Abstract
Background
The purpose of this paper was to systematically evaluate the application value of artificial intelligence in predicting mortality among COVID-19 patients.
Methods
The PubMed, Embase, Web of Science, CNKI, Wanfang, China Biomedical Literature, and VIP databases were systematically searched from inception to October 2022 to identify studies that evaluated the predictive effects of artificial intelligence on mortality among COVID-19 patients. The retrieved literature was screened according to the inclusion and exclusion criteria. The quality of the included studies was assessed using the QUADAS-2 tools. Statistical analysis of the included studies was performed using Review Manager 5.3, Stata 16.0, and Meta-DiSc 1.4 statistical software. This meta-analysis was registered in PROSPERO (CRD42022315158).
Findings
Of 2193 studies, 23 studies involving a total of 25 AI models met the inclusion criteria. Among them, 18 studies explicitly mentioned training and test sets, and 5 studies did not explicitly mention grouping. In the training set, the pooled sensitivity was 0.93 [0.87, 0.96], the pooled specificity was 0.94 [0.87, 0.97], and the area under the ROC curve was 0.98 [0.96, 0.99]. In the validation set, the pooled sensitivity was 0.84 [0.78, 0.88], the pooled specificity was 0.89 [0.85, 0.92], and the area under the ROC curve was 0.93 [1.00, 0.00]. In the subgroup analysis, the areas under the summary receiver operating characteristic (SROC) curves of the artificial intelligence models KNN, SVM, ANN, RF and XGBoost were 0.98, 0.98, 0.94, 0.92, and 0.91, respectively. The Deeks funnel plot indicated that there was no significant publication bias in this study (P > 0.05).
Interpretation
Artificial intelligence models have high accuracy in predicting mortality among COVID-19 patients and have high prognostic value. Among them, the KNN, SVM, ANN, RF, XGBoost, and other models have the highest levels of accuracy.
Similar content being viewed by others
Introduction
The ongoing COVID-19 pandemic poses enormous challenges to global public health, health care systems, and economies. As of December 21, 2022, 649 million people have been diagnosed with COVID-19, and more than 6 million related deaths have occurred worldwide [1]. Although COVID-19 mortality rates have been significantly reduced as vaccination rates have increased and several treatments have been proposed for COVID-19, the progress of the disease has been rapid due to the high complexity of its characteristics. For patients with underlying diseases or those who cannot be treated in a timely manner, the disease tends to progress faster, and the mortality rate is higher [2, 3]. Therefore, effective and accurate outcome predictions and effective and personalized patient management are increasingly important. However, there is still a lack of tools for predicting the risk of death in COVID-19 patients.
Artificial intelligence (AI) is a fusion technology developed based on computer science, cybernetics, information theory, and other disciplines. It can be used in health care applications such as disease diagnosis, prognostic judgement, image analysis, and big data collection. With the rapid development of AI technology, AI algorithms are gradually being applied in various medical fields, such as (1) disease diagnosis, (2) patient morbidity or mortality risk assessment, (3) disease outbreak prediction and surveillance, and (4) health policy and planning [4, 5].
Several studies have shown that AI has high diagnostic value for the early identification of high-risk patients with COVID-19, improving patient prognosis and helping rapid clinical prescreening and triage [6,7,8,9]. However, evidence-based medical studies for predicting mortality among COVID-19 patients are currently unavailable. In this study, a meta-analysis on AI prediction of mortality in COVID-19 patients was conducted to guide the early clinical identification of groups with high mortality risk.
Methods
The present meta-analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (PRISMA) guidelines [10]. For further details (Supplementary Material 1 and 2), this meta-analysis has been registered in PROSPERO (CRD42022315158).
