an IDU state of “None,” “Active,” and “Former.” An “Active” state confers additional risk of acquiring infections from the act of injection. In addition, those with IDU have a lower likelihood of starting antiretroviral therapy (ART) in people with HIV compared to those not in the “Active” state. People in the “None” and “Former” states – which can include people who orally ingest, snort, or smoke drugs – have the same probability of acquiring infections regardless of their drug use state. As this model focuses on PWID, we assume all people start in the “Active” IDU state. People who move into the “Remission” opioid use state also move into the “Former” IDU state.
Serious bacterial infections
People can be in either an “Active” serious bacterial infection state or have “None.” They enter the model in the “None” state. For people with an “Active” serious bacterial infection, they are assigned one of 4 types of infections: “CNS Infection,” “Endocarditis,” “Osteomyelitis,” and “Skin and Soft Tissue Infection.” The type of infection confers an additional mortality risk only for the month in which the infection occurs. The probability of acquiring or being cured of an infection as well as the type of infection depends on the current IDU state; data were derived from published literature (Table 1) [4, 25]. Only one bacterial infection can be experienced in a given month, although simulated people remain at risk for additional infections in subsequent months.
HIV
People enter the model in a “With HIV” or “Without HIV” state, informed from published national HIV prevalence data (Table 1) [26]. Those in the “Without HIV” state have a monthly probability of moving to the “With HIV” state based on their IDU state with inputs derived from national HIV incidence estimates [26]. People in the “With HIV” state cannot return to the “Without HIV” state. Those in the “With HIV” state are assigned a CD4 state of “High” (CD4 ≥ 500 cells/mm3), “Medium” (CD4 200–499 cells/mm3), or “Low” (CD4 < 200 cells/mm3); the current CD4 state determines an additional monthly mortality risk due to HIV. People can transition between CD4 states based on if they are receiving ART – those not on ART can only transition to a lower CD4 state and those on ART can transition to lower or higher CD4 states based on published data [10, 27]. The likelihood of starting or stopping ART depends on a person’s IDU state as well as if they are aware of their HIV status since only those aware of their HIV state can start ART [26, 28].
HCV
People enter the model in either a “With HCV” or “Without HCV” state, and they transition monthly between the states based on their IDU state (Table 1) [20]. Those in the “Without HCV” state have a monthly probability of moving to the “With HCV” state based on their IDU state, with inputs derived from national HCV incidence estimate [29]. When in the “With HCV” state, there is a monthly probability for transitioning in the Liver state, which uses the METAVIR score to stage liver fibrosis severity from “F0” through “F4”. Those with a Liver state of “F4” incur a probability of developing decompensated cirrhosis or hepatocellular carcinoma, which both confer increased liver-related mortality.
Model validation and calibration
We used a multistep approach for model validation based on guidelines from the International Society for Pharmacoeconomics and Outcomes Research and the Society for Medical Decision Making [13].
Face validation
We first ensured face validity through detailed review of the model structure, underlying assumptions, and derived inputs with experts in simulation modeling of infectious diseases and opioid use who were not involved in the model’s construction.
Internal validation and calibration
We next internally validated the model by comparing model results to data used as model inputs for each health condition to check the validity of the model structure, and calibrated model parameters as needed to meet target estimates. For example, we isolated the HIV health condition to ensure HIV incidence and prevalence occurred as expected according to input specifications, which did not require calibration (Supplement B). However, we used national estimates of annual fatal opioid overdose rates from 2015 to 2019 to calibrate monthly transition probabilities between health states within the drug use behavior health condition.
