Abstract
Background
High-dose methotrexate (HD-MTX) is a potent chemotherapeutic agent used to treat pediatric acute lymphoblastic leukemia (ALL). HD-MTX is known for cause delayed elimination and drug-related adverse events. Therefore, close monitoring of delayed MTX elimination in ALL patients is essential.
Objective
This study aimed to identify the risk factors associated with delayed MTX elimination and to develop a predictive tool for its occurrence.
Methods
Patients who received MTX chemotherapy during hospitalization were selected for inclusion in our study. Univariate and least absolute shrinkage and selection operator (LASSO) methods were used to screen for relevant features. Then four machine learning (ML) algorithms were used to construct prediction model in different sampling method. Furthermore, the performance of the model was evaluated using several indicators. Finally, the optimal model was deployed on a web page to create a visual prediction tool.
Results
The study included 329 patients with delayed MTX elimination and 1400 patients without delayed MTX elimination who met the inclusion criteria. Univariate and LASSO regression analysis identified eleven predictors, including age, weight, creatinine, uric acid, total bilirubin, albumin, white blood cell count, hemoglobin, prothrombin time, immunological classification, and co-medication with omeprazole. The XGBoost algorithm with SMOTE exhibited AUROC of 0.897, AUPR of 0.729, sensitivity of 0.808, specificity of 0.847, outperforming the other models. And had AUROC of 0.788 in external validation.
Conclusion
The XGBoost algorithm provides superior performance in predicting the delayed elimination of MTX. We have created a prediction tool to assist medical professionals in predicting MTX metabolic delay.
Similar content being viewed by others
Introduction
Acute lymphoblastic leukemia (ALL) is a prevalent neoplasm in childhood. The incidence of ALL in children below 15 years of age is 0.004%, which accounts for about 35% of all cases of pediatric malignancies [1,2,3]. Epidemiological studies of ALL indicate that the cumulative incidence is 1/2000 under the age of 15 [4].
Methotrexate (MTX) is a crucial antineoplastic agent in ALL therapy, which inhibits the synthesis of tumor cells by restraining dihydrofolate reductase. In clinical practice, high-dose methotrexate (HD-MTX) can significantly increase the blood drug concentration and permeate blood-brain and blood-testis barriers, so it is recommended as a common chemotherapy approach for ALL treatments. Although HD-MTX is deemed an effective ALL treatment, prolonged exposure to HD-MTX can cause hepatotoxicity, nephrotoxicity, and neurotoxicity [2, 5,6,7,8]. A study from China revealed the rate of delayed MTX elimination was as high as 12.1%, which is a non-negligible rate [9]. A clinical trial has demonstrated that 2-12% of patients develop acute kidney injury (AKI) despite appropriate support during HD-MTX treatment [2, 10]. Furthermore, the severity of adverse reactions of MTX is linked to the concentration and duration of drug exposure. Since the liver and immune system of children are not yet fully developed, their tolerance and metabolic capacity to potential liver toxicity of drugs are inadequate. Therefore, children are more prone to delayed MTX elimination, which could affect their prognosis or lead to other adverse outcomes. Consequently, it is crucial to find ways to reduce the delayed elimination of MTX and the incidence of side effects.
To address the problem of delayed MTX elimination, the current approach is to monitor MTX concentration at 24 h, 48 h, and 72 h post-administration and to administer calcium leucovorin rescue agent and urine alkalization if necessary to accelerate MTX elimination. However, the risk of delayed elimination cannot be predicted based on patient’s signs and data before medication. Therefore, early warning and timely intervention are crucial to effectively reduce the risk of delayed MTX elimination and prevent serious adverse drug reactions.