Literature search strategy and screening
The PubMed, Embase, CNKI, Wanfang, China Biomedical Literature Database, VIP, and Web of Science electronic databases were searched from inception to October 2022. Searches were performed by a combination of subject headings and keywords. The search terms included “Artificial Intelligence”, “Machine Intelligence”, “Machine learning”, “AI”, “deep learning”, “random forest”, “Mortality”, “diagnosis”, “SARS-CoV-2”, and “Covid-19”. Two independent researchers (XY and LHX) screened the articles according to the inclusion criteria and performed preliminary screening by reading the titles and abstracts. If a title or abstract could not be judged, the full text was examined to determine whether the article met the inclusion criteria. Disagreements between the researchers were resolved by consulting a third senior expert. For specific retrieval strategies, see Supplementary Material 3.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the study had to be in English and peer-reviewed; (2) the results of machine learning algorithms and predictions of mortality in COVID-19 patients were provided; (3) the data had to be complete with information on sample size, sensitivity values, and specificity values; (4) the total number of patients with COVID-19 was provided; (5) the study subjects were patients who were confirmed positive for COVID-19 by reverse transcription-polymerase chain reaction (RT–PCR), and there was no age limit; (6) the machine learning models and predictors used in the predictions were clearly described; and (7) a clear overview of the sources of the datasets used in the study was provided.
The exclusion criteria were as follows: (1) documents for which true positive values, false-positive values, true negative values, and false-negative values could not be obtained directly or indirectly; (2) reviews, conference reports, case studies, and animal experiments; and (3) duplicate publications.
Data extraction and literature quality assessment
Two researchers independently extracted the following data from the included literature: author, publication year, study population, study type, number of training sets and validation set (if there was no clear grouping in the text, we used the total sample for analysis). In addition, the number of deaths and survivors in the validation set were counted. For the studies for which a fourfold table could not be constructed, we calculated the number of deaths and survivors through the sample mortality rate. Regarding the machine learning models, each model included indicators, study locations, true-positive values, false-positive values, false-negative values, true-negative values, sensitivity values, and specificity values (for studies where there were multiple AI models in the validation set, we primarily analysed the model with the best overall performance). The QUADAS-2 tool was used to evaluate the quality of the included literature and the possibility of bias, and inconsistencies were resolved through by consulting a third investigator [11].
Statistical analysis
Statistical analysis was performed using RevMan 5.3 for Mac, Stata 16.0 for Mac, and Meta-DiSc software. Threshold effect heterogeneity analysis was performed using Meta-DiSc 1.4 software, and the magnitude of heterogeneity was assessed by the I2 statistic. If the effect sizes of the studies were homogeneous, the fixed effects model was used; if there was heterogeneity, the random effects model was used. If there was obvious heterogeneity among the studies, the source of heterogeneity was further judged by sensitivity analysis, threshold effect, and nonthreshold effect analyses. The Sen merge, Spe merge, PLR merge, NLR merge, and DOR merge and their 95% confidence intervals (95% CI) were calculated separately by Stata 16.0 for Mac, the SROC curve was drawn, and the AUC was calculated. The Deeks test was used to evaluate the publication bias of the included studies. If P < 0.05, the included studies were considered to have publication bias.
Results
Literature search results and characteristics of the included studies
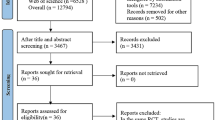
A total of 2193 studies were retrieved from the databases, and 0 studies were identified via manual search. After importing the studies into EndNote literature management software to check for duplicate studies, reading the abstracts and excluding relevant literature according to the exclusion criteria, 23 studies were finally included. The specific literature screening process and results are shown in Fig. 1. Table 1 shows the detailed characteristics of the 23 studies, which were conducted across a total of 12 countries and regions. Twenty-five AI models were used. There were 14 multicentre studies and 9 single-centre studies. Twenty-two studies were retrospective, and one study was cross-sectional. Fifteen studies distinguished between training and validation sets, and 5 studies did not explicitly mention grouping.
