Abstract
Background
In recent years, several large studies have assessed the costs of national infant immunization programs, and the results of these studies are used to support planning and budgeting in low- and middle-income countries. However, few studies have addressed the costs and cost-effectiveness of interventions to improve immunization coverage, despite this being a major focus of policy attention. Without this information, countries and international stakeholders have little objective evidence on the efficiency of competing interventions for improving coverage.
Methods
We conducted a systematic literature review on the costs and cost-effectiveness of interventions to improve immunization coverage in low- and middle-income countries, including both published and unpublished reports. We evaluated the quality of included studies and extracted data on costs and incremental coverage. Where possible, we calculated incremental cost-effectiveness ratios (ICERs) to describe the efficiency of each intervention in increasing coverage.
Results
A total of 14 out of 41 full text articles reviewed met criteria for inclusion in the final review. Interventions for increasing immunization coverage included demand generation, modified delivery approaches, cash transfer programs, health systems strengthening, and novel technology usage. We observed substantial heterogeneity in costing methods and incompleteness of cost and coverage reporting. Most studies reported increases in coverage following the interventions, with coverage increasing by an average of 23 percentage points post-intervention across studies. ICERs ranged from $0.66 to $161.95 per child vaccinated in 2017 USD. We did not conduct a meta-analysis given the small number of estimates and variety of interventions included.
Conclusions
There is little quantitative evidence on the costs and cost-effectiveness of interventions for improving immunization coverage, despite this being a major objective for national immunization programs. Efforts to improve the level of costing evidence—such as by integrating cost analysis within implementation studies and trials of immunization scale up—could allow programs to better allocate resources for coverage improvement. Greater adoption of standardized cost reporting methods would also enable the synthesis and use of cost data.
Similar content being viewed by others
Background
A large body of evidence has demonstrated the effectiveness and cost-effectiveness of infant immunization for reducing the burden of vaccine-preventable diseases [1, 2]. Routine immunization programs, supplemented by periodic campaigns, cover the majority of the target population in most countries, yet in many settings coverage remains below program goals [3]. Expanding coverage is a major objective of immunization programs, both to increase the magnitude of health benefits from vaccination and to reduce disparities in outcomes among underserved populations [4]. The remaining gaps in target coverage are especially prominent in low- and middle-income countries (LMICs), and so these settings have been a focus for ongoing efforts to improve immunization efforts with both vertical and horizontal programs.
Several recent studies have been undertaken to describe the costs of providing national immunization services [5,6,7,8,9,10,11,12]. This research provides precise estimates of the cost of providing services at current coverage levels in a range of countries, and describes variation in the costs and operating practices of individual immunization sites. However, these studies do not provide direct evidence on the costs or cost-effectiveness of strategies and interventions used to scale up immunization coverage. Without this information, countries and the international stakeholders have little quantitative evidence on the best ways to use scarce resources to achieve immunization objectives. One possible source of information is studies that report the costs and effects of specific coverage-enhancing interventions.
We conducted a systematic review on the costs and effects of interventions to improve immunization coverage in LMICs, including research reported in the grey literature. This review updates past initiatives to survey the evidence on interventions to improve immunization coverage in low-income, high-burden settings [1, 2]. Several recent and historical studies have reviewed evidence related to immunization coverage improvements, but the majority of these have limited their scope to reporting effects on coverage or related programmatic outcomes, and excluded costs [13,14,15,16,17,18,19]. Three reviews have considered both costs and effectiveness. In 2004, Pegurri, et al. summarized evidence in the peer-reviewed literature [1] and Batt, et al. reviewed the relevant grey literature [2]. Both of these reviews identified a limited number of LMIC studies meeting inclusion criteria, and concluded that heterogeneity in methods adopted by these studies prevented quantitative synthesis of results. Both reviews found that few studies reported costs, with 10 out of 60 and 15 out of 34 identified studies including costs, respectively. A recent review by Ozawa, et al. including articles through 2016, adopted a broader scope, including peer-reviewed studies from both low- and high-income country settings, and covering all age groups, but excluding grey literature [20]. While this study was able to undertake some quantitative synthesis of results, the authors acknowledged difficulties due to the heterogeneity of methods and reporting adopted by included studies, and the majority of estimates in their final sample came from high-income settings.
