Abstract
Background
A significant number of late middle-aged adults with depression have a high illness burden resulting from chronic conditions which put them at high risk of hospitalization. Many late middle-aged adults are covered by commercial health insurance, but such insurance claims have not been used to identify the risk of hospitalization in individuals with depression. In the present study, we developed and validated a non-proprietary model to identify late middle-aged adults with depression at risk for hospitalization, using machine learning methods.
Methods
This retrospective cohort study involved 71,682 commercially insured older adults aged 55–64 years diagnosed with depression. National health insurance claims were used to capture demographics, health care utilization, and health status during the base year. Health status was captured using 70 chronic health conditions, and 46 mental health conditions. The outcomes were 1- and 2-year preventable hospitalization. For each of our two outcomes, we evaluated seven modelling approaches: four prediction models utilized logistic regression with different combinations of predictors to evaluate the relative contribution of each group of variables, and three prediction models utilized machine learning approaches - logistic regression with LASSO penalty, random forests (RF), and gradient boosting machine (GBM).
Results
Our predictive model for 1-year hospitalization achieved an AUC of 0.803, with a sensitivity of 72% and a specificity of 76% under the optimum threshold of 0.463, and our predictive model for 2-year hospitalization achieved an AUC of 0.793, with a sensitivity of 76% and a specificity of 71% under the optimum threshold of 0.452. For predicting both 1-year and 2-year risk of preventable hospitalization, our best performing models utilized the machine learning approach of logistic regression with LASSO penalty which outperformed more black-box machine learning models like RF and GBM.
Conclusions
Our study demonstrates the feasibility of identifying depressed middle-aged adults at higher risk of future hospitalization due to burden of chronic illnesses using basic demographic information and diagnosis codes recorded in health insurance claims. Identifying this population may assist health care planners in developing effective screening strategies and management approaches and in efficient allocation of public healthcare resources as this population transitions to publicly funded healthcare programs, e.g., Medicare in the US.
Similar content being viewed by others
Introduction
Patients with depression often develop chronic medical illnesses, including cardiovascular disease, diabetes, and chronic obstructive pulmonary disease at an earlier age than their non-depressed counterparts [1]. The reasons are complex, and include health factors such as poor diet, poorer adherence to self-management regimens, obesity, sedentary lifestyles and smoking, as well as physiologic abnormalities occurring during depression, including high corticosteroid levels, pro-inflammatory states, and other metabolic factors [1]. It is well-established that there is a bidirectional relationship between depression and chronic illness, where chronic medical illnesses increase the likelihood of developing depression, and factors such as health-related distress, functional impairments, and symptom burden associated with these conditions may worsen depression [1].
Improving healthcare outcomes in late middle-aged adults with depression (aged between 55 and 64 years) is a priority area because during this time chronic conditions such as cardiovascular disease, stroke, and cancer often become apparent [2,3,4,5]. Patients with chronic medical conditions and comorbid depression have high numbers of hospitalizations, frequent emergency hospital admissions, long hospital stays, high risk of readmission, utilization and cost of general medical services, poor adherence to self-care regimens, [1, 6,7,8,9,10,11] and a tendency to experience greater somatic symptom burden [1, 12]. Late middle-aged adults also face unique social and health-related factors. In addition to chronic medical illness, they or members of their household begin to plan for retirement, a stressful life transition that may affect mental well-being [13]. Perceived poor health status has been found to be a predictor of loneliness in late middle-aged adults [14]. In the U.S., late middle-aged patients are approaching the age of eligibility for the publicly funded Medicare insurance program. Identifying high-risk individuals in this age group with chronic diseases and co-morbid depression has the potential to improve their healthcare trajectories and reduce costs through efficient allocation of healthcare resources and targeted care management programs [15, 16].
In addition to reducing costs, preventing hospitalization earlier in the life course can improve patient experiences [17, 18]. Hospitalization is not without risks. Complications from diagnostic and therapeutic procedures, reactions to therapeutic drugs, hospital acquired infections, [19,20,21] and functional decline are all risks of hospitalization [22,23,24,25]. Efforts to identify patients at risk for hospitalization have largely focused on re-hospitalization within several weeks after admission, rather than a first or future hospitalization within the next year or longer [26,27,28,29]. Predictive modeling of first instances of preventable hospitalization among patients with depression represents a promising avenue for identifying patients who may be at high risk for adverse health outcomes.