Artificial intelligence (AI) has been widely used in the medical field. In previous studies, machine learning (ML) was used to classify diseases and analyze the survival of prognosis [11, 12]. Researchers not only extracted disease features for building models, but also achieved high accuracy. This can reduce the fluctuation of patient incidence rate and save on medical costs. Therefore, it is necessary to apply ML to predict the metabolic delay of methotrexate. Researchers, such as Wang Yang [13], Yang Fan [14], and Min Zhang [7], have begun using ML to develop prediction models for delayed MTX elimination. However, previous studies have encountered various issues such as small sample sizes, inadequate representation, limited model construction methods, and insufficient comparability. Additionally, predictive indicators failed to fully consider patient clinical data and relevant clinical laboratory indicators.
This study aims to assess the potential correlation between premedication indicators and delayed elimination of MTX by integrating electronic medical data from multiple centers. Furthermore, a prediction model will be developed using ML methods and a web-based tool to offer an early warning for the delayed elimination of MTX in clinical settings.
Methods
Study design and population
This retrospective study included MTX dosing information, combination medications and laboratory test indicators from seven affiliated medical institutions of Chongqing Medical University from 2011 to 2017. In addition, for external verification, we used MTX medication data from ALL children in Children’s Hospital affiliated to Chongqing Medical University from 2018 to 2021. Inclusion criteria were: (1) patients ≤ 18 years; (2) ALL with risk classification, morphotyping, and immunological classification; (3) chemotherapy with MTX during hospitalization; (4) MTX blood concentration was measured during hospitalization and not longer than 7 days after administration. Exclusion criteria were: (1) missing clinical data; (2) missing ALL risk levels and patient’s weight. According to clinical guidelines and previous literature, the elimination delay of MTX was defined as C24h ≥ 10.0 µmol/L, C48h ≥ 1.0 µmol/L, and C72h ≥ 0.1 µmol/L in this study [2, 8, 9, 13, 15,16,17].
Feature selection
We consulted the variables that were influential in previous studies on delayed MTX elimination, as evidenced in Additional Table 1. The variables in this study comprised demographic characteristics, clinical features, combination medications, and laboratory test data. The demographic variables included age, gender, and weight, whereas clinical features encompassed emesis, hydrops, immunological classification, ALL risk level, the dosage of MTX, and cell morphological classification. Combination medications consisted of omeprazole, ofloxacin, levofloxacin, and benzylpenicillin sodium. The laboratory test variables included total bilirubin (TBIL), creatinine (Cr), uric acid (UA), albumin (ALB), alanine aminotransferase (ALT), urine PH-value (PH), pressure-controlled ventilator (PCV), white blood cell (WBC), platelet count (PLT), hemoglobin (HGB), prothrombin time (PT), lactate dehydrogenase (LDH), fibrinogen (FIB), cerebrospinal fluid (CSF) transparency, and Pandy’s test.
Statistical analysis
The patients were randomly divided into a training set and a test set at a ratio of 7:3 using a random number table. The training set was utilized to select predictors and construct the prediction model, while the test set was used to evaluate the performance of the model. All statistical analyses were conducted in R for Windows (version 3.6.1, https://www.r-project.org/) and SPSS 25.0 (IBM Corporation, Armonk, NY, USA). The random forest algorithm was used to fill in missing values that were less than 30%.
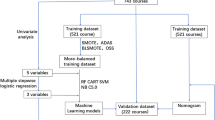
Initially, the normality of continuous variables was assessed using the Shapiro-Wilk test. The t-test was utilized for normal data, while the Mann-Whitney test was used for non-normal data in the univariate analysis. Additionally, the Pearson chi-square test was used for categorical variables. The significant indicators selected by univariate analysis were further filtered using the least absolute shrinkage and selection operator (LASSO) regression method. To address the issue of imbalanced data sets, we conducted three different sampling methods on imbalanced datasets. Oversampling, under-sampling and Synthetic Minority Oversampling Technique (SMOTE) was employed to balance the data sets. ML-based prediction models were constructed using the predictors filtered by LASSO. In the model construction, four ML models were developed, including extreme gradient boosting (XGBoost), random forest classifier (RFC), adaptive boosting (AdaBoost), and light gradient boosting machine (LightGBM). The grid search algorithm was employed to determine the optimal parameters of the model. The area under the receiver operating characteristic curve (AUROC) and the area under the precision-recall curve (AUPR) were used to evaluate the model performance. Additionally, SHapley Additive exPlanation (SHAP) was utilized to interpret the chosen model and complete SHAP visualization. The entire statistical analysis process is shown in Fig. 1. In the previous research on the prediction model of MTX delayed elimination, in addition to using ML, logistic regression was also used. So, we also build a logistic regression nomogram to compare its performance with optimal ML. Finally, we use an external validation set to ensure the generalization and consistency of the model.