Literature quality evaluation
According to the QUADAS-2 tool, the overall risk of bias in patient selection was unclear in 2 studies. All of the risks of bias in the index test and the reference standard test were low. All 23 studies had an unclear risk of bias for the flow and timing domains. In terms of overall concerns regarding applicability, only two studies had an unclear risk of bias in patient selection. The remaining concerns regarding applicability presented low risks (Supplementary Material 4).
Results of the meta-analysis
Validation set (best model pooling)
In the validation set, the best predictive model of the 23 studies assessed the performance of AI in predicting mortality in COVID-19 patients. The overall pooled AUROC was 0.92 [1.00, 0.00]. Additionally, the sensitivity, specificity, PLR, NLR, and diagnostic odds ratio were 0.82 [0.69, 0.91], 0.89 [0.79, 0.95], 7.57 [4.06, 14.09], 0.20 [0.11, 0.35], and 38.33 [18.23, 80.59], respectively (Figs. 2, 3, 4 and 5).
Validation set (all model pooling)
In the validation set, a total of 25 models from 23 studies evaluated the performance of AI in predicting mortality in COVID-19 patients [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The overall pooled AUROC was 0.93 [1.00, 0.00]. Additionally, the sensitivity, specificity, PLR, NLR, and diagnostic odds ratio were 0.84 [0.78, 0.88], 0.89 [0.85, 0.92], 7.37 [5.38, 10.1], 0.18 [0.13, 0.25], and 40.15 [23.79, 67.74], respectively (Supplementary material 5; Figure S1-S4).
Training set
In the training set, a total of 14 models from 5 studies evaluated the performance of AI in predicting mortality in COVID-19 patients. The overall pooled AUROC was 0.98 [0.96, 0.99]. Additionally, the sensitivity, specificity, PLR, NLR, and diagnostic odds ratio were 0.93 [0.87, 0.96], 0.94 [0.87, 0.97], 15.08 [6.89, 33.01], 0.07 [0.04, 0.14], and 202.41 [49.05, 835.20], respectively (Supplementary material 5; Figure S5-S8).
Subgroup Analysis results
-
(1)
In the subgroup analysis of each AI model, we found that the areas under the summary receiver operating characteristic (SROC) curves of KNN, SVM, ANN, RF, XGBoost, LR, DNN, GBM, and DT were 0.98, 0.98, 0.94 0.92, 0.91, 0.86, 0.83, 0.50, and 0.50, respectively. Subgroup analysis was not possible due to the small number of studies that included other models. (Table 2)
-
(2)
In the subgroup analysis of mortality, the areas under the summary receiver operating characteristic (SROC) curve of 0-10%, 10-20%, and > 20% were 0.96, 0.80, and 0.95, respectively. (Table 2)
-
(3)
In the subgroup analysis of the study centres, the areas under the summary receiver operating characteristic (SROC) curves of the multicentre and single-centre studies were 0.93 and 0.88, respectively. (Table 2)
-
(4)
In the regional subgroup analysis, the area under the summary receiver operating characteristic (SROC) curve for Asian and non-Asian regions was 0.94 and 0.84, respectively. (Table 2)
-
(5)
In subgroup analyses with in-hospital mortality as the outcome measure, the overall pooled AUROC was 0.85. Additionally, the sensitivity, specificity, PLR, NLR, and diagnostic odds ratio were 0.76 [0.75, 0.78], 0.85 [0.85, 0.86], 4.14 [3.28, 5.24], 0.33 [0.26, 0.40], and 14.50 [10.28, 20.45], respectively. (Table 2)
Heterogeneity analysis
The results of the heterogeneity test found significant heterogeneity among the studies; a random effects model was used for meta-analysis. Spearman’s correlation coefficient for log sensitivity and log specificity was 0.054 (p = 0.81), suggesting no threshold effect. After excluding the threshold effect heterogeneity, we conducted a sensitivity analysis. After removing each study in turn, the results showed no significant difference between the combined effect size and the total combined effect after removing a single study, indicating that the results were stable and reliable (Supplementary material 5. Figure S9).
