Abstract
Background
Smoking among cancer survivors can increase the risk of cancer reoccurrence, reduce treatment effectiveness and decrease quality of life. Cancer survivors without health insurance have higher rates of smoking and decreased probability of quitting smoking than cancer survivors with health insurance. This study examines the associations of the Affordable Care Act (ACA) Medicaid insurance expansion with smoking cessation assistance and quitting smoking among cancer survivors seen in community health centers (CHCs).
Methods
Using electronic health record data from 337 primary care community health centers in 12 states that expanded Medicaid eligibility and 273 CHCs in 8 states that did not expand, we identified adult cancer survivors with a smoking status indicating current smoking within 6 months prior to ACA expansion in 2014 and ≥ 1 visit with smoking status assessed within 24-months post-expansion. Using an observational cohort propensity score weighted approach and logistic generalized estimating equation regression, we compared odds of quitting smoking, having a cessation medication ordered, and having ≥6 visits within the post-expansion period among cancer survivors in Medicaid expansion versus non-expansion states.
Results
Cancer survivors in expansion states had higher odds of having a smoking cessation medication order (adjusted odds ratio [aOR] = 2.54, 95%CI = 1.61-4.03) and higher odds of having ≥6 office visits than those in non-expansion states (aOR = 1.82, 95%CI = 1.22-2.73). Odds of quitting smoking did not differ significantly between patients in Medicaid expansion versus non-expansion states.
Conclusions
The increased odds of having a smoking cessation medication order among cancer survivors seen in Medicaid expansion states compared with those seen in non-expansion states provides evidence of the importance of health insurance coverage in accessing evidence-based tobacco treatment within CHCs. Continued research is needed to understand why, despite increased odds of having a cessation medication prescribed, odds of quitting smoking were not significantly higher among cancer survivors in Medicaid expansion states compared to non-expansion states.
Similar content being viewed by others
Introduction
Approximately 30% of cancer deaths in the United States (US) are caused by tobacco use and smoking [1]. Smoking among cancer survivors can increase the risk of cancer reoccurrence, reduce treatment effectiveness and survival time, and decrease quality of life [2]. Conversely, quitting smoking after a cancer diagnosis is associated with greater response to cancer treatment and reduced risk of other health conditions (e.g., heart disease, chronic obstructive pulmonary disease, stroke) [3]. The 2020 US Surgeon General’s report on smoking cessation suggests that quitting smoking after a cancer diagnosis can significantly reduce all-cause mortality relative to continued smoking [4].
A study using data from the 2017 National Health Interview Survey (NHIS) found 13% of adult cancer survivors reported current smoking [5] and approximately 44% of cancer survivors who previously smoked quit after cancer diagnosis. Indeed, cancer survivors are less likely to currently smoke and are more likely to report former smoking than those with no history of cancer [6]. A 2000-2017 national US study found higher probability of reporting a smoking cessation event after cancer diagnosis among cancer survivors who are older, those diagnosed as having a smoking-related cancer (vs. non-smoking-related), individuals with an undergraduate degree or a postgraduate degree (vs. high school diploma or GED), and individuals with obesity. Individuals living below the federal poverty level (FPL) have a lower probability of reporting a smoking cessation event after cancer diagnosis than those living above the FPL [7].
Cancer survivors without health insurance have higher rates of smoking and decreased probability of quitting smoking than cancer survivors with insurance [8,9,10]. A study using the NHIS from 2008 through 2017 data found a decreasing trend in smoking rates for cancer survivors with private insurance (17% in 2008/2009 to 12% in 2016/2017). This same study reported strikingly higher rates of smoking among uninsured cancer survivors across this time period, with 43% reporting current smoking in both 2008/2009 and 2016/2017 [11]. An earlier study using data from the 2009 Behavioral Risk Factor Surveillance System survey (ages 18-64) reported similar findings, with 41% prevalence of smoking among cancer survivors who did not have health insurance compared to 20% among those with health insurance. Further, uninsured cancer survivors had 2-fold greater odds of not quitting smoking compared to those with health insurance [10]. Therefore, insurance coverage could play a role in increasing access to smoking cessation assistance and increased cessation among cancer survivors.
The Patient Protection and Affordable Care Act (ACA) mandate called for the expansion of Medicaid coverage (the US public health insurance program for people with low incomes) to all adults earning less than or equal to 138% of the federal poverty level (FPL). Following a Supreme Court ruling, states were allowed to choose whether or not to expand Medicaid. As of February 2022, 39 states had adopted the Medicaid expansion and 12 states had not [12].