Our review returns to the approach of the earlier reviews with a focus on LMIC settings. In our review, we extracted data for studies conducted after the period covered by the Pegurri, et al. and Batt, et al. reviews (prior to 2003), as earlier studies were already included in these prior reviews, and following the reasoning that older research would be less relevant to contemporary planning and budgeting decisions. These earlier reviews stressed the importance of the grey literature in documenting findings in this field, and consequently we included both the peer-reviewed and grey literature in our review [1, 2]. The objective of this review was to describe the incremental cost and effectiveness of interventions to increase coverage of infant immunization in LMICs, as defined by World Bank income group. The primary outcomes of interest were the incremental costs and incremental changes in target population coverage associated with a coverage-improvement intervention, as compared to routine program performance.
Methods
This systematic review was registered with PROSPERO, an international prospective register of systematic reviews (record number 69586). We included interventions directed solely at increasing infant immunization coverage, as well as interventions designed to improve multiple aspects of immunization performance including coverage. The target age group for infants was defined as age 1 year and below; therefore, measles, mumps, rubella, and varicella vaccination interventions were included. We only included studies that reported empirical data, and excluded modeled analyses to minimize the introduction of additional bias into any summary results. We excluded interventions designed to improve health service delivery generally (e.g., improvements in access to primary health care). We also excluded studies that reported changes in immunization coverage but did not describe specific interventions used to impact infant immunization coverage. Studies with no data on intervention costs were excluded, as were those targeting adult immunization and animal studies. We included studies published between January 2003 (the end date of the period covered by older reviews [1, 2]) and May 2019.
Search strategy and extraction
We identified eligible studies in the published literature by searching the following electronic databases, without language limitations: CEA Registry, Cochrane Library, EconLit, Embase, PubMed, Social Science Research Network (SSRN), and Web of Science [21,22,23]. We also reviewed the reference lists of recent reviews of coverage improvement interventions [13, 20] as well as selected studies found in the initial title search [24,25,26,27,28,29,30].
We identified eligible studies in the grey literature by searching relevant databases and repositories, including World Health Organization (WHO) regional databases Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS) and African Index Medicus (AIM), ELDIS, the World Bank working papers, GreyNet, and Grey Literature Report [31]. Reports found to have a matching publication in the published literature were excluded.
We adopted previously developed sensitivity- and specificity-optimized search strategies for identifying healthcare cost studies and economic evaluations [31,32,33,34]. We tested the robustness of our search strategy by applying it to the timeframe of the Pegurri, et al. review in PubMed and confirming that all studies included in the earlier review were identified by our search terms. Additional file 1 lists the complete search terms utilized for each of the databases. Search terms fell into four categories: (1) immunization terms; (2) coverage terms; (3) cost terms; and (4) LMIC terms. Some databases limited the number of terms that could be searched; in such cases, the number of search terms was consolidated and, when necessary, distinct searches were performed for each category. Due to these limitations, LMIC search terms could not be included in every database.
Record titles and abstracts were independently screened by one of two reviewers (CM and AP) to identify studies that met the inclusion criteria. Reports not meeting study inclusion criteria were excluded. Uncertainty about study inclusion was resolved by a third independent investigator (NM).
From each of the included studies, we extracted information on the type of vaccination coverage intervention and study design; urban vs. rural setting; campaign vs. routine delivery; vaccination delivery platform; the baseline and endline coverage or incremental coverage, where applicable; the intervention costs and intervention cost per person exposed; and the incremental cost-effectiveness ratio. This information was extracted for each unique intervention or country in a study. For example, if a study analyzed two different interventions in two different countries, we extracted information related to each of the four observations.
Analysis
Quality evaluation
We evaluated the methodological quality and risk of bias of included studies using the Consensus on Health Economic Criteria (CHEC) list [35]. We excluded the CHEC list items that were specific to modeling analyses, as our review focused on empirical analyses. For each of the relevant items on the checklist, we assigned studies a score of zero or one (Additional file 2) [35]. A score of “0” indicated that the selected element was not present in the article; “1” indicated the element was present.