This study sought to demonstrate the feasibility of identifying late middle-aged adults with depression who are at increased risk of hospitalization. Specifically, we developed and validated predictive models of risk of preventable hospitalization (1-year and 2-year risk) in late middle-aged adults with depression. We used commercial insurance claims that are national in scope from four of the largest insurers in the U.S. – Aetna, Humana, Kaiser Permanente, and UnitedHealthcare. We used the diagnostic categories used in the CMS HCC risk adjustment system [30] together with the Psychiatric Case-Mix System (PsyCMS) developed in the Veterans Affairs health system, [31] as well as demographic characteristics, and prior healthcare utilization measures to capture health status and prior healthcare utilization of the patient population. Given the lack of access to care in many rural areas and the complex ways that sex influences chronic and mental health conditions, [32, 33] we examined how the relationship between chronic and mental health conditions and preventable hospitalization varied by sex and rural/urban residence.
Methods
Data source
This retrospective cohort study used claims data from the Health Care Cost Institute (HCCI) [34]. The HCCI data include de-identified claims from four of the nation’s largest health insurers (Aetna, Humana, Kaiser Permanente and UnitedHealthcare) for U.S. residents of all 50 states. The Institutional Review Board of Weill Cornell Medicine approved this study. The informed consent was waived by Weill Cornell Medicine Institutional Review Board because it involved secondary data analysis using deidentified data. All methods were carried out in accordance with relevant guidelines and regulations.
Establishment of the study cohort
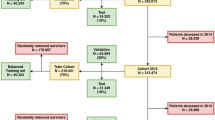
Enrollees in a commercial insurance plan who were aged 55–64 were considered for inclusion in our study sample. Additionally, enrollees were required to have continuous medical benefits coverage for at least 36-months from January 2011 through December 2013 to be included in the study sample to accurately capture their medical history and risk of hospitalization in the study period. The 36 months of the study period were split into (1) Year 1 (the “base year”); and (2) Year 2 (to capture the 1-year risk of preventable hospitalization); and (3) Years 2 and 3 combined (to capture the 2-year risk of preventable hospitalization). Enrollees were excluded if they had a hospice or nursing home claim in the base year or if they did not have at least one medical claim during the base year.
In order to identify enrollees with depression, we used a validated method for identifying depression using administrative data [35, 36]. We required at least one inpatient claim for depression, or two outpatient or two physician claims with a diagnosis of depression, or one outpatient or physician claim for depression plus at least one antidepressant medication fill during the base year. The following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to identify individuals with a depression diagnosis in the base year: 296.20, 296.21, 296.22, 296.23, 296.24, 296.25, 296.26, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36, 300.4, and 311. To identify whether enrollees had a prescription fill for an antidepressant medication during the base year, we searched available prescription drug claims for NDC codes associated with an antidepressant medication, using the HEDIS Antidepressant Medication Management list produced by the National Committee for Quality Assurance that was in effect during the enrollee’s base year.
The population of community-dwelling adults aged 55 to 64 with 36 months of continuous enrollment in a commercial insurance plan with diagnosed depression who met our inclusion criteria was 71,682.
Measures
All predictors of preventable hospitalization were measured in the base year only. Candidate predictors included demographic characteristics and prior healthcare utilization measures, as well as binary variables to indicate the presence of hierarchical condition category (HCC) and Psychiatric Case-Mix System (PsyCMS) conditions. Each of these measures is described in greater detail below.
Demographic characteristics
Due to the effect of sex in the course and development of medical and mental health conditions, we included a binary variable to capture sex in our models. A binary variable was used to indicate whether the individual is a resident of a metropolitan core-based statistical area, as defined by the U.S. White House Office of Management and Budget and using population counts collected in the decennial Census [37]. In this approach, Metropolitan Statistical Areas (MSAs) are defined as having a Core-Based Statistical Area (CBSA) with least one urbanized area with a population of at least 50,000. The MSA is comprised of the central county or counties containing the core plus adjacent outlying counties that have a high degree of economic integration with the core area, [37] where at least 25% of the population commute to or from the core urban area for work [38]. MSAs have also been used as a method for grouping hospitals, and they tend to be stable over time [38].
Prior healthcare utilization measures
Variables that capture prior healthcare utilization often improve model performance [29]. We included several dichotomous markers to capture prior healthcare utilization in the base year: any hospitalization, hospitalization for a psychiatric condition, any emergency department use, and emergency department use for a psychiatric condition.