Results
Study population
In our research’s dataset (1729 cases), there were 329 and 1400 cases with and without metabolic delays, respectively. After proportionally dividing the dataset with a ratio of 7:3, the training set (1210 cases) comprised 230 patients with metabolic delay and 980 patients without metabolic delay. In the test set (519 cases), 99 patients experienced metabolic delay and 420 patients did not. The external validation set includes 1090 data cases.
Feature selection and data preprocessing
Upon conducting the Shapiro-Wilk test, it was found that all variables were non-normally distributed (as per Additional Table 2). Consequently, we employed the Mann-Whitney U test to compare the continuous variables. Our analysis revealed that age, weight, Cr, UA, TBIL, ALB, ALT, PCV, WBC, HGB, LDH, and PT were statistically significant between the two groups. Furthermore, the Chi-square test indicated that immunological classification, ALL risk level, and co-medication with omeprazole displayed significant differences between the two groups (as illustrated in Table 1). We subsequently performed a LASSO regression analysis on the 15 significant predictors identified through univariate analysis. The paths of the coefficients with different log-transformed λ values in LASSO regression model was displayed in Fig. 2, which clearly demonstrates the significance of several variables, with the influence on delayed MTX elimination increasing as the line moves closer to zero. Moreover, the cross-validation error plot of the LASSO regression model was depicted in Fig. 3. To create a more simplified model, we selected the top 11 variables that had the greatest impact on the outcome. Ultimately, the LASSO method identified eleven indicators, including age, weight, Cr, UA, TBIL, ALB, WBC, HGB, PT, immunological classification, and co-medication with omeprazole, which were used to develop our predictive models.
Coefficient regression graph
The horizontal coordinate is the magnitude of the λ value in the LASSO regression model. As the λ value changes, the later the coefficient is compressed to zero the more influential the variable is. The graphs show that age, TBIL, and Immunological Classification are highly significant
Cross validation curve
The dashed lines indicate the particular λ values, Lambda.min and Lambda.1se. The former represents higher accuracy using the corresponding number of features, i.e., a few more features are used; the latter represents the most straightforward model constructed, i.e., fewer features are used
Model evaluation and interpretation
The variables selected previously were utilized as input variables to establish a prediction model for delayed MTX elimination, with the occurrence of delayed MTX elimination being designated as the outcome event (yes = 1, no = 0). Ultimately, a total of 230 patients with delayed MTX elimination and 980 patients without delayed MTX elimination were included in the training set to develop the predictive model. The test set was then used to validate the predictive ability of the established model. The performance of the delayed MTX elimination risk prediction models with different sampling methods are showed in Additional Table 3.
We chose the XGBoost model sampled by SMOTE as the optimal model for this study. The AUROC performance of the delayed MTX elimination risk prediction model with SMOTE is illustrated in Fig. 4. The AUPR value is more sensitive to sample distribution, and the precision-recall (P-R) curve to showcase the model’s precision and recall performance (Fig. 5). The AUROC value of XGBoost using SMOTE is 0.897(0.857–0.937) and it had an area under the P-R curve (AUPR) of 0.729. In addition, XGBoost sensitivity in SMOTE is 0.808. The higher the sensitivity, the better the model’