Publication bias detection
The results of the Deeks test showed that p = 0.67 (p > 0.05), indicating no publication bias in the included literature (Fig. 6).
Discussion
COVID-19 continues to spread, and global epidemic prevention work has basically brought about the stage of “normalization”. However, as new strains continue to emerge, the death toll from COVID-19 continues to climb, putting enormous pressure on the global health care system. At present, the MEWS [35], APACHE [36], Simplified Acute Physiology Score (SAPS II) [37], Sepsis-related Organ Failure Assessment (SOFA) [38], and Rapid SOFA Score [39] are often used clinically as tools to estimate the death rate of COVID-19, but these scales fail to provide accurate and reliable predictions of mortality in patients with COVID-19 and have limited specificity and sensitivity. Therefore, finding predictive tools with higher diagnostic accuracy is crucial for improving the prognosis of patients with COVID-19.
The advent of AI has generated rapid progress in the diagnosis and prognosis of COVID-19. AI has gradually attracted the attention of clinicians with its large, advanced algorithms in image recognition, data analysis, decision assistance, and other aspects [40, 41]. Therefore, in this study, we conducted the first meta-analysis of algorithm performance in AI prediction of COVID-19 patient mortality.
In the literature included in this meta-analysis, the dataset used by the AI model was divided into a training set and a validation set. The sensitivity of the training set was 0.93 [0.87, 0.96], the specificity was 0.94 [0.87, 0.97], and the AUC was 0.98 [0.96, 0.99]. The sensitivity of the validation set was 0.84 [0.78, 0.88], the specificity was 0.89 [0.85, 0.92], and the AUC was 0.93 [1.00, 0.00]. Compared with the training set, the diagnostic performance of the validation set was slightly reduced, but the difference was not obvious, and the AUC value was still high. This indicates that AI predicts the mortality of patients with high accuracy, and the results are repeatable and reliable.
A subgroup analysis was performed according to different AI models from the studies we included. The results show that among the various models, KNN, SVM, and ANN performed the best, and their AUCs were 0.98, 0.98, and 0.94, respectively. This seems to contradict the general pattern in machine learning: XGBoost tends to perform better than the KNN and SVM models under the same conditions. By analysing the experimental process of comparative literature, we explain this phenomenon from two perspectives. First, from the perspective of data characteristics, the input data in the above research are all low-dimensional (the maximum number of features is 48). SVM and KNN are suitable for dealing with such problems. In contrast, XGBOOST is better suited to handle more complex relationships between data features and targets, and overfitting problems can occur in the case of fewer features. Second, the difference in data quality affects the model performance. The original data used by SVM and KNN do not have the problem of missing data, while the original data of other models do have the problem of missing data, and the processing methods are too simple, such as simple mean and median replacement. As a result, the latter prediction is less than satisfactory. For example, in Prathamesh Parchure’s study, the proportion of missing data ranges from 53.5 to 89.0%, and more than 60% of missing data will make it difficult for any data interpolation method to obtain satisfactory results [42]. An C et al. and Sun L et al. also proposed that the SVM algorithm has high sensitivity and specificity for predicting the mortality of COVID-19 patients with high accuracy and stability [43, 44].
In addition, these AI models predict mortality based on all or part of the clinical characteristics, including demographics (e.g., age, sex, ethnicity), comorbidities (e.g., diabetes, heart disease), symptoms (e.g., cough, fever), vital signs (e.g., heart rate, oxygen saturation), laboratory tests (e.g., blood glucose, creatinine, haemoglobin), imaging measures (e.g., X-ray), and disease treatment and clinical course (e.g., artificial ventilation, length of hospital stay, medications). Algorithms with high AUC values use more predictors. We found that the most commonly adopted predictors of mortality were age, C-reactive protein, and comorbidities. Previously published clinical studies have shown that age, C-reactive protein, and comorbidities play important roles in predicting mortality in patients with COVID-19 [43, 45,46,47].