Hierarchical condition categories (HCCs)
We used the condition categories defined in Version 12 of the CMS MA HCC model, as this version was in use for claims incurred during the study observation window. This version of the CMS HCC model contains 70 HCCs (A list of these HCCs can be found in Supplementary Table 1) [39]. Hierarchies are imposed among related condition categories so that a person is coded for only the most severe occurrence among related diseases. For unrelated categories, the HCCs are additive, where an individual may be coded for none, one, or multiple HCCs. Finally, the hierarchical versions of the conditions are used in predictions [40].
PsyCMS psychiatric condition categories
We used the 46 condition categories defined using the PsyCMS Case-Mix System (A list of these PsyCMS psychiatric condition categories can be found in Supplementary Table 2). The PsyCMS system uses hierarchies to reduce overlap among closely related diagnosis codes [31]. The hierarchies employed in PsyCMS reduce overlap among closely related diagnoses by assigning individuals to the single category most likely to drive mental health and substance use utilization [31]. These hierarchies were developed based on the clinical assessment of severity, medical diagnostic criteria, and greater specificity [31].
Study outcomes
The outcome variables of interest were the 1- and 2-year risk of having a preventable hospitalization. We defined preventable hospitalization using the ambulatory care sensitive conditions adapted from the Prevention Quality Indicators put forth by the Agency for Healthcare Research and Quality [17, 18]. Binary variables were created to capture whether enrollees experienced an inpatient hospitalization with a primary diagnosis for one of the following conditions: diabetes short-term complications, diabetes long-term complications, uncontrolled diabetes, lower-extremity amputation, chronic obstructive pulmonary disease or asthma, hypertension, heart failure, dehydration, bacterial pneumonia, urinary tract infection, congestive heart failure, and perforated appendix, with certain conditions (e.g., asthma in younger adults and low birth weight) not included in our measure as they do not apply to our adult population. A list of the ICD-9 diagnoses corresponding to these conditions is available through AHRQ) [41].
Data analysis
We developed four prediction models using logistic regression (Models 1 through 4), with different combinations of predictors to evaluate the relative contribution of each group of variables. Model 1 included demographics (sex and whether enrollee was a resident of a metropolitan CBSA) and variables to capture whether the enrollee experienced selected healthcare utilization events in the base year (any hospitalization, hospitalization for a psychiatric condition, any emergency department use, and emergency department use for a psychiatric condition); Model 2 included demographics and HCC conditions; Model 3 included demographics and PsyCMS conditions; and Model 4 included demographics in addition to HCC and PsyCMS conditions.
We then included all predictors and utilized various machine learning algorithms in our remaining three models (Models 5 through 7). Models 5 through 7 included all of the variables used in the previous four models (demographics, prior utilization, HCC conditions, and PsyCMS conditions), with the addition of two-way interactions sex with other all other predictors and metropolitan CBSA resident status with all other predictors. Model 5 utilized logistic regression with Least Absolute Shrinkage and Selection Operator (LASSO) penalty; [42] Model 6 utilized random forests (RF); [43] and Model 7 utilized gradient boosting machine (GBM) [44]. We chose two classes of ML algorithms: a regression-based method (LASSO) that models additive effects of predictors along with simple two-way interactions of predictors with sex and a marker for rural/urban residence; and methods based on decision trees (GBM and RF) which model complex higher order interactions involving predictors. The LASSO machine learning algorithm applies shrinkage to the coefficients in the model, with a penalty on the sum of the absolute magnitudes of the regression coefficients, giving better prediction accuracy due to reduced variance [45]. RF and GBM are similar in that both produce ensembles of tree learners, but with differences in how they arrive at the final aggregate tree learner. RF starts by generating multiple bootstrapped samples from the original data, then fits uncorrelated decision trees and combines them using a technique called bagging, whereas GBM combines simple prediction models to produce a complex aggregate model using boosting [46, 47].