The ACA mandate also required that insurers cover certain preventive services, including smoking cessation interventions, with no cost sharing for newly eligible Medicaid beneficiaries in states that expanded Medicaid [13]. This provided the opportunity to have evidence-based tobacco treatment included as a covered benefit for millions of adult smokers not eligible for Medicaid pre-expansion [14]. Findings are mixed from studies examining the impact of the ACA on smoking-related outcomes among populations not limited to cancer survivors [15,16,17,18,19,20,21]. We are not aware of any studies that assessed smoking cessation assistance and quitting smoking among cancer survivors after the implementation of the ACA.
Therefore, this retrospective observational cohort study sought to examine the odds of smoking cessation assistance and cessation among cancer survivors who were patients of community health centers (CHCs) in states that expanded Medicaid via the ACA compared to those in non-expansion states. CHC settings are important to study given that they provide primary care services, including smoking cessation services, to many uninsured or Medicaid-insured patients with higher rates of smoking than the general population [22, 23]. National quality performance data show that CHCs have high rates of assessing and treating tobacco use among their patients [24, 25]. We tested the hypotheses that cancer survivors in ACA expansion states who reported current smoking prior to the ACA expansion would have higher odds of smoking cessation, higher odds of having a cessation medication ordered, and increased primary care utilization post-ACA compared to those in non-expansion states.
Methods
Data sources
We used electronic health record (EHR) data from the Accelerating Data Value Across a National Community Health Center Network (ADVANCE) Clinical Research Network (CRN) [26], a member of PCORnet. The ADVANCE CRN’s data warehouse integrates outpatient EHR data from several data networks. This study used data from OCHIN (not an acronym) and Health Choice Network (HCN).
Study setting and population
In this observational cohort study, we included 337 primary care CHCs across 12 states that expanded Medicaid eligibility to ≤138% of the FPL for all adults including those without dependent children as of 1/1/2014 (California, Hawaii, Massachusetts, Maryland, Minnesota, New Mexico, Nevada, Ohio, Oregon, Rhode Island, Washington, and Wisconsin) and 273 CHCs in 8 non-expansion states (Alaska, Florida, Indiana, Kansas, Missouri, Montana, North Carolina, and Texas). We included Wisconsin as an expansion state because it opened Medicaid coverage to adults earning up to 100% of the FPL (near the threshold of ≤138% of the FPL). We included Alaska, Indiana, and Montana as non-expansion states because they did not expand Medicaid until late in our post-ACA study period (expanded 9/1/2015, 2/1/2015, and 1/1/2016 respectively). Our designation of states as expansion vs. non-expansion are similar to other studies assessing the impact of the ACA on health care outcomes and utilization [6, 17, 27,28,29].
We assessed patients 12 months pre-ACA (1/1/2013-12/31/2013) and 24 months post-ACA Medicaid expansion (1/1/2014-12/31/2015). We identified cancer survivors through their medical histories, encounter diagnoses, and problem-list records up to the date of their last pre-ACA visit. We included cancer survivors aged 19-64 throughout the entire study period, who had ≥1 pre-ACA visit to a study CHC and whose last recorded tobacco use status during this time period indicated current smoking (e.g., current every day, current some-day, heavy), and who had ≥1 post-ACA visit with a documented smoking assessment. Based on the US National Cancer Institute’s definition, we consider a cancer survivor to be anyone alive who has ever had a cancer diagnosis regardless of where they are in the course of their disease [30]. We excluded pregnant women as rates of care utilization and cessation treatment recommendations differ for this subgroup.
Variables
Primary outcomes
Outcomes included quitting smoking (≥1 report of quitting), provision of smoking cessation medication (≥1 prescription of a cessation medication) and utilization of primary care (≥6 vs. < 6 visits) in the 24-month post-period. The EHR presents a discrete data field for smoking status at each primary care encounter, which can be confirmed, updated, or not reviewed. Smoking cessation (‘quit’) at ≥1 visit during the post-period was coded as a binary yes/no variable. Using methods similar to prior EHR-based studies [31,32,33,34,35], a person was identified as ‘quit’ if there was at least one measurement documented in the post-period that indicated the patient’s status changed from one indicating current smoking to one indicating former smoking (regardless of whether the patient had a subsequent status that indicated a return to smoking). For smoking cessation medication provision, we extracted orders for bupropion, varenicline, and all nicotine replacement products from EHR medication orders. As a proxy for utilization of care, we extracted data on the number of post-period office visits per patient (≥6 vs. < 6 visits) based on previous studies [31, 36].