A previous study by Escobar GJ et al. showed that race was not associated with mortality after COVID-19 infection [48]. We divided the included studies into two groups, Asian and non-Asian, for subgroup analysis and found that the AUC value of the Asian group was 0.94, and the AUC value of the non-Asian group was 0.84. The AI model performed better in the diagnosis of the Asian group. This suggests that when our AI model is extended to people in different regions, the stability of prediction may change to a certain extent due to differences in local medical care levels and new disease prevention and control measures. This suggests that when the model is popularized and applied, it should be adjusted and calibrated according to changes in factors such as regions to improve the accuracy of the diagnosis of the target population.
To further explore the heterogeneity of the studies, we used sensitivity analysis to remove each study one by one, and the results did not change significantly. There was no significant difference between the combined effect size and the total combined effect size after removing a single study with large heterogeneity. The results are stable and reliable.
AI prediction of the mortality of COVID-19 patients can help clinicians make decisions on the length of hospital stay and whether to upgrade according to the risk stratification of predicted patient mortality. In the context of the COVID-19 pandemic, especially in the case of ventilator shortages, it can help medical resource management teams allocate resources and optimize patient management [13, 28]. At present, pathology and radiotherapy guidelines for patients with COVID-19 need to be supplemented. AI model prediction of mortality in COVID-19 patients can help pathologists and radiologists more accurately interpret pathological imaging results to aid diagnosis and treatment. COVID-19 patients who receive AI to accurately predict mortality can also decide whether to discharge or receive palliative care according to the level of their own prediction results to make more appropriate decisions [16].
Our research also has certain limitations. First, the number of studies we included is relatively limited. Due to the lack of relevant articles on AI models based on imaging features, we did not include them in the analysis. We hope that more studies will be conducted in the future with the ability to develop and validate models with imaging features. Second, there were as many as 25 AI models in our included articles, which we believe may be a major source of heterogeneity. In our included studies, the baseline variables (e.g., demographic characteristics, vital signs, comorbidities, laboratory tests) included in each model differed to some extent, which may also be a source of some of the heterogeneity (Supplementary material 6). In addition, the use of “English” as the sole language for searches leads to potential bias due to the large number of studies in other languages involved in COVID-19 studies. Finally, and most importantly, none of our included studies addressed the vaccination status of the included population, which has a strong impact on mortality in COVID-19 patients. It is hoped that future studies will include vaccines as a parameter in AI models to improve their application value in COVID-19 patients.
Conclusion
Compared with traditional COVID-19 mortality prediction tools, the AI model has higher accuracy in predicting the mortality of COVID-19 patients, better predictive performance, and higher prognostic value. Among them, KNN, SVM, RF, ANN, XGBoost, and other models have higher accuracy.
Data Availability
All data supporting the conclusions presented in this article are included in this published article.
Abbreviations
- LR:
-
logistic regression
- SVM:
-
support vector machine
- RF:
-
random forest
- KNN:
-
K Nearest Neighbors
- GBM:
-
Gradient boosting machine
- Cat boost:
-
categorical boosting
- RPART:
-
Recursive Partitioning and Regression Trees
- SVM:
-
Support Vector Machine
- DNN:
-
Deep Neural Network
- ANN:
-
artificial neural network
- XGBoost:
-
eXtreme Gradient Boosting
- ADABoost:
-
Adaptive boosting
- CNN:
-
convolutional neural network
- SVC-RBF:
-
Support Vector Classifier - Radial Basis Function
- DT:
-
Decision Trees
- ABC:
-
Ada-Boost-Classifier
- QDA:
-
Quadratic Discriminant Analysis
- AI:
-
Artificial intelligence
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- CI:
-
confidence interval
- SEN:
-
sensitivity
- SPE:
-
specificity
- NLR:
-
negative likelihood ratio
- PLR:
-
positive likelihood ratio
- DOR:
-
diagnostic odds ratio
- SEN:
-
sensitivity
- SPE:
-
specificity
- TP:
-
true positive
- FP:
-
false positive
- TN:
-
true negative
- FN:
-
false negative
- AUC:
-
area under the curve
- SROC:
-
summary receiver operating characteristic
References
WHO. WHO Coronavirus disease (COVID-19) pandemic. 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed December 21, 2022, 9, 2022.
Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–5.
Toniolo M, Negri F, Antonutti M, Masè M, Facchin D. Unpredictable fall of severe Emergent Cardiovascular Diseases Hospital admissions during the COVID-19 pandemic: experience of a single large Center in Northern Italy. J Am Heart Assoc. 2020;9(13):e017122.
Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20(5):e262–73.
Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395(10236):1579–86.
Khan IU, Aslam N, Aljabri M, Aljameel SS, Kamaleldin MMA, Alshamrani FM, Chrouf SMB. Computational intelligence-based model for Mortality Rate Prediction in COVID-19 patients. Int J Environ Res Public Health. 2021;18(12).
Vaid A, Somani S, Russak AJ, De Freitas JK, Chaudhry FF, Paranjpe I, Johnson KW, Lee SJ, Miotto R, Richter F, et al. Machine learning to Predict Mortality and critical events in a cohort of patients with COVID-19 in New York City: Model Development and Validation. J Med Internet Res. 2020;22(11):e24018.
Schiaffino S, Codari M, Cozzi A, Albano D, Alì M, Arioli R, Avola E, Bnà C, Cariati M, Carriero S, et al. Machine learning to predict in-hospital mortality in covid-19 patients using computed tomography-derived pulmonary and vascular features. J Personalized Med. 2021;11(6).
Bae J, Kapse S, Singh G, Gattu R, Ali S, Shah N, Marshall C, Pierce J, Phatak T, Gupta A, et al. Predicting mechanical ventilation and mortality in covid-19 using radiomics and deep learning on chest radiographs: a multi-institutional study. Diagnostics. 2021;11(10).
Lee SW, Koo MJ. PRISMA 2020 statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematics: Life Cycle Committee Recommendations. Life Cycle. 2022;2:e9.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Das AK, Mishra S, Saraswathy Gopalan S. Predicting CoVID-19 community mortality risk using machine learning and development of an online prognostic tool. PeerJ. 2020;8:e10083.
Yu L, Halalau A, Dalal B, Abbas AE, Ivascu F, Amin M, Nair GB. Machine learning methods to predict mechanical ventilation and mortality in patients with COVID-19. PLoS ONE. Apr 2021;116(4):e0249285.
Tezza F, Lorenzoni G, Azzolina D, Barbar S, Leone LAC, Gregori D. Predicting in-hospital mortality of patients with covid-19 using machine learning techniques. J Personalized Med. 2021;11(5).
Santos-Lozano A, Calvo-Boyero F, López-Jiménez A, Cueto-Felgueroso C, Castillo-García A, Valenzuela PL, Arenas J, Lucia A, Martín MA. Can routine laboratory variables predict survival in COVID-19? An artificial neural network-based approach. Clin Chem Lab Med. 2020;58(12):e299–e302.
Parchure P, Joshi H, Dharmarajan K, Freeman R, Reich DL, Mazumdar M, Timsina P, Kia A. Development and validation of a machine learning-based prediction model for near-term in-hospital mortality among patients with COVID-19. BMJ Support Palliat Care. 2020.
Murri R, Lenkowicz J, Masciocchi C, Iacomini C, Fantoni M, Damiani A, Marchetti A, Sergi PDA, Arcuri G, Cesario A, et al. A machine-learning parsimonious multivariable predictive model of mortality risk in patients with Covid-19. Sci Rep. 2021;11(1):21136.
Li X, Ge P, Zhu J, Li H, Graham J, Singer A, Richman PS, Duong TQ. Deep learning prediction of likelihood of ICU admission and mortality in COVID-19 patients using clinical variables. PeerJ. 2020;8:e10337.