Calculating incremental coverage
The primary outcomes of interest were the incremental costs and incremental changes in target population coverage associated with a coverage-improvement intervention, as compared to routine program performance. We calculated incremental coverage (defined as the percentage point change in vaccination coverage for the target group) based on the reported study design and outcomes. For studies that measured coverage at baseline and at the intervention conclusion, in both control (ctl) and intervention (int) groups (pre-post with control, including randomized control trials), incremental coverage was calculated as:
In studies which measured coverage only at the intervention conclusion (post-test with control), incremental coverage was calculated as:
In studies that measured coverage before and after the intervention without a control group (pre-post without control), incremental coverage was calculated as:
Calculating incremental intervention cost
For studies that measured costs at baseline and at the intervention conclusion, incremental intervention costs were calculated as:
If studies included only incremental intervention costs, with no baseline costs stated, the stated intervention costs were listed. All costs were converted to 2017 USD using local inflation according to the consumer price index and local currency to USD exchange rates [36, 37].
Calculating incremental cost effectiveness ratios (ICERs)
ICERs were calculated as:
Some studies evaluated interventions that combined multiple health services, some of which were not focused on immunization [38, 39]. The costs reported by these studies were not broken out by immunization activities vs. other areas. For these studies, the ICER assumes that the costs of all included interventions are attributable solely to increasing immunization coverage, while the incremental coverage benefit is specific to immunization.
In other studies, the currency years of costs were unclear. We contacted the authors of these studies to request clarification of cost currency years, where possible, and assumed a currency year according to the year of the intervention otherwise. Nevertheless, several studies remained in which the ICER could not be calculated with the cost and coverage data provided.
Results
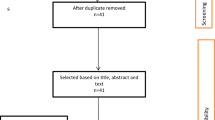
A total of 2325 records were identified from 15 databases (Fig. 1), with an additional 4 records obtained from reference lists of other articles. After removing 114 duplicates, 2215 titles were screened for eligibility, of which 1629 were removed. Of the 586 records remaining, 545 were removed after abstract review, leaving 41 articles which received full-text review. Of these, 27 were excluded for not meeting study inclusion criteria. We examined the LMIC studies included in the Ozawa review and added two relevant articles that we had previously excluded [40, 41]. Fourteen studies were included in the final analysis [38–51].
Table 1 describes general characteristics of the 14 studies. One study describes interventions from two countries (Mexico and Nicaragua); these were considered separate observations when extracting cost and effectiveness data [44]. More than half of the observations (n = 10) were conducted in Asia, with the remaining in Africa (Ethiopia, Guinea-Bissau, and Madagascar) and Central America (Mexico and Nicaragua). Observations were nearly evenly split with regard to setting: 6 were conducted in rural areas; 3 in urban; 3 in mixed settings and 3 were not specified. About half of the interventions (n = 7) were delivered in a mobile outreach format; 4 were delivered in a fixed (i.e., health facility) format, and 4 interventions utilized both a mobile and fixed format. Additionally, 10 interventions were administered within routine vaccination systems, while 5 were administered using a campaign format. One study utilized both routine and campaign formats. Intervention duration ranged from 2 weeks to 5 years.
More than half of the studies (n = 9) reported randomized controlled trials, with the remaining studies reporting non-randomized pre-post evaluations (1 with control group, 3 without control groups) or cross-sectional designs. The majority of interventions were aimed at approaches for vaccine delivery (n = 5) or approaches to encourage additional vaccine uptake (i.e., demand generation; n = 5). Additional studies were aimed at health systems strengthening or the introduction of novel vaccine technologies (e.g., syringes). The interventions targeted coverage improvements for a range of vaccines, with DPT3 (diphtheria-pertussis-tetanus vaccine third dose) and measles vaccination most commonly addressed (Fig. 2).
Coverage and cost information
Table 2 summarizes coverage and cost data in the reviewed studies. If relevant values were not provided directly by studies, values were derived from the study data, such as incremental coverage, intervention cost (converted to 2017 USD), intervention cost in 2017 USD per person exposed, and ICERs. Intervention and baseline costs were primarily presented as total costs, with some studies presenting cost per person exposed [50] or cost per community health worker trained [49].