Independent variable
Our independent variable was Medicaid expansion status: patients from CHCs in states that expanded versus did not expand.
Patient characteristics
To describe patients living in expansion and non-expansion states and to develop weights to control for differences in expansion groups, we used the following, EHR-derived pre-ACA covariates: sex, age as of 1/1/2014, race/ethnicity (Hispanic, non-Hispanic white, Non-Hispanic black, Non-Hispanic other, Missing), household income as percent of FPL as of 1/1/2014, location of patient’s primary clinic (urban/rural), number of ambulatory visits in 2013, insurance status at visits in 2013 (all private insurance; all or some visits with public insurance; discontinuously insured; all uninsured), and the Charlson Comorbidity Index [37]. We excluded both cancer and depression diagnosis from the Charlson Comorbidity Index since all patients in the study were cancer survivors and we included depressive disorders as a separate variable in our model since bupropion can be used for both depressive disorders and as a smoking cessation aid. We excluded 19 patients who were missing clinic location data.
Analysis
To analyze whether cancer survivors in expansion states who reported current smoking prior to the ACA expansion would have better smoking cessation outcomes than those in non-expansion states, we used a propensity score (PS) weighted approach.
Propensity score weighting
We calculated inverse probability of treatment weights (IPTW) to control for differences in pre-ACA patient-level characteristics between the expansion and non-expansion groups. In this approach, patients are weighted by the inverse of the probability of being sampled from the treatment (i.e., expansion) group. We first fit a logistic regression model for expansion status including all patient characteristics described above as covariates, to obtain each patient’s PS or probability of being in an expansion state. We then calculated stabilized IPTWs to create a pseudo-population close to our original sample size. To assess balance before and after weighting, we computed standardized differences, as they are not unduly influenced by sample sizes. We considered covariates with absolute standardized mean differences (ASMD) ≤0.1 in the weighted sample to be free of residual imbalance that would influence final models. We calculated effective sample size, which was the approximate number of observations under simple random sampling that would produce a variation equivalent to that of the IPTW sample.
Generalized estimating equation logistic regression
Using the PS-weighted sample, we computed adjusted odds ratios (aOR) and predicted marginal probabilities of quitting smoking, having a cessation medication order, or having ≥6 ambulatory visits in the post-period. We used generalized estimating equation (GEE) logistic regression models to account for clustering of patients within CHCs. To account for potential differences in data handling, we included EHR network (i.e. OCHIN vs. HCN) as the only covariate in our GEE models. All GEE models assumed an exchangeable correlation structure and applied a robust sandwich variance estimator to account for possible misspecification [38]. All analyses were conducted using SAS v9.3. The study was approved by the Oregon Health & Science University Institutional Review Board.
Results
Table 1 displays the patient characteristics in Medicaid expansion and non-expansion states, before and after PS weighting. Our final sample included 476 cancer survivors from non-expansion states and 2441 from expansion states. The majority of our study sample were older (50-64 years of age), female, non-Hispanic white, publicly insured or uninsured, seen in urban clinics, and had multiple comorbidities. After IPTW, the characteristics of patients in expansion versus non-expansion states were well balanced. IPTW adjusted rates for quit status, smoking cessation medication orders, and ≥ 6 visits over 24 months in the overall study sample were 15.5, 28.5 and 59.2%, respectively.
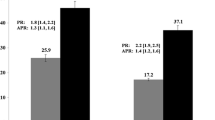
Figure 1 presents aORs for our study outcomes as estimated by the GEE models. Table 2 also displays aORs, as well as predicted probabilities. Patients in expansion states had 2-fold greater odds of having a smoking cessation medication order (aOR = 2.54, 95% CI = 1.61-4.03), and 82% higher odds of having ≥6 visits than cancer survivors in non-expansion states (aOR = 1.82, 95%CI = 1.22-2.73). Patients in expansion states had a non-significant elevation in odds of quitting smoking compared to patients from non-expansion states (aOR = 1.82, 95%CI = 0.84-3.95).