Kar S, Chawla R, Haranath SP, Ramasubban S, Ramakrishnan N, Vaishya R, Sibal A, Reddy S. Multivariable mortality risk prediction using machine learning for COVID-19 patients at admission (AICOVID). Sci Rep. 2021;11(1):12801.
Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2021;34(3):522–31.
Garrafa E, Vezzoli M, Ravanelli M, Farina D, Borghesi A, Calza S, Maroldi R. Early prediction of in-hospital death of COVID-19 patients: a machine-learning model based on age, blood analyses, and chest x-ray score. Elife. 2021;10:e70640.
Banoei MM, Dinparastisaleh R, Zadeh AV, Mirsaeidi M. Machine-learning-based COVID-19 mortality prediction model and identification of patients at low and high risk of dying. Crit Care. 2021;25(1):328.
Ko H, Chung H, Kang WS, Park C, Kim DW, Kim SE, Chung CR, Ko RE, Lee H, Seo JH, et al. An artificial intelligence model to predict the mortality of COVID-19 patients at hospital admission time using routine blood samples: development and validation of an ensemble model. J Med Internet Res. 2020;22(12).
Lin JK, Chien TW, Wang LY, Chou W. An artificial neural network model to predict the mortality of COVID-19 patients using routine blood samples at the time of hospital admission: development and validation study. Med (Baltim). 2021;100(28):e26532.
Abdulaal A, Patel A, Charani E, Denny S, Mughal N, Moore L. Prognostic modeling of COVID-19 using Artificial Intelligence in the United Kingdom: Model Development and Validation. J Med Internet Res. 2020;22(8):e20259.
Moghaddam-Tabrizi F, Omidi T, Mahdi-Akhgar M, Bahadori R, Valizadeh R, Farrokh-Eslamlou H. Identification of risk factors associated with mortality among patients with covid-19 using random forest model: a historical cohort study. Acta Medica Iranica. 2021;59(8):457–65.
Aljouie AF, Almazroa A, Bokhari Y, Alawad M, Mahmoud E, Alawad E, Alsehawi A, Rashid M, Alomair L, Almozaai S, et al. Early Prediction of COVID-19 Ventilation requirement and mortality from routinely collected baseline chest radiographs, Laboratory, and Clinical Data with Machine Learning. J Multidisciplinary Healthc. 2021;14:2017–33.
Naseem M, Arshad H, Hashmi SA, Irfan F, Ahmed FS. Predicting mortality in SARS-COV-2 (COVID-19) positive patients in the inpatient setting using a novel deep neural network. Int J Med Informatics. 2021;154.
Moulaei K, Ghasemian F, Bahaadin-Beigy K, Sarbi RE, Taghiabad ZM. Predicting mortality of covid-19 patients based on data mining techniques. J Biomedical Phys Eng. 2021;11(5):653–62.
Ryan L, Lam C, Mataraso S, Allen A, Green-Saxena A, Pellegrini E, Hoffman J, Barton C, McCoy A, Das R. Mortality prediction model for the triage of COVID-19, pneumonia, and mechanically ventilated ICU patients: a retrospective study. Annals of Medicine and Surgery. 2020;59:207–16.
Ikemura K, Bellin E, Yagi Y, Billett H, Saada M, Simone K, Stahl L, Szymanski J, Goldstein DY, Gil MR. Using automated machine learning to predict the mortality of patients with COVID-19: prediction model development study. J Med Internet Res. 2021;23(2).
Chi PENG, Ge-yao QI, Chen-xu ZHANG, Yu-feng GUO. JIN Zhi-chaoPrediction of in-hospital clinical outcomes of coronavirus disease 2019 patients based on machine learning algorithms. Acad J Sec Mil Med Univ. 2021;42(10):1115–23.