The overall sample of 71,682 was split into two random groups, with 2/3 of the sample used as a training set for model development and the remaining as the test set to estimate prediction accuracy. Because of the low frequency of the observed outcomes, we randomly chose the nonevents to match the events 1:1 to create a balanced training set for each outcome. For the analysis involving the 1-year risk of preventable hospitalization, the training set included 1,462 beneficiaries and the test set included 23,893 beneficiaries; for analyses involving 2-year risk of preventable hospitalization, the training set included 2,362 beneficiaries and the test set included 23,894 beneficiaries. All predictors were binary and those that had a frequency ratio (i.e., the ratio of the frequency of the most common category and the other category) greater than 20 were removed from analysis because of the low information content in these predictors. All models were trained on the training set and the prediction accuracy (area under the receiver operating characteristics curve or AUC) was estimated on the test set. All logistic regression models were examined for calibration (agreement between predicted and observed frequencies) using the Brier score and visual inspection, then, if needed, re-calibrated coefficients were obtained in the training data. Among the machine learning algorithms, Model 5 (the LASSO model) had the highest AUC in the training set and a five-fold cross-validation in the training set was used to select the tuning parameter of the LASSO model that maximized the AUC.
Results
In our sample, 1.5% experienced preventable hospitalization in the 1-year prediction window (n = 1,096), and 2.8% experienced preventable hospitalization within the 2-year prediction window (n = 1,974). Approximately 70% of the sample was female, and all were aged 55–64 during the observation period. Nearly 90% resided in a metropolitan core-based statistical area.
In Table 1 we present a comparison of the model performance for the seven alternative modeling approaches for our two outcome measures, with corresponding Figs. 1 and 2 which show a graphical representation of the ROC curves. The sensitivity and specificity of our predictive models for 1- and 2-year risk of preventable hospitalization were obtained by choosing a threshold for the risk prediction functions that maximized the Youden index [48].
Receiver operating characteristic curves comparing accuracy of five models in predicting 1-year risk of preventable inpatient hospitalization
Model Legend:
Model 1 = Dem. + prior utilization
Model 2 = Dem. + HCC
Model 3 = Dem. + PsyCMS
Model 4 = Dem., HCC + PsyCMS
Model 5 = Dem., prior utilization, HCC, PsyCMS, interaction effects, with LASSO
Receiver operating characteristic curves comparing accuracy of five models in predicting 2-year risk of preventable inpatient hospitalization
Model Legend:
Model 1 = Dem. + prior utilization
Model 2 = Dem. + HCC
Model 3 = Dem. + PsyCMS
Model 4 = Dem., HCC + PsyCMS
Model 5 = Dem., prior utilization, HCC, PsyCMS, interaction effects, with LASSO
For the one-year risk of preventable hospitalization, our best performing model was a machine learning model (Model 5, referred to as the Final Model in Table 1) with an AUC of 0.803. This model utilized the machine learning approach of logistic regression with LASSO penalty, and included demographic characteristics, prior healthcare utilization variables, HCC conditions, PsyCMS conditions, and interaction effects between sex and all other variables in the model and between the rural/urban indicator and all other variables in the model. For the 1-year risk of preventable hospitalization, using Model 5, a sensitivity of 72% and a specificity of 76% were obtained under the optimum threshold 0.453. Model 1 included only demographic variables and prior healthcare utilization variables and achieved an AUC of 0.746; Model 2 included demographic variables and HCC conditions (AUC = 0.793); Model 3 included demographic variables and PsyCMS conditions (AUC = 0.585); Model 4 included demographic variables, HCC conditions and PsyCMS conditions (AUC = 0.782); Model 6 utilized the machine learning approach of random forests and included demographic characteristics, prior healthcare utilization variables, HCC conditions, PsyCMS conditions, and all higher-order interactions (AUC = 0.718); and Model 7 utilized the machine learning approach of GBM and included demographic characteristics, prior healthcare utilization variables, HCC conditions, PsyCMS conditions, and all higher-order interactions (AUC = 0.765).
As for the 2-year risk of preventable hospitalization, we similarly found the best performing model was the one that utilized the machine learning approach of logistic regression with Least Absolute Shrinkage and Selection Operator (LASSO) penalty (AUC = 0.793) (Model 5, referred to as the Final Model in Table 1), which had a sensitivity of 76% and a specificity of 71% under the optimum threshold 0.452. It performed better than Model 1 (AUC = 0.706); Model 2 (AUC = 0.775); Model 3 (AUC = 0.588); Model 4 (AUC = 0.784); Model 6 (AUC = 0.729); and Model 7 (AUC = 0.770).