Adjusted odds ratios for quit status, smoking cessation medication ordered and ≥ 6 visits over 24 months comparing Medicaid expansion vs. non-expansion states (reference group) among cancer survivors. Note: OR: odds ratio; LCL: lower 95% confidence limit; UCL: upper 95% confidence limit. Results based on propensity score weighted logistic generalized estimating equation regression accounting for the following covariates: sex, age as of 1/1/2014, race/ethnicity, household income as percent of federal poverty level as of 1/1/2014, rurality/urbanity of patient’s primary clinic, number of ambulatory visits in 2013, insurance status in 2013, Charlson Comorbidity Index excluding cancer and depression as of 1/1/2014; depressive disorder as of 1/1/2014
Discussion
The ACA expansion resulted in health insurance coverage for millions of US adults not previously Medicaid-eligible [14]. In the 24-months post-ACA expansion, cancer survivors from Medicaid expansion states had significantly higher odds of having a smoking cessation medication ordered and more primary care visits compared with those in non-expansion states. Research shows increased use of cessation treatments when out-of-pocket costs for these services are reduced or eliminated [39]; thus, removing cost-sharing among patients eligible via Medicaid expansion likely resulted in the higher rates of medication use. Insurance coverage also likely led to the increased visits among patients in Medicaid expansion states, which could have resulted in more opportunities to assess tobacco use and assist patients in quitting.
Despite these increased odds of smoking cessation medication orders among cancer survivors in expansion versus non-expansion states, we did not observe statistically significant differences in odds of quitting. These findings are in contrast to those of a previous study that found higher odds of quitting smoking among adult patients of CHCs in expansion states compared with those in non-expansion states; however, this prior study included the entire patient population and subanalyses for cancer survivors were not performed [20]. We postulate potential reasons for this finding. While the adjusted odds of quitting were higher for those in Medicaid expansion states (aOR = 1.82, 95%CI = 0.84-3.95), the lack of statistical significance could be due to low cell counts of those who quit, as shown in the wide confidence intervals.
It also is possible that current smoking cessation interventions, including pharmacotherapy, may not be as effective among cancer survivors compared to patients without a history of cancer. One meta-analysis of randomized controlled trials designed to promote smoking cessation among cancer survivors supports this conclusion; however, the quality of the included interventions was mixed and the authors caution in drawing firm conclusions based on the present evidence [40]. It also might be that cancer survivors need more intensive, tailored treatment to increase their odds of quitting smoking. Cancer survivors are more likely to experience poorer mental health than patients without cancer, including fear of cancer recurrence [41,42,43], which could impact smoking cessation outcomes [44, 45]. Given the association between smoking and negative affect, some cancer survivors might benefit from medications to treat the physical dependence, as well as more intensive or longer-term counseling to address the unique concerns and stressors of cancer survivors. A recent intervention study demonstrated that integrating evidence-based, sustained tobacco treatment (which included long-term telephone counseling) into the care of patients with cancer around the time of diagnosis can be effective [46]. Continued research is needed to identify and test smoking cessation interventions among cancer survivors, especially those in underserved communities.
The importance of CHCs for smoking cessation assistance among cancer survivors should also be highlighted. In the overall study sample, 30% of cancer survivors received smoking cessation medications from their CHC. The American College of Surgeons emphasizes that providing a high level of quality care to cancer survivors requires coordination of care among many medical disciplines, including primary care providers [47]. Much recent work has focused on increasing tobacco treatment in oncology settings, with cancer centers across the country receiving funds through the National Cancer Institute Cancer Moonshot Initiative to support this work [48]. While progress has been made in the oncology setting, reach has remained low [48]. Further, many patients return to primary care clinics rather than continue to receive ongoing care from cancer centers once treatment is complete [49,50,51]. Some consider, not only the diagnosis of cancer [3], but also the time period after active treatment to be a “teachable moment” (e.g., the time frame following a health event in which a patient is most conducive to behavioral changes) when cancer survivors might be focused on reducing the likelihood of cancer recurrence. This could lead to increased quit attempts [52]. Primary care clinics have the opportunity to address smoking cessation during this critical time. CHCs, the majority of which also have behavioral health services onsite [53], and insurance coverage are critical resources to ensure access to comprehensive treatment for smoking among this high-risk population.