We calibrated unknown variables within certain health conditions using the approach from Vanni et al. (Table 2) [30]. First, we identified parameters within each health condition to vary for the calibration process. Second, we selected calibration targets within each health condition. Third, we selected Pearson’s Chi-square as the goodness of fit (GOF) measure. This measure was selected to account for the level of certainty in the observed data while also balancing the complexity of the model and processing time to run the model. Fourth, we used Latin hypercube sampling as a parameter search strategy. This approach uses a probability density function and divided intervals with the same probability. A parameter value is selected within each interval for each parameter. Fifth, we established convergence criteria for each parameter being calibrated, which defines when the GOF parameters have been successfully met. We considered the convergence criteria being met when model outputs were within the 99% confidence interval of the observed calibration target values. Sixth, we established conducting 100 samples as a stopping rule for terminating the calibration process to balance identifying the ideal parameter set while also accounting for model processing time. Finally, we used the parameter sets meeting the convergence criteria and minimized the GOF measure for the external validation process.
Target estimates for internal validation and calibration
We calibrated the model to publicly available data. We identified parameter subsets that best fit the calibration data targets through an indirect, iterative process with Latin hypercube sampling and a GOF goal to minimize Pearson’s \({\chi }^{2}\) while within the 99% confidence interval of the calibration target data.
Target estimates for the annual fatal overdose rates from 2015 to 2019 were calculated using the number of fatal opioid overdoses identified in the Centers for Disease Control and Prevention (CDC) Wide-ranging Online Data for Epidemiologic Research (WONDER) database divided by an adjusted estimate for the number of people who misuse opioids in the US from the National Survey on Drug Use and Health (NSDUH) (Table 3) [1, 24]. Compared to published opioid overdose death rates for the general US population, which were estimated to increase from 10.4 in 2015 to 21.4 in 2020 per 100,000 people, we calibrated towards higher opioid overdose death rate targets for a population of people who misuse opioids that included people who misuse prescription opioids and/or used heroin [1]. While the number of people who misuse opioids can be challenging to estimate, this approach more accurately reflects the at-risk population for fatal opioid overdoses. We applied a multiplier from a capture-recapture analysis that more accurately reflects the prevalence of OUD to account for the underreporting of opioid misuse in the NSDUH [31]. We also accounted for the exclusion of people experiencing homelessness or incarceration in the NSDUH by estimating the number of people with substance use disorder within these populations (see Supplement Table B1) [32, 33].
Target estimates for HIV incidence from 2015 to 2019 were calculated by the annual number of new HIV infections attributed to IDU divided by an estimated number of active PWID without HIV (Supplement Table B2) [5, 26]. Target estimates for HCV incidence from 2015 to 2019 were calculated by the estimated number of new HCV infections in people ages 18–40 (as a proxy for IDU) divided by the estimated age-related population of PWID (Supplement Table B3) [17, 29, 34, 35]. Target estimates for serious bacterial infection hospitalization rates from 2015 to 2017 were calculated by the estimated annual number of hospitalizations divided by the number of active PWID (Supplement Table B4) [4]. Active PWID was defined as having reported IDU within the past 12 months.
External validation
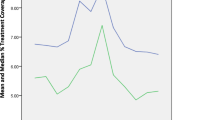
To externally validate the model, we integrated all health conditions within the model and compared model output for the year 2020 to estimated targets from the published literature for that year (Fig. 2).
Estimates of clinical outcomes
We provided estimates of clinical outcomes over 5 years (2015–2019). To estimate the total number of fatal injection-related overdoses, we first averaged model results for the annual fatal opioid overdose rates that used parameter subsets meeting the convergence criteria. Next, we multiplied the rate by the annual estimate for the number of people who misuse opioids to calculate the number of fatal opioids overdoses each year. Lastly, we applied multipliers for the proportion of people who reported IDU upon admission for opioid use within the Treatment Episode Data Set-Admissions (TEDS-A) dataset (as a proxy for the proportion of opioid overdose deaths due to IDU) and for the proportion of injection-related deaths from opioids to calculate the total number of injection-related overdose deaths (Supplemental Table B6) [36].
The number of new HIV and HCV infections among PWID from 2015 to 2019 were estimated by applying model results for annual HIV and HCV incidence to the estimated PWID population for each year.