Our modeling approach allowed for the identification of main effects and interaction effects that influence the odds of preventable hospitalization. Figures 3 and 4 present risk factors identified for preventable hospitalization together with the odds of preventable hospitalization for 1- and 2-year risk of preventable hospitalization in our final models.
Risk factors identified by LASSO for 1-year risk of preventable hospitalization: The risk factors selected by the LASSO model (Model 5) are presented along with their Odds Ratio and 95% CI. Risk factors above the two dashed line correspond to the interaction between Ischemic or unspecified stroke and Rural residence. Marginal estimates of risk are presented for the four categories of the interaction effect adjusting for other risk factors in the model
Risk factors identified by LASSO for 2-year risk of preventable hospitalization: The risk factors selected by the LASSO model (Model 5) are presented along with their Odds Ratio and 95% CI. Risk factors above the two dashed lines correspond to the interaction between Polyneuropathy and Rural residence and between Gender and ER utilization. Marginal estimates of risk are presented for the four categories of each interaction effect adjusting for other risk factors in the model
In our analysis involving the 1-year risk of preventable hospitalization, the following factors significantly increased the odds of preventable hospitalization: having an inpatient hospitalization in the base year, the presence of diabetes without complications, the presence of diabetes with neurologic or other specified manifestation, rheumatoid arthritis and inflammatory connective tissue disease, polyneuropathy, congestive heart failure, vascular disease, and chronic obstructive pulmonary disease (see Fig. 3). For the 1-year risk of preventable hospitalization, there was an interaction effect between ischemic or unspecified stroke and rural residence, with the marginal estimates of risk for the four categories of this interaction effect depicted in the top portion of Fig. 3. Among urban enrollees, ischemic or unspecified stroke increased the odds of preventable hospitalization, adjusting for other risk factors in the model.
In our analysis for the 2-year risk of preventable hospitalization, the following factors significantly increased the odds of experiencing a preventable hospitalization: having an inpatient hospitalization in the base year, having a diagnosis of HIV/AIDS, diabetes with neurologic or other unspecified manifestation, diabetes without complications, rheumatoid arthritis and inflammatory connective tissue disease, congestive heart failure, vascular disease, chronic obstructive pulmonary disease, and renal failure, and having a mental health diagnosis of adjustment reaction decreased the odds of preventable hospitalization (see Fig. 4). For the 2-year risk of preventable hospitalization outcome, significant interactions were observed between the rural/urban indicator and polyneuropathy and between sex and prior emergency department utilization, as depicted in the top portions of Fig. 4. Among individuals with prior emergency department use, females had an even higher odds of preventable hospitalization compared to males, and among individuals with polyneuropathy, rural residence increased the odds of preventable hospitalization.
Discussion
We developed custom calibrated models to assess the feasibility of predicting preventable hospitalization in late middle-aged adults with depression. Our model development process relied on large set of clinical, demographic, and prior utilization variables that provided a rich description of the enrollees. The risk factors identified by our modeling approach are consistent with earlier literature [49,50,51]. This study demonstrates the feasibility of identifying late middle-aged adults with depression who are at high risk of hospitalization using data from health insurance claims.
We considered several machine learning algorithms for predicting both 1-year and 2-year risk of preventable hospitalization and our best performing was a logistic regression with LASSO penalty. The LASSO method performed better than the other candidate machine learning approaches of RF and GBM. This observation indicates that a regression-based model with additive effects of predictors along with hypothesized simple interaction effects of all predictors with sex and rural/urban residence sufficiently captures the variability in the two outcomes, without the need of including complex higher-order interaction effects by using the RF or GBM approaches.
Unsurprisingly, several chronic, prevalent comorbidities were strongly associated with high risk for preventable hospitalization, including diabetes, congestive heart failure, vascular disease and chronic obstructive pulmonary disease. In models for both 1- and 2-year risk of preventable hospitalization, diabetes (either without complications or with neurologic or other specified manifestations), rheumatoid arthritis and inflammatory tissue disease, congestive heart failure, vascular disease, chronic obstructive pulmonary disease, and a prior hospitalization in the base year, were risk factors for preventable hospitalization in late middle-aged adults with depression. These observations are consistent with previous literature [49,50,51,52]. Certain risk factors were associated with only 1-year risk of hospitalization (i.e., ischemic or unspecified stroke) and other factors were associated with only 2-year risk of hospitalization (i.e., adjustment reaction, prior emergency department use, HIV/AIDS, and renal failure). Our models revealed distinct differences in risk factors by sex and urban/rural residence. In particular, ischemic or unspecified stroke was associated with a high risk of 1-year preventable hospitalization in patients residing in urban settings. Polyneuropathy was associated with a high risk of 2-year hospitalization in rural patients. Having a history of prior emergency department visit in the base year was a risk factor for 2-year hospitalization for males.