Most studies reported increases in vaccination coverage following the interventions. In our review coverage increased by an average of 23 percentage points post-intervention across the studies, with rates ranging from 8 [38] to 72 [40] percentage points. Interventions aimed at improving and increasing delivery mechanisms saw the largest incremental coverage increases, at an average of 36 percentage points post-intervention [40, 43, 45, 47, 50]. While some studies did not provide baseline coverage, there was no apparent relationship between incremental coverage and baseline coverage, even when looking across intervention types. There was no discernable pattern between low-income and middle-income country studies.
Given the small total number and methodological heterogeneity of included studies, we decided not to attempt a quantitative synthesis of results. For those studies reporting coverage improvements with the intervention, ICERs ranged from $0.66 [43] to $161.95 [51] per child vaccinated in 2017 USD. There was also variation in the intervention costs reported. Studies most commonly reported site-level immunization costs (46%, n = 6). The next most common reported costs were supply chain and management costs (38%, n = 5), other costs (36%, n = 5), and vaccine costs (31%, n = 4).
Quality ratings
Additional file 2 lists the quality scores each study received, according to the CHEC list, out of a total of 17 possible points. Scores ranged from 12 to 17, with a mean score of 14. Studies with the highest overall scores (averaging greater than or equal to 14 out of 17) were more likely to include appropriate costs and outcomes, as well as incremental analysis. Seven out of 14 included studies were missing items required for the incremental analysis that we conducted, indicating a risk of bias within the calculated ICERs.
Discussion
We reviewed the recent literature describing the incremental cost and impact of efforts to improve immunization program coverage in LMIC, and identified 14 studies that containing sufficient cost and coverage data to be included in this study. The interventions reviewed in these studies covered a wide range of geographic settings, vaccines, intervention types, delivery mechanisms and scales. About half of the studies were randomized controlled trials, and the average study score on the CHEC list was 14 out of 17. The majority of included studies reported increases in vaccination coverage following the examined interventions, similar to the results of the Pegurri, et al. 2004 and Batt, et al. 2004 reviews [1, 2], which found coverage improvements of 27 percentage points and 20 percentage points on average, respectively.
Although all reviewed studies provided costs of the intervention itself, only 2 studies also considered changes in the costs of providing routine immunization services, despite the fact that such changes are a likely consequence of efforts to increase coverage. Eleven studies (79%) provided adequate data to calculate ICERs. From these, 14 ICERs of different interventions were calculated, ranging from $0.66 [43] to $161.95 [51] per child vaccinated in 2017 USD. In several cases, calculations of ICERs relied on substantial assumptions. Given the considerable differences in settings and perspectives, as well as different methods and cost categories included, an observed difference between ICERs may well reflect an artifact of the different study designs rather than a true feature of the interventions under study. Therefore, estimates should be compared with caution, and with full knowledge of the methodological and contextual differences between two studies. Funding devoted to coverage improvements may not be utilized efficiently in the absence of better evidence on optimal approaches.
Several factors prevented ICERs from being calculated in all studies. The interventions described in some studies [38, 44] contained a package of health services (immunization and other health activities). However, intervention costs were given only at an aggregate level and therefore immunization ICERs could not be calculated. Additionally, in one study the size of the population exposed to the intervention was not stated [38]. Similarly, in one study the costs were calculated at the level of health workers, so cost per exposed child could not be determined [49]. Due to the small total number and methodological heterogeneity of the extracted studies, we were unable to conduct a quantitative synthesis with cost estimates stratified by intervention type and country category.
Despite the 15-year gap since previous systematic reviews focusing on LMICs, there is still a scarcity of evidence on the cost-effectiveness of options for improving immunization coverage [1, 2, 20]. Studies rarely report estimates of the incremental change in both cost and coverage. Several of the studies that do provide such information failed to report key features of the study methods, which limits the utility of their results. In particular, future studies should include detailed discussions of the intervention type (i.e., aimed at demand generation, delivery mechanisms, etc.), target population, baseline and endline coverage, specific intervention costs, changes in costs of routine service provision as well as information on effect modifiers that could affect incremental costs and coverage. Such information should be reported at a level of granularity that other programs would be able to interpret and modify those costs to fit their individual setting. These details are necessary to navigate the heterogeneity of the studies and to directly compare and synthesize the results to produce generalizable conclusions. The biggest challenge is that efforts to scale up immunization coverage often lack provision for a costing study, which represents a huge missed opportunity to understand the best use of scarce resources for improving coverage. We challenge the immunization community at country, regional, and global levels to incorporate costing studies into scaling up efforts.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article (and its additional files).