Results
Internal validation and calibration
For the drug use behavior health condition, we identified 3 independent parameters to undergo calibration. Out of 100 parameter sets derived by Latin Hypercube sampling for monthly transition probabilities among drug use behavior states, model results for 3 parameter sets fell within the 99% confidence interval of the annual opioid overdose death target data (Fig. 2). For parameter sets meeting the convergence criteria, the monthly probability of transitioning within the drug use behavior state ranged from 0.22 to 0.27% for the “Low-risk” to “Medium-risk” state, 0.06–0.32% for the “Low-risk” to “OUD” state, and 2.80–3.80% for the “Medium-risk” to “OUD” state. We identified the best-fitting parameter set with the lowest Pearson’s Chi-square (Supplement Table B5). Additional parameter sets for internal validation of severe bacterial infection hospitalization rates, HIV and HCV incidence can be found in Supplement B (Figures B1-B4). Model results for HIV and HCV incidence were within the 99% confidence interval of target estimates.
External validation
Of the 3 parameter sets meeting the convergence criteria for fatal opioid overdose rate, 2 sets produced a model estimate that fell within the 99% confidence interval for the 2020 target rate (Fig. 2).
Estimates of clinical outcomes
From 2015 to 2019, the model estimated 223,288 fatal opioid overdoses. Using data from TEDS-A that approximated 48.9% of these deaths were injection-related and 91.2% of injection-related deaths are from opioids (Supplemental Table B7), we estimated 119,816 injection-related overdose deaths over this period. The model estimated 17,137 new HIV infections and 143,765 new HCV infections over this period as well.
Discussion
We described the structure of a novel multimorbidity microsimulation model of injection drug use and associated infections, detailing the process for input derivation, model calibration and validation against national US data. The model produced well-fitting results to key calibration and validation targets that include fatal opioid overdose rates, HIV incidence, and HCV incidence.
The model estimated approximately 120,000 injection-related overdose deaths from 2015 to 2019. Hall et al. estimated 28,257 injection-related overdose deaths for 2018, [36] slightly above our model’s estimate of 24,957 injection-related overdose deaths for that year. Our model’s estimate of more than 17,000 new HIV infections among PWID from 2015 to 2019 is similar to HIV surveillance estimates of 18,700 (range 15,100–22,400) over the same time period (using transmission categories of IDU with or without men who have sex with men) [26]. Similarly, our model’s estimate of nearly 144,000 new HCV infections among PWID was comparable to surveillance estimates of 158,000 (range 125,000–539,000) among people aged 18–40, which serves as a proxy for new HCV infections from IDU [29].
The model’s value is in its ability to address the syndemic of drug use and associated infections by incorporating drug overdose with injection-related viral and bacterial infections, which is particularly important for PWID who may experience many infections concurrently. In the absence of preventive measures, undiagnosed asymptomatic HIV and HCV each propagate transmission of the other, and treatment delays worsen the long-term response as well as survival. This is reflected in our input parameters that incorporate higher monthly probabilities for developing a new bacterial infection, HIV, HCV infection in PWID (Table 1). As PWID seek medical care for acute symptomatic bacterial infections, an opportunity arises for earlier HIV and HCV diagnoses, linkage to care, and treatment of OUD, a key contributor to IDU. Other models of IDU have focused on a viral infection (HIV and HCV) or a bacterial infection (e.g., endocarditis), while this model integrates multiple infections with an emphasis on the multimorbidity of IDU [9,10,11,12].
The model also distinguishes substance use and substance use disorders (e.g., OUD) from the act of injection, which will allow us to look at interventions with different goals. For example, syringe service programs focus on reducing infections associated with the act of injection without addressing the drug use (i.e., harm reduction). Whereas medications for OUD would address OUD (but not necessarily non-opioid substance use disorder), where IDU may or may not decrease. This model allows us to look at the impact of medications for OUD on its own, syringe service programs on its own, and an integrated syringe service program with MOUD that could synergistically impact IDU and OUD together.