Limitations
We had follow-up smoking status for patients who had a return clinic visit, and therefore, cannot determine the quit status of patients who did not return for various reasons (e.g., transferred to another clinic, death). That said, a previous study found that about 80% of CHC patients with a chronic health problem return for ≥1 visit within a three-year period [54]. We also did not have detailed information on quit attempts, such as how long the patient remained quit or when the patient quit in relation to date of cancer diagnosis. Some evidence shows that quit attempts decline as the time since diagnosis increases [55]. We were unable to examine cessation by type of cancer as some counts were low or type of cancer was missing. Research suggests that smoking-related cancer survivors have higher current smoking prevalence [5, 56] and that odds of quitting smoking after a cancer diagnosis may vary by whether the person is a survivor of a smoking- or non-smoking-related cancer [5, 57]. We might not have captured use of nicotine replacement therapy that does not require a prescription. We also were unable to assess if bupropion was prescribed for smoking cessation or depression; however, all patients in the study sample reported current smoking and our models controlled for depressive disorders. We also only had access to medication orders, not receipt and/or use of the medications. We could not assess the impact of Medicaid expansion on the provision of cessation counseling among this population as these data were unavailable. While we were able to balance groups based on some known correlates of being uninsured and/or smoking (e.g., % of FPL, depressive disorders, race and ethnicity, clinic rurality, baseline assess to care), we were unable to control for all potential confounders, including state-level factors such as tobacco-related policies. Finally, due to use of diagnoses codes and problem lists to identify cancer survivors and only moderate agreement between CHC EHRs and cancer registries [49], some cancer survivors were likely not identified.
Conclusions
Cancer survivors in ACA Medicaid expansion states had more than twice the odds of having a smoking cessation medication prescribed compared with those from non-expansion states, providing evidence of the importance of insurance coverage in accessing evidence-based tobacco treatment within the CHC setting. Our study findings support the need for continued efforts to ensure health insurance coverage for primary care-based tobacco treatment for socioeconomically disadvantaged cancer survivors. Further, research is needed to understand why, despite increased odds of having a cessation medication prescribed, odds of quitting were not significantly higher among cancer survivors in Medicaid expansion states. If our findings are replicated, interventions tailored to the specific needs of cancer survivors might be warranted.
Availability of data and materials
Raw data underlying this article were generated from multiple agencies and institutions; restrictions apply to the availability and re-release of data under cross-institution agreements.
Abbreviations
- ACA:
-
Affordable Care Act
- CHCs:
-
Community Health Centers
- NHIS:
-
National Health Interview Survey
- FPL:
-
Federal poverty level
- EHR:
-
Electronic Health Record
- ADVANCE:
-
Accelerating Data Value Across a National Community Health Center Network
- CRN:
-
Clinical Research Network
- HCN:
-
Health Choice Network
- IPTW:
-
Inverse probability of treatment weights
- ASMD:
-
Absolute standardized mean differences
- aOR:
-
Adjusted odds ratios
- GEE:
-
Generalized estimating equation
References
Jacobs EJ, Newton CC, Carter BD, et al. What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Ann Epidemiol. 2015;25(3):179–82.e1. https://doi.org/10.1016/j.annepidem.2014.11.008.
Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19(8):1941–8. https://doi.org/10.1158/1078-0432.Ccr-13-0666.
Jassem J. Tobacco smoking after diagnosis of cancer: clinical aspects. Translational Lung Cancer Res. 2019;8(Suppl 1):S50–8. https://doi.org/10.21037/tlcr.2019.04.01.
Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. Smoking cessation: a report of the surgeon general. 2020.
Gritz ER, Talluri R, Fokom Domgue J, Tami-Maury I, Shete S. Smoking behaviors in survivors of smoking-related and non–smoking-related cancers. JAMA Netw Open. 2020;3(7):e209072. https://doi.org/10.1001/jamanetworkopen.2020.9072.
Simon K, Soni A, Cawley J. The impact of health insurance on preventive care and health behaviors: evidence from the first two years of the ACA Medicaid expansions. J Policy Anal Manage. 2017;36(2):390–417.
Talluri R, Fokom Domgue J, Gritz ER, Shete S. Assessment of trends in cigarette smoking cessation after Cancer diagnosis among US adults, 2000 to 2017. JAMA Netw Open. 2020;3(8):e2012164. https://doi.org/10.1001/jamanetworkopen.2020.12164.
Swoboda CM, Walker DM, Huerta TR. Likelihood of Smoking Among Cancer Survivors: An Updated Health Information National Trends Survey Analysis. Nicotine Tob Res. 2019;21(12):1636–43. https://doi.org/10.1093/ntr/ntz007.