Abbreviations
- AIM:
-
African index medicus
- CHEC:
-
Consensus on health economic criteria
- DPT3:
-
Diphtheria-pertussis-tetanus vaccine third dose
- ICER:
-
Incremental cost-effectiveness ratio
- LILACS:
-
Literatura Latino-Americana e do Caribe em Ciências da Saúde
- LMIC:
-
Low- and middle-income country
- SSRN:
-
Social Science Research Network
- USD:
-
United States dollar
- WHO:
-
World Health Organization
References
Pegurri E, Fox-Rushby JA, Walker DG. The effects and costs of expanding the coverage of immunisation services in developing countries: a systematic literature review. Vaccine. 2004;23(13):1624–35.
Batt K, Fox-Rushby JA, Castillo-Riquelme M. The costs, effects and cost-effectiveness of strategies to increase coverage of routine immunizations in low- and middle-income countries: systematic review of the grey literature. Bull World Health Organ. 2004;82(9):689–96.
World Health Organization. Global vaccine action plan 2011–2020. Geneva; 2013. Available at: http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/. Accessed 1 Mar 2018
World Health Organization. Immunization, vaccines and biologicals: GIVS goals. 2009 Available at: http://www.who.int/immunization/givs/goals/en/. Accessed 1 Mar 2018
Ahanhanzo CD, Huang XX, Le Gargasson JB, et al. Determinants of routine immunization costing in Benin and Ghana in 2011. Vaccine. 2015;33(Suppl 1):A66–71.
Brenzel L, Young D, Walker DG. Costs and financing of routine immunization: approach and selected findings of a multi-country study (EPIC). Vaccine. 2015;33(Suppl 1):A13–20.
Geng F, Suharlim C, Brenzel L, Resch SC, Menzies NA. The cost structure of routine infant immunization services: a systematic analysis of six countries. Health Policy Plan. 2017;32(8):1174–84.
Goguadze K, Chikovani I, Gaberi C, et al. Costs of routine immunization services in Moldova: findings of a facility-based costing study. Vaccine. 2015;33(Suppl 1):A60–5.
Janusz CB, Castaneda-Orjuela C, Molina Aguilera IB, et al. Examining the cost of delivering routine immunization in Honduras. Vaccine. 2015;33(Suppl 1):A53–9.
Le Gargasson JB, Nyonator FK, Adibo M, Gessner BD, Colombini A. Costs of routine immunization and the introduction of new and underutilized vaccines in Ghana. Vaccine. 2015;33(Suppl 1):A40–6.
Menzies NA, Suharlim C, Geng F, Ward ZJ, Brenzel L, Resch SC. The cost determinants of routine infant immunization services: a meta-regression analysis of six country studies. BMC Med. 2017;15(1):178.
Schutte C, Chansa C, Marinda E, et al. Cost analysis of routine immunisation in Zambia. Vaccine. 2015;33(Suppl 1):A47–52.
Johri M, Perez MC, Arsenault C, et al. Strategies to increase the demand for childhood vaccination in low- and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ. 2015;93(5):339–46c.
LaFond A, Kanagat N, Steinglass R, Fields R, Sequeira J, Mookherji S. Drivers of routine immunization coverage improvement in Africa: findings from district-level case studies. Health Policy Plan. 2015;30(3):298–308.
Nelson KN, Wallace AS, Sodha SV, Daniels D, Dietz V. Assessing strategies for increasing urban routine immunization coverage of childhood vaccines in low and middle-income countries: a systematic review of peer-reviewed literature. Vaccine. 2016;34(46):5495–503.