We hope that this multimorbidity model will allow us to inform health policy guidance in the US, for example, by assessing packages of interventions that can reduce drug overdose deaths (e.g., medications for OUD, safe consumptions sites), reduce harms associated with specific injection practices (e.g., syringe service programs), prevent HIV (i.e., pre-exposure prophylaxis), and improve linkage to care for chronic viral infections such as HIV and HCV. This model can also assess the impact of an intervention on multiple infections concurrently, such as the reduction of bacterial infections, HIV, and HCV with syringe service programs.
There lacks a standard approach to model validation in the development process. Best practice guidelines developed by the International Society for Pharmacoeconomics and Outcomes Research and the Society for Medical Decision Making highlight the importance of transparency and validation in model development so that decisionmakers can trust the model output when making informed healthcare decisions [13]. Other health models have transparently reported model development with a systematic approach to calibration and validation. Zang et al. reported on the development and calibration of a dynamic compartmental model of HIV transmission among 6 US cities [15].
While we used a systematic approach for selecting calibration targets outlined by Vanni et al., there is no standardized approach to model calibration and validation [30]. A standardized approach may be challenging given the limited availability of data to both calibrate and validate the model. For example, we used national estimates of annual fatal opioid overdose rates from 2015 to 2019 to calibrate the model and separately validated the model with data from 2020.
Our analysis has limitations. First, calibration targets of national estimates are derived from cross-sectional surveys that may undercount people who use drugs and PWID due to reporting and selection biases. We addressed these biases by adjusting for the number of people who misuses opioids in the US and using a 99% confidence interval for calibration targets to fall within given uncertainty of the data. Second, the model was calibrated to overall opioid overdose deaths in the US given a lack of injection-specific data. Newly published data estimating the proportion of injection-related overdoses for opioid and non-opioid use will be utilized in future analyses [36]. Third, the model does not account for differences in clinical outcomes by race/ethnicity due to differing rates of access to prevention and treatment measures, which will be the focus of a future analysis. Fourth, the model does not account for the impact of COVID-19 on IDU. In our next planned set of policy-focused analyses, we will consider this impact as well as the impact from shifts in opioid usage (e.g., increasing fentanyl use) when projecting future clinical outcomes. We can account for the accelerated overdose deaths from fentanyl using data from the CDC WONDER database, where D’Orsogna et al. found that fentanyl-related fatal overdose rates for 2020 exceeded projections by 30% [37]. Fifth, our model may undercount clinical outcomes when all people start in an active IDU state, thus not accounting for those in remission from OUD or have a history of IDU who may relapse. Our focus for this analysis were people with active IDU, but future analyses can incorporate those who relapse to better account for additional clinical and economic outcomes. Sixth, this analysis focuses on selected infections of clinical and public health interests in the US; we exclude other injection-related infections that may play a more significant role in other countries such as tuberculosis or hepatitis B. Seventh, HCV treatment for PWID is not included in the model and will be incorporated into future analyses. Eighth, as with all simulation models, the model necessarily simplifies complex biosocial processes while also retaining key parameters that drive outcomes for IDU and associated infections. We internally validated our model with experts in simulation modeling of HIV, HCV, and opioid use to maintain equipoise between the simplifying assumptions and reflecting the complex interactions between drug use and its associated infections. Finally, our calibration approach of Latin hypercube sampling risks convergence on local optima (i.e., the most favorable solution among neighboring set of solutions, but not the most favorable solution among all possible solutions). To address this, we average model results from all 3 parameter sets that met the convergence criteria.