Munera N, Garcia-Gallo E, Gonzalez Á, Zea J, Fuentes YV, Serrano C, Ruiz-Cuartas A, Rodriguez A, Reyes LF. A novel model to predict severe COVID-19 and mortality using an artificial intelligence algorithm to interpret chest radiographs and clinical variables. ERJ Open Res. 2022;8(2):00010–2022.
Peng H, Hu C, Deng W, Huang L, Zhang Y, Luo B, Wang X, Long X, Huang X. Incubation period, clinical and lung CT features for early prediction of COVID-19 deterioration: development and internal verification of a risk model. BMC Pulm Med. 2022;22(1):188.
Subbe CP, Slater A, Menon D, Gemmell L. Validation of physiological scoring systems in the accident and emergency department. Emerg Med J. 2006;23(11):841–5.
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–36.
Le Gall JR, Lemeshow S, Saulnier F. A new simplified Acute Physiology score (SAPS II) based on a European/North american multicenter study. JAMA. 1993;270(24):2957–63.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–8.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Pourhomayoun M, Shakibi M. Predicting mortality risk in patients with COVID-19 using machine learning to help medical decision-making. Smart Health (Amst). 2021;20:100178.
Ryan L, Lam C, Mataraso S, Allen A, Green-Saxena A, Pellegrini E, Hoffman J, Barton C, McCoy A, Das R. Mortality prediction model for the triage of COVID-19, pneumonia, and mechanically ventilated ICU patients: a retrospective study. Ann Med Surg (Lond). 2020;59:207–16.
Barzi F, & Woodward M. Imputations of missing values in practice: results from imputations of serum cholesterol in 28 cohort studies. Am J Epidemiol. 2004;160(1):34–45.
An C, Lim H, Kim DW, Chang JH, Choi YJ, Kim SW. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide korean cohort study. Sci Rep. 2020;10(1):18716.
Sun L, Song F, Shi N, Liu F, Li S, Li P, Zhang W, Jiang X, Zhang Y, Sun L, et al. Combination of four clinical indicators predicts the severe/critical symptom of patients infected COVID-19. J Clin Virol. 2020;128:104431.
Clinical characteristics. day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73.
Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, Worrall D, Giguel F, Piechocka-Trocha A, Atyeo C, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11(1):5493.
Becerra-Muñoz VM, Núñez-Gil IJ, Eid CM, García Aguado M, Romero R, Huang J, Mulet A, Ugo F, Rametta F, Liebetrau C, et al. Clinical profile and predictors of in-hospital mortality among older patients hospitalized for COVID-19. Age Aging. 2021;50(2):326–34.
Escobar GJ, Adams AS, Liu VX, Soltesz L, Chen YI, Parodi SM, Ray GT, Myers LC, Ramaprasad CM, Dlott R, et al. Racial disparities in COVID-19 testing and outcomes: Retrospective Cohort Study in an Integrated Health System. Ann Intern Med. 2021;174(6):786–93.
Acknowledgements
This work was supported by the Technology Department and Natural Science Foundation for Distinguished Young Scholars of Heilongjiang Province (JQ2021H003).
Funding
This work was supported by the Technology Department and Natural Science Foundation for Distinguished Young Scholars of Heilongjiang Province (JQ2021H003).
Author information
Authors and Affiliations
Contributions
Yu Xin, Yuxin Zhou, and Qing Yang wrote the manuscript. Hongxu Li, XutongQu and Hongying Wang performed the literature review. Wenjing Mu, Hongyu Liu and Zipeng Zhuo performed the statistical analysis. Changsong Wang, Haitao Liu and Kaijiang Yu revised the text. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors are responsible for the content of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xin, Y., Li, H., Zhou, Y. et al. The accuracy of artificial intelligence in predicting COVID-19 patient mortality: a systematic review and meta-analysis. BMC Med Inform Decis Mak 23, 155 (2023). https://doi.org/10.1186/s12911-023-02256-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-023-02256-7