Oyo-Ita A, Wiysonge CS, Oringanje C, Nwachukwu CE, Oduwole O, Meremikwu MM. Interventions for improving coverage of childhood immunisation in low- and middle-income countries. Cochrane Database Syst Rev. 2016;7:Cd008145.
Ryman TK, Dietz V, Cairns KL. Too little but not too late: results of a literature review to improve routine immunization programs in developing countries. BMC Health Serv Res. 2008;8:134.
Sridhar S, Maleq N, Guillermet E, Colombini A, Gessner BD. A systematic literature review of missed opportunities for immunization in low- and middle-income countries. Vaccine. 2014;32(51):6870–9.
Steinglass R. Routine immunization: an essential but wobbly platform. Global Health Sci Pract. 2013;1(3):295–301.
Ozawa S, Yemeke TT, Thompson KM. Systematic review of the incremental costs of interventions that increase immunization coverage. Vaccine. 2018;36(25):3641–9.
Aguiar-Ibanez R, Nixon J, Glanville J, et al. Economic evaluation databases as an aid to healthcare decision makers and researchers. Expert Rev Pharmacoecon Outcomes Res. 2005;5(6):721–32.
Alton V, Eckerlund I, Norlund A. Health economic evaluations: how to find them. International journal of technology assessment in health care. Fall. 2006;22(4):512–7.
Royle P, Waugh N. Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for clinical excellence appraisal system. Health Technol Asses. 2003;7(34):iii, ix–x, 1-51.
Brenzel L. Can investments in health systems strategies lead to changes in immunization coverage? Expert Rev vaccin. 2014;13(4):561–72.
Bassani DG, Arora P, Wazny K, Gaffey MF, Lenters L, Bhutta ZA. Financial incentives and coverage of child health interventions: a systematic review and meta-analysis. BMC Public Health. 2013;13(Suppl 3):S30.
Ranganathan M, Lagarde M. Promoting healthy behaviours and improving health outcomes in low and middle income countries: a review of the impact of conditional cash transfer programmes. Prev Med. 2012;55 Suppl:S95–s105.
Babigumira JB, Morgan I, Levin A. Health economics of rubella: a systematic review to assess the value of rubella vaccination. BMC Public Health. 2013;13:406.
Martin S, Lopez AL, Bellos A, et al. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ. 2014;92(12):881–93.
Owusu-Addo E, Cross R. The impact of conditional cash transfers on child health in low- and middle-income countries: a systematic review. Int J Public Health. 2014;59(4):609–18.
Patel AR, Nowalk MP. Expanding immunization coverage in rural India: a review of evidence for the role of community health workers. Vaccine. 2010;28(3):604–13.
Glanville J, Paisley S. Identifying economic evaluations for health technology assessment. Int J Technol Assess Health Care. 2010;26(4):436–40.
Glanville J, Kaunelis D, Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. Int J Technol Assess Health Care. 2009;25(4):522–9.
McKinlay RJ, Wilczynski NL, Haynes RB. Optimal search strategies for detecting cost and economic studies in EMBASE. BMC Health Serv Res. 2006;6:67.
Wilczynski NL, Haynes RB, Lavis JN, Ramkissoonsingh R, Arnold-Oatley AE. Optimal search strategies for detecting health services research studies in MEDLINE. CMAJ. 2004;171(10):1179–85.
Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Asses Health Care. 2005;21(2):240–5.
World Bank. World development indicators. Washington, DC: The World Bank; 2018. (Last updated: 31-Jan-2019). Available at: http://data.worldbank.org/
International Monetary Fund. World Economic Outlook. Washington, DC. (Last updated: 31-Oct-2018). Available at: https://www.imf.org/external/pubs/ft/weo/2018/02/weodata/index.aspx; 2018. Accessed 2 Feb 2019.
Carnell MA, Dougherty L, Pomeroy AM, Karim AM, Mekonnen YM, Mulligan BE. Effectiveness of scaling up the ‘three pillars’ approach to accelerating MDG 4 progress in Ethiopia. J Health Popul Nutr. 2014;32(4):549–63.
Pandey P, Sehgal AR, Riboud M, Levine D, Goyal M. Informing resource-poor populations and the delivery of entitled health and social services in rural India: a cluster randomized controlled trial. JAMA. 2007;298(16):1867–75.