This study demonstrates the feasibility of identifying groups of individuals among late middle-aged adults with depression who are at high risk of hospitalization. Algorithms, similar to ours, may serve to identify groups of individuals at high risk who may benefit from screening and services that can be proactively provided in outpatient settings [17, 18]. Such interventions include referrals to care management services, [53, 54] including care coordination, disease management programs, complex care management, disease-specific self-management education, health maintenance reminders, provider decision support tools, telephone support, and 24-hour consultation telephone lines [55]. A collaborative care model [56] may be another option for adults with chronic medical illness and depression at high risk of future hospitalizations. In this model, primary care and behavioral health services are integrated to address mental health and medical conditions concurrently [56]. Risk stratification algorithms have been used in other healthcare and led to successful interventions. Algorithms for identifying patients at risk for frailty have been used to target interventions that prevent, delay or treat frailty [57]. Similarly, algorithms have been used to identify individuals in need of assistance for emergency disaster preparedness [58].
A strength of our study is the use of insurance claims from several large U.S. insurers rather than insurance claims from a single insurer or geographic region. Our risk prediction models were based on diagnoses, prior utilization and demographic characteristics routinely captured in health records and do not rely on survey-based tools, screenings, or clinical assessments. Although information derived from clinical examination, patient interview, or medical record reviews serve important purposes for research investigations, data derived from claims databases are attractive because they are readily available and are less costly than other approaches [59]. Unlike other studies which assign diagnosis codes to diagnosis clusters known as Aggregated Diagnosis Groups (ADGs), [29, 60] our approach does not use proprietary algorithms that would make it difficult for researchers to fully explore data, and does not require a user license and a fee [29, 60].
Our study has several limitations. Our selection criterion of 36 months of continuous enrollment allowed us to take a long-term view of enrollees’ health outcomes. However, this selection criterion may limit the generalizability of our findings to other patient populations. Patients under the age of 65 years with continuous enrollment in commercial insurance are likely different than other patient populations, such as older patients enrolled in Medicare or patients younger than 65 years of age with interrupted employment that may be non-eligible for commercial health insurance. Another limitation is that diagnostic codes may change over time. For this reason, our findings require replication based on newer health insurance claims data. Future work may incorporate more detailed information involving sociodemographic characteristics, when available. Another limitation of our approach is that we relied on the claims’ coding process during the base year to identify the enrollees’ illnesses; in some cases enrollees may have a mental health or medical illness but not receive a diagnosis, and in other cases enrollees may be incorrectly diagnosed as having a condition.
In conclusion, this study demonstrates that our predictive modeling approach, using diagnoses, prior utilization and other demographic characteristics readily available in claims data, can be used to identify older adults with depression at high risk for preventable hospitalization. As the U.S. population ages, there is an increasing medical burden of individuals with depression. Our approach may assist health care planners in identifying various populations at risk for hospitalization that would be suitable for screening strategies and targeted referral processes and interventions to improve depression and chronic disease management.
Data Availability
The datasets generated and/or analyzed during the current study are available from a third party (Health Care Cost Institute, HCCI) but restrictions apply to the availably of these data, which were used under license for the current study, and so are not publicly available. Data are however available from Samprit Banerjee upon reasonable request and with permission of Health Care Cost Institute (HCCI).
References
Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23.
Percentage of Adults Aged ≥18 Years with Diagnosed Heart Disease, by Urbanization Level and Age Group — National Health Interview Survey, United States, 2020. MMWR Morb Mortal Wkly Rep 2022;71:778. https://doi.org/10.15585/mmwr.mm7123a4
Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8(3):319–29.
Centers for Disease Control and Prevention. United States Cancer Statistics: Highlights from 2019 Incidence. USCS Data Brief, no. 29. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2022.
Martin LG, Freedman VA, Schoeni RF, Andreski PM. Trends in disability and related chronic conditions among people ages fifty to sixty-four. Health Aff (Millwood). 2010;29(4):725–31.