Gallaway MS, Glover-Kudon R, Momin B, et al. Smoking cessation attitudes and practices among cancer survivors - United States, 2015. J Cancer Surviv. 2019;13(1):66–74. https://doi.org/10.1007/s11764-018-0728-2.
Burcu M, Steinberger EK, Sorkin JD. Health care access and smoking cessation among cancer survivors: implications for the affordable care act and survivorship care. J Cancer Surviv. 2016;10(1):1–10. https://doi.org/10.1007/s11764-015-0446-y.
Zhao Y, Zheng-Lin B, Wang B, Hu X-C, Jiang C. Insurance disparity in the United States cancer survivors’ smoking rates: a trend study from NHIS 2008-2017. J Clin Oncol. 2019;37(15_suppl):1553. https://doi.org/10.1200/JCO.2019.37.15_suppl.1553.
Kaiser Family Foundation. State Health Facts: Status of State Action on the Medicaid Expansion Decision. 2018. https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D.
McAfee T, Babb S, McNabb S, Fiore MC. Helping smokers quit--opportunities created by the Affordable Care Act. N Engl J Med. 2015;372(1):5–7. https://doi.org/10.1056/NEJMp1411437.
DiGiulio A, Haddix M, Jump Z, et al. State Medicaid Expansion Tobacco Cessation Coverage and Number of Adult Smokers Enrolled in Expansion Coverage - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(48):1364–9. https://doi.org/10.15585/mmwr.mm6548a2.
Donahoe JT, Norton EC, Elliott MR, Titus AR, Kalousová L, Fleischer NL. The affordable care act Medicaid expansion and smoking cessation among low-income smokers. Am J Prev Med. 2019;57(6):e203–10. https://doi.org/10.1016/j.amepre.2019.07.004.
Cotti C, Nesson E, Tefft N. Impacts of the ACA Medicaid expansion on health behaviors: evidence from household panel data. Health Econ. 2019;28(2):219–44. https://doi.org/10.1002/hec.3838.
Koma JW, Donohue JM, Barry CL, Huskamp HA, Jarlenski M. Medicaid coverage expansions and cigarette smoking cessation among low-income adults. Med Care. 2017;55(12):1023–9. https://doi.org/10.1097/mlr.0000000000000821.
Yip D, Gubner N, Le T, Williams D, Delucchi K, Guydish J. Association of Medicaid Expansion and Health Insurance with receipt of smoking cessation services and smoking behaviors in substance use disorder treatment. J Behav Health Serv Res. 2020;47(2):264–74. https://doi.org/10.1007/s11414-019-09669-1.
Maclean JC, Pesko MF, Hill SC. Public insurance expansions and smoking cessation medications. Econ Inq. 2019;57(4):1798–820. https://doi.org/10.1111/ecin.12794.
Bailey SR, Marino M, Ezekiel-Herrera D, et al. Tobacco cessation in affordable care act Medicaid expansion states versus non-expansion states. Nicotine Tob Res. 2020;22(6):1016–22. https://doi.org/10.1093/ntr/ntz087.
Valvi N, Vin-Raviv N, Akinyemiju T. Current smoking and quit-attempts among US adults following Medicaid expansion. Prev Med Rep. 2019;15:100923. https://doi.org/10.1016/j.pmedr.2019.100923.
Flocke SA, Hoffman R, Eberth JM, et al. The Prevalence of Tobacco Use at Federally Qualified Health Centers in the United States, 2013. Prev Chronic Dis. 2017;14:E29. https://doi.org/10.5888/pcd14.160510.
Bailey SR, Heintzman J, Jacob RL, Puro J, Marino M. Disparities in smoking cessation assistance in US primary care clinics. Am J Public Health. 2018;108(8):1082–90. https://doi.org/10.2105/ajph.2018.304492.
Huguet N, Hodes T, Holderness H, Bailey SR, DeVoe JE, Marino M. Community health Centers' performance in Cancer screening and prevention. Am J Prev Med. 2022;62(2):e97–e106. https://doi.org/10.1016/j.amepre.2021.07.007.