Khan IA, Saha A, Chowdhury F, et al. Coverage and cost of a large oral cholera vaccination program in a high-risk cholera endemic urban population in Dhaka, Bangladesh. Vaccine. 2013;31(51):6058–64.
Soeung SC, Grundy BM, Ly CK, et al. Improving immunization coverage through budgeted microplans and sub-national performance agreements: early experience from Cambodia. Asia Pac J Public Health. 2006;18(1):29–38.
Andersson N, Cockcroft A, Ansari NM, et al. Evidence-based discussion increases childhood vaccination uptake: a randomised cluster controlled trial of knowledge translation in Pakistan. BMC Int Health Hum Rights. 2009;9(Suppl 1):S8.
Banerjee AV, Duflo E, Glennerster R, Kothari D. Improving immunisation coverage in rural India: clustered randomised controlled evaluation of immunisation campaigns with and without incentives. BMJ. 2010;340:c2220.
Barham T, Brenzel LE, Maluccio JA. Beyond 80%: are there new ways of increasing vaccination coverage? Evaluation of CCT programs in Mexico and Nicaragua. SSRN Electron J. 2007.
Byberg S, Fisker AB, Thysen SM, et al. Cost-effectiveness of providing measles vaccination to all children in Guinea-Bissau. Glob Health Action. 2017;10(1):1329968.
Drain PK, Ralaivao JS, Rakotonandrasana A, Carnell MA. Introducing auto-disable syringes to the national immunization programme in Madagascar. Bull World Health Organ. 2003;81(8):553–60.
Hayford K, Uddin MJ, Koehlmoos TP, Bishai DM. Cost and sustainability of a successful package of interventions to improve vaccination coverage for children in urban slums of Bangladesh. Vaccine. 2014;32(20):2294–9.
Levin CE, Nelson CM, Widjaya A, Moniaga V, Anwar C. The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull World Health Organ. 2005;83(6):456–61.
Owais A, Hanif B, Siddiqui AR, Agha A, Zaidi AK. Does improving maternal knowledge of vaccines impact infant immunization rates? A community-based randomized-controlled trial in Karachi, Pakistan. BMC Public Health. 2011;11:239.
Rainey JJ, Bhatnagar P, Estivariz CF, et al. Providing monovalent oral polio vaccine type 1 to newborns: findings from a pilot birth-dose project in Moradabad district, India. Bull World Health Organ. 2009;87(12):955–9.
Powell-Jackson T, Fabbri C, Dutt V, Tougher S, Singh K. Effect and cost-effectiveness of educating mothers about childhood DPT vaccination on immunisation uptake, knowledge, and perceptions in Uttar Pradesh, India: a randomised controlled trial. PLoS Med. 2018;15(3):e1002519.
Vassall A, Sweeney S, Kahn J, et al. Reference Case for Estimating the Costs of Global Health Services and Interventions. 2017 Last Updated: September 12, 2017. Available at: https://ghcosting.org/pages/standards/reference_case. Accessed 13 February 2019
Wilkinson T, Sculpher MJ, Claxton K, et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health. 2016;19(8):921–8.
Acknowledgements
Not applicable.
Funding
Bill & Melinda Gates Foundation. An employee of the Bill & Melinda Gates Foundation participated as a co-investigator and author on this study. The funder of the study had no role in study design, or data collection, analysis, or interpretation. The funders were given the opportunity to review this paper prior to publication, but the final decision on the content of the publication was taken by the authors. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
CM, NM, and SR conceptualized and designed the study. CM, NM, and AP conducted the review. CM and AP analyzed the included articles and drafted the initial manuscript. LB, EC, NM, SR, and CS critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Search terms for each database. (DOCX 16 kb)
Additional file 2:
Consensus on Health Economic Criteria (CHEC) list for health economic evaluations35 and study scores. (DOCX 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Munk, C., Portnoy, A., Suharlim, C. et al. Systematic review of the costs and effectiveness of interventions to increase infant vaccination coverage in low- and middle-income countries. BMC Health Serv Res 19, 741 (2019). https://doi.org/10.1186/s12913-019-4468-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-019-4468-4