Prina AM, Cosco TD, Dening T, Beekman A, Brayne C, Huisman M. The association between depressive symptoms in the community, non-psychiatric hospital admission and hospital outcomes: a systematic review. J Psychosom Res. 2015;78(1):25–33.
Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54(3):216–26.
Himelhoch S, Weller WE, Wu AW, Anderson GF, Cooper LA. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Med Care. 2004;42(6):512–21.
Unutzer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older; A 4-year prospective study. JAMA. 1997;277(20):1618–23.
Katon WJ, Lin E, Russo J, Unutzer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60(9):897–903.
Huang BY, Cornoni-Huntley J, Hays JC, Huntley RR, Galanos AN, Blazer DG. Impact of depressive symptoms on hospitalization risk in community-dwelling older persons. J Am Geriatr Soc. 2000;48(10):1279–84.
Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29(2):147–55.
Kim JE, Moen P. Retirement transitions, gender, and psychological well-being: a life-course, ecological model. J Gerontol B Psychol Sci Soc Sci. 2002;57(3):P212–222.
Franssen T, Stijnen M, Hamers F, Schneider F. Age differences in demographic, social and health-related factors associated with loneliness across the adult life span (19–65 years): a cross-sectional study in the Netherlands. BMC Public Health. 2020;20(1):1118.
Quinn KL, Stall NM, Yao Z, et al. The risk of death within 5 years of first hospital admission in older adults. CMAJ. 2019;191(50):E1369–e1377.
Ko DT, Alter DA, Austin PC, et al. Life expectancy after an index hospitalization for patients with heart failure: a population-based study. Am Heart J. 2008;155(2):324–31.
AHRQ Quality Indicators -- Guide to Prevention Quality Indicators: Hospital Admission for Ambulatory Care Sensitive Conditions. Rockville, MD: Agency for Healthcare Research and Quality, 2001. AHRQ Pub. No. 02-R0203
Davies S, McDonald KM, Schmidt E, Schultz E, Geppert J, Romano PS. Expanding the uses of AHRQ’s prevention quality indicators: validity from the clinician perspective. Med Care. 2011;49(8):679–85.
Rubins HB, Moskowitz MA. Complications of care in a medical intensive care unit. J Gen Intern Med. 1990;5(2):104–9.
Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16(10):1033–8.
Schimmel EM. The hazards of hospitalization. 1964. Qual Saf Health Care 2003;12(1):58–63.
Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–69.
Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292(17):2115–24.
Mudge AM, O’Rourke P, Denaro CP. Timing and risk factors for functional changes associated with medical hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2010;65(8):866–72.
Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–9.
Donze J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–8.
Goldfield NI, McCullough EC, Hughes JS, et al. Identifying potentially preventable readmissions. Health Care Financ Rev. 2008;30(1):75–91.
Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–98.
Lemke KW, Weiner JP, Clark JM. Development and validation of a model for predicting inpatient hospitalization. Med Care. 2012;50(2):131–9.
Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–41.
Sloan KL, Montez-Rath ME, Spiro A 3, et al. Development and validation of a psychiatric case-mix system. Med Care. 2006;44(6):568–80.
Kulshreshtha A, Goyal A, Dabhadkar K, Veledar E, Vaccarino V. Urban-rural differences in coronary heart disease mortality in the United States: 1999–2009. Public Health Rep. 2014;129(1):19–29.
Pruitt J 3rd, Moracho-Vilrriales C, Threatt T, Wagner S, Wu J, Romero-Sandoval EA. Identification, prevalence, and treatment of painful diabetic neuropathy in patients from a rural area in South Carolina. J Pain Res. 2017;10:833–43.
Newman D, Herrera CN, Parente ST. Overcoming barriers to a research-ready national commercial claims database. Am J Manag Care. 2014;20(Spec No. 17)eSP25–30.
Townsend L, Walkup JT, Crystal S, Olfson M. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):163–73.
Fiest KM, Jette N, Quan H, et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:289.
Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U.S. epidemiologic studies. J Urban Health. 2006;83(2):162–75.
Everson J, Hollingsworth JM, Adler-Milstein J. Comparing methods of grouping hospitals. Health Serv Res. 2019;54(5):1090–8.