Health Resources & Services Administration HCP. Uniform Data System Clinical Quality Measures 2021. 2022. https://bphcdata.net/wp-content/uploads/2020/07/ClinicallMeasuresHandout2020.pdf. Accessed 14 March 2022
DeVoe JE, Gold R, Cottrell E, et al. The ADVANCE network: accelerating data value across a national community health center network. J Am Med Inform Assoc. 2014;21(4):591–5. https://doi.org/10.1136/amiajnl-2014-002744.
Angier HE, Marino M, Springer RJ, Schmidt TD, Huguet N, DeVoe JE. The Affordable Care Act improved health insurance coverage and cardiovascular-related screening rates for cancer survivors seen in community health centers. Cancer. 2020;126(14):3303–11. https://doi.org/10.1002/cncr.32900.
Huguet N, Angier H, Rdesinski R, et al. Cervical and colorectal cancer screening prevalence before and after affordable care act Medicaid expansion. Prev Med. 2019;124:91–7. https://doi.org/10.1016/j.ypmed.2019.05.003.
Angier H, Ezekiel-Herrera D, Marino M, et al. Racial/ethnic disparities in health insurance and differences in visit type for a population of patients with diabetes after Medicaid expansion. J Health Care Poor Underserved. 2019;30(1):116–30. https://doi.org/10.1353/hpu.2019.0011.
National Cancer Institute. Dictionary of cancer terms. 2022. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivor
Bailey SR, Hoopes MJ, Marino M, et al. Effect of gaining insurance coverage on smoking cessation in community health centers: a cohort study. J Gen Intern Med. 2016;31(10):1198–205. https://doi.org/10.1007/s11606-016-3781-4.
Bailey SR, Stevens VJ, Fortmann SP, et al. Long-term outcomes from repeated smoking cessation assistance in routine primary care. Am J Health Promot. 2018;32(7):1582–90. https://doi.org/10.1177/0890117118761886.
Silfen SL, Cha J, Wang JJ, Land TG, Shih SC. Patient characteristics associated with smoking cessation interventions and quit attempt rates across 10 community health centers with electronic health records. Am J Public Health. 2015;105(10):2143–9. https://doi.org/10.2105/ajph.2014.302444.
Barnett PG, Chow A, Flores NE, Sherman SE, Duffy SA. Changes in veteran tobacco use identified in electronic medical records. Am J Prev Med. 2017;53(1):e9–e18. https://doi.org/10.1016/j.amepre.2017.01.009.
Melzer AC, Pinsker EA, Clothier B, et al. Validating the use of veterans affairs tobacco health factors for assessing change in smoking status: accuracy, availability, and approach. BMC Med Res Methodol. 2018;18(1):39. https://doi.org/10.1186/s12874-018-0501-2.
Dunlop S, Coyte PC, McIsaac W. Socio-economic status and the utilisation of physicians' services: results from the Canadian National Population Health Survey. Soc Sci Med. 2000;51(1):123–33.
Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–40. https://doi.org/10.1016/j.jclinepi.2008.01.006.
Overall JE, Tonidandel S. Robustness of generalized estimating equation (GEE) tests of significance against misspecification of the error structure model. Biom J. 2004;46(2):203–13.
Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update: Clinical Practice Guideline 2008.
Sheeran P, Jones K, Avishai A, et al. What works in smoking cessation interventions for cancer survivors? A meta-analysis. Health Psychol. 2019;38(10):855–65. https://doi.org/10.1037/hea0000757.
Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14(8):721–32. https://doi.org/10.1016/s1470-2045(13)70244-4.
Liu J, Butow P, Beith J. Systematic review of interventions by non-mental health specialists for managing fear of cancer recurrence in adult cancer survivors. Support Care Cancer. 2019;27(11):4055–67. https://doi.org/10.1007/s00520-019-04979-8.
Carreira H, Williams R, Müller M, Harewood R, Stanway S, Bhaskaran K. Associations between breast Cancer survivorship and adverse mental health outcomes: a systematic review. J Natl Cancer Inst. 2018;110(12):1311–27. https://doi.org/10.1093/jnci/djy177.
Guimond AJ, Croteau VA, Savard MH, Bernard P, Ivers H, Savard J. Predictors of smoking cessation and relapse in Cancer patients and effect on psychological variables: an 18-month observational study. Ann Behav Med. 2017;51(1):117–27. https://doi.org/10.1007/s12160-016-9834-4.
Streck JM, Luberto CM, Muzikansky A, et al. Examining the effects of stress and psychological distress on smoking abstinence in cancer patients. Prev Med Rep. 2021;23:101402. https://doi.org/10.1016/j.pmedr.2021.101402.