Conclusion
Injection drug use has converged multiple epidemics into a syndemic in the US, including opioid use, HIV, HCV, and serious bacterial infections. There are limited national data on clinical outcomes for PWID, and in the absence of clinical trials and nationally representative longitudinal observational studies of PWID, simulation models are critical in providing expected outcomes for decisionmakers. This validated and calibrated model will next be used to project clinical outcomes and costs. We hope that this microsimulation model of injection drug use will be an important tool for decision-makers; it will allow them to project long-term clinical outcomes beyond the horizon of traditional clinical studies, as well as short- and long-term costs, and thus to evaluate the cost-effectiveness of interventions that reduce drug overdoses and other harms associated with injection drug use.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hedegaard H, Miniño AM, Spencer MR, Warner M. Drug overdose deaths in the United States, 1999–2020. NCHS Data Brief 2021;(426):1–8.
Crepaz N, Hess KL, Purcell DW, Irene Hall H. Estimating national rates of HIV infection among MSM, persons who inject drugs, and heterosexuals in the United States. AIDS. 2019;33(4):701–8.
Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163(5):412–20.
McCarthy NL, Baggs J, See I, et al. Bacterial infections associated with substance use disorders, large cohort of United States Hospitals, 2012–2017. Clin Infect Dis. 2020;71(7):e37–44.
Lansky A, Finlayson T, Johnson C, et al. Estimating the number of persons who inject drugs in the united states by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS ONE. 2014;9(5):e97596.
Thein H-H, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–91.
Islam S, Piggott DA, Moriggia A, et al. Reducing injection intensity is associated with decreased risk for invasive bacterial infection among high-frequency injection drug users. Harm Reduct J. 2019;16(1):38.
Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608.
Barocas JA, Eftekhari Yazdi G, Savinkina A, et al. Long-term infective endocarditis mortality associated with injection opioid use in the United States: a modeling study. Clin Infect Dis. 2021;73(11):e3661–9.
Krebs E, Zang X, Enns B, et al. Ending the HIV epidemic among persons who inject drugs: a cost-effectiveness analysis in six US cities. J Infect Dis. 2020;222(Suppl 5):301–11.
Barbosa C, Fraser H, Hoerger TJ, et al. Cost-effectiveness of scaling-up HCV prevention and treatment in the United States for people who inject drugs. Addiction. 2019;114(12):2267–78.
Bernard CL, Brandeau ML, Humphreys K, et al. Cost-effectiveness of HIV preexposure prophylaxis for people who inject drugs in the United States. Ann Intern Med. 2016;165(1):10–9.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM modeling Good Research Practices Task Force–7. Med Decis Making. 2012;32(5):733–43.
Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling Good Research Practices Task Force–6. Value Health. 2012;15(6):835–42.
Zang X, Krebs E, Min JE, et al. Development and calibration of a dynamic HIV transmission model for 6 US Cities. Med Decis Making. 2020;40(1):3–16.
R Core Team. R: A language and environment for statistical computing [Internet]. 2022;Available from: https://www.R-project.org/.
Bradley H, Hall E, Asher A, et al. Estimated number of people who inject drugs in the United States. Clin Infect Dis. 2022;76(1):96–102.
National Center for Health Statistics. Mortality Data -- Vital Statistics NCHS’ Multiple Cause of Death Data, 1959–2017. National Center for Health Statistics, Hyattsville, Maryland. Available from: https://www2.nber.org/data/vital-statistics-mortality-data-multiple-cause-of-death.html.
Hessol NA, Buchbinder SP, Colbert D, et al. Impact of HIV infection on mortality and accuracy of AIDS reporting on death certificates. Am J Public Health. 1992;82(4):561–4.
Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161(3):170–80.
Engels EA, Yanik EL, Wheeler W, et al. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis. 2017;65(4):636–43.
Edwards JK, Cole SR, Breger TL, et al. Mortality among persons entering HIV care compared with the general U.S. population: an observational study. Ann Intern Med. 2021;174(9):1197–206.
Collaboration HIV-CAUSAL, Ray M, Logan R, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24(1):123–37.