Evaluation of the CMS-HCC Risk Adjustment Model: Final Report. Authors: Pope GC, Kautter J, Ingber MJ, Freeman S, Sekar R, Newhart C. Federal Project Officer: Melissa A. Evans, PhD. RTI International. CMS Contract No. HHSM-500-2005-00029I TO 0006. March 2011
Rosen AK, Loveland SA, Anderson JJ, Hankin CS, Breckenridge JN, Berlowitz DR. Diagnostic cost groups (DCGs) and concurrent utilization among patients with substance abuse disorders. Health Serv Res. 2002;37(4):1079–103.
AHRQI Software Version 6.0 AHRQQI™ Version v6.0 ICD-9-CM, Prevention Quality Indicator 90. Available at https://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V60-ICD09/TechSpecs/PQI_90_Prevention_Quality_Overall_Composite.pdf. Accessed December 8 2019.
Hastie T, Tibshirani R, Friedman J. Linear Methods for Regression. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. New York:Springer; 2009.
Hastie T, Tibshirani R, Friedman J. Random Forests. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. New York:Springer; 2009.
Hastie T, Tibshirani R, Friedman J. Boosting and Additive Trees. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. New York:Springer; 2009.
R T. Regression shrinkage and selection via the Lasso J Roy Stat Soc 1996;58(1):267–88.
Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Statist. 2001;29(5):1189–232.
Breiman L. Random forests. Mach Learn. 2001;45(1):5–32.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–72.
Davydow DS, Katon WJ, Lin EH, et al. Depression and risk of hospitalizations for ambulatory care-sensitive conditions in patients with diabetes. J Gen Intern Med. 2013;28(7):921–9.
Dai W, Brisimi TS, Adams WG, Mela T, Saligrama V, Paschalidis I. Prediction of hospitalization due to heart diseases by supervised learning methods. Int J Med Inform. 2015;84(3):189–97.
Kuo CF, Burns PB, Chen JS, Wang L, Chung KC. Risk of preventable hospitalization before and after diagnosis among rheumatoid arthritis patients compared to non-rheumatoid arthritis controls. Joint Bone Spine. 2020;87(2):149–56.
Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep. 2008;10(3):258–64.
Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19(9):725–32.
Hendrikx RJ, Drewes HW, Spreeuwenberg M, Ruwaard D, Struijs JN, Baan CA. Which Triple Aim related measures are being used to evaluate population management initiatives? An international comparative analysis. Health Policy. 2016;120(5):471–85.
Mosley DG, Peterson E, Martin DC. Do hierarchical condition category model scores predict hospitalization risk in newly enrolled Medicare advantage participants as well as probability of repeated admission scores? J Am Geriatr Soc. 2009;57(12):2306–10.
Raney LE. Integrating primary care and behavioral health: the role of the psychiatrist in the Collaborative Care Model. Focus (Am Psychiatr Publ). 2017;15(3):354–60.
Sternberg SA, Bentur N, Abrams C, et al. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18(10):e392–397.
Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a Claims-based Frailty Indicator Anchored to a well-established Frailty phenotype. Med Care. 2017;55(7):716–22.
Kinosian B, Wieland D, Gu X, Stallard E, Phibbs CS, Intrator O. Validation of the JEN frailty index in the National Long-Term Care Survey community population: identifying functionally impaired older adults from claims data. BMC Health Serv Res. 2018;18(1):908.
Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins aggregated diagnosis groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49(10):932–9.
Acknowledgements
Not applicable.
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under the following Award Numbers: P50 MH113838-01; R01 MH105384; and T32MH073553. SB, GA and JP are partially supported by P50 MH113838 and R01 MH105384. LE was supported by T32MH073553 for this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Study Concept and Design: S Banerjee, J Pathak, L Evans Acquisition of Data: Y Wu, L Evans, W Xi Analysis and Interpretation of Data: S Banerjee, W Xi, Y Wu, L Evans, M Kim Drafting and Revision of the Manuscript: L Evans, S Banerjee, Y Wu, W Xi, A Ghosh, G Alexopoulos.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of Weill Cornell Medicine approved this study. The informed consent was waived by Weill Cornell Medicine Institutional Review Board because it involved secondary data analysis using deidentified data. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable, as we have not presented any material with identifiable information and/or media.
Competing interests
The author(s) declare(s) that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Evans, L., Wu, Y., Xi, W. et al. Risk stratification models for predicting preventable hospitalization in commercially insured late middle-aged adults with depression. BMC Health Serv Res 23, 621 (2023). https://doi.org/10.1186/s12913-023-09478-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-023-09478-5