Park ER, Perez GK, Regan S, et al. Effect of sustained smoking cessation counseling and provision of medication vs shorter-term counseling and medication advice on smoking abstinence in patients recently diagnosed with Cancer: a randomized clinical trial. JAMA. 2020;324(14):1406–18. https://doi.org/10.1001/jama.2020.14581.
American College of Surgeons. Cancer program standards: Ensuring patient-centered care. 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx
D'Angelo H, Rolland B, Adsit R, et al. Tobacco treatment program implementation at NCI Cancer centers: Progress of the NCI Cancer moonshot-funded Cancer center cessation initiative. Cancer Prev Res (Phila). 2019;12(11):735–40. https://doi.org/10.1158/1940-6207.Capr-19-0182.
Hoopes M, Voss R, Angier H, et al. Assessing Cancer history accuracy in primary care electronic health records through Cancer registry linkage. J Natl Cancer Inst. 2021;113(7):924–32. https://doi.org/10.1093/jnci/djaa210.
Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029–36. https://doi.org/10.1007/s11606-009-1058-x.
Kenzik KM. Health care use during cancer survivorship: review of 5 years of evidence. Cancer. 2019;125(5):673–80. https://doi.org/10.1002/cncr.31852.
Frazelle ML, Friend PJ. Optimizing the teachable moment for health promotion for Cancer survivors and their families. J Adv Pract Oncol. 2016;7(4):422–33.
National Association of Community Health Centers. Community Health Center Chartbook. 2021. https://www.nachc.org/wp-content/uploads/2020/01/Chartbook-2020-Final.pdf
Huguet N, Kaufmann J, O'Malley J, et al. Using Electronic Health Records in Longitudinal Studies: Estimating Patient Attrition. Med Care. 2020;58(Suppl 6 1):S46–s52. https://doi.org/10.1097/mlr.0000000000001298.
Paul CL, Tzelepis F, Boyes AW, D'Este C, Sherwood E, Girgis A. Continued smoking after a cancer diagnosis: a longitudinal study of intentions and attempts to quit. J Cancer Surviv. 2019;13(5):687–94. https://doi.org/10.1007/s11764-019-00787-5.
Underwood JM, Townsend JS, Tai E, White A, Davis SP, Fairley TL. Persistent cigarette smoking and other tobacco use after a tobacco-related cancer diagnosis. J Cancer Surviv. 2012;6(3):333–44. https://doi.org/10.1007/s11764-012-0230-1.
Westmaas JL, Alcaraz KI, Berg CJ, Stein KD. Prevalence and correlates of smoking and cessation-related behavior among survivors of ten cancers: findings from a nationwide survey nine years after diagnosis. Cancer Epidemiol Biomark Prev. 2014;23(9):1783–92. https://doi.org/10.1158/1055-9965.Epi-14-0046.
Acknowledgements
This work was conducted with the Accelerating Data Value Across a National Community Health Center Network (ADVANCE) Clinical Research Network (CRN). OCHIN leads the ADVANCE network in partnership with Health Choice Network, Fenway Health, and Oregon Health & Science University. ADVANCE is funded through the Patient-Centered Outcomes Research Institute (PCORI), contract number RI-CRN-2020-001.
Funding
This work was supported by the National Cancer Institute, grant numbers P50CA244289 and R01CA204267, and the National Health, Lung, and Blood Institute, grant number R01HL136575.
Author information
Authors and Affiliations
Contributions
SRB contributed to conceptualization and design of the study and wrote the first draft of the article. RV conducted statistical analysis, drafted the methods section and provided substantive revisions to the article. MM and SHV provided statistical consultation and substantive revisions to the article. HA, KC-B, NH, and JED edited and provided substantive revisions to the article. All authors have provided final approval of the submitted version of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Oregon Health & Science University Institutional Review Board (IRB00011858). A waiver of informed consent was obtained for this study as the research involves minimal risk, does not adversely affect the rights of subjects, and could not be practicably carried out without the waiver.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bailey, S.R., Voss, R., Angier, H. et al. Affordable Care Act Medicaid expansion and access to primary-care based smoking cessation assistance among cancer survivors: an observational cohort study. BMC Health Serv Res 22, 488 (2022). https://doi.org/10.1186/s12913-022-07860-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-07860-3