US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration., Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health (NSDUH), 2015–2020 [accessed 2022 Sep 12];Available from: https://www.datafiles.samhsa.gov/.
Kim J-H, Fine DR, Li L, et al. Disparities in United States hospitalizations for serious infections in patients with and without opioid use disorder: a nationwide observational study. PLoS Med. 2020;17(8):e1003247.
Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report 2021 [accessed 2021 Sep 4];26(1). Available from: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
Long EF, Mandalia R, Mandalia S, Alistar SS, Beck EJ, Brandeau ML. Expanded HIV testing in low-prevalence, high-income countries: a cost-effectiveness analysis for the United Kingdom. PLoS ONE. 2014;9(4):e95735.
Wu K, Tie Y, Dasgupta S, Beer L, Marcus R. Injection and non-injection drug use among adults with diagnosed HIV in the United States, 2015–2018. AIDS Behav. 2021;26(4):1026–38.
Centers for Disease Control and Prevention. Viral Hepatitis Surveillance 2019. U.S. Department of Health and Human Services; 2021 [accessed 2022 Oct 10]. Available from: https://www.cdc.gov/hepatitis/statistics/2019surveillance/index.htm.
Vanni T, Karnon J, Madan J, et al. Calibrating models in economic evaluation: a seven-step approach. PharmacoEconomics. 2011;29(1):35–49.
Barocas JA, White LF, Wang J, et al. Estimated prevalence of opioid use disorder in Massachusetts, 2011–2015: a capture-recapture analysis. Am J Public Health. 2018;108(12):1675–81.
Tsai J. Lifetime and 1-year prevalence of homelessness in the US population: results from the national epidemiologic survey on Alcohol and related Conditions-III. J Public Health. 2018;40(1):65–74.
Maruschak LM, Bronson J, Alper M. Alcohol and drug use and treatment reported by prisoners. (Rep. No. NCJ #252641). Washington, DC: United States Department of Justice, Bureau of Justice Statistics. Available from https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/adutrpspi16st.pdf.
Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2019;114(1):150–66.
Grebely J, Page K, Sacks-Davis R, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59(1):109–20.
Hall EW, Rosenberg ES, Jones CM, Asher A, Valverde E, Bradley H. Estimated number of injection-involved drug overdose deaths, United States, 2000–2018. Drug Alcohol Depend. 2022;234(109428):109428.
D’Orsogna MR, Böttcher L, Chou T. Fentanyl-driven acceleration of racial, gender and geographical disparities in drug overdose deaths in the United States. PLOS Glob Public Health. 2023;3(3):e0000769.
Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622–2.
Acknowledgements
We would like to thank Fatma Shebl, PhD for her assistance in deriving mortality input parameters.
Funding
This research received funding from the National Institute of Allergy and Infectious Diseases (T32 AI007433 to JJC), the Massachusetts General Hospital (Executive Committee on Research Fund for Medical Discovery to JJC, Scholar Award in Population and Health Care Research to ALC), the Doris Duke Foundation (Physician Scientist Fellowship to JJC). The content is solely the responsibility of the authors, and the study’s findings and conclusions do not necessarily represent the official views of the National Institutes of Health or other funders. Funders were not involved in the study design; in the collection, analysis, or interpretation of data; or in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
JJC is responsible for the conceptualization, design, data collection, and draft manuscript preparation. JJC and PPM developed the model. JJC, ALC, and JC analyzed and interpreted the results. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Mass General Brigham Institutional Review Board determined this study does not meet their definition of human subjects research (Protocol 2021P002525).
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chiosi, J.J., Mueller, P.P., Chhatwal, J. et al. A multimorbidity model for estimating health outcomes from the syndemic of injection drug use and associated infections in the United States. BMC Health Serv Res 23, 760 (2023). https://doi.org/10.1186/s12913-023-09773-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-023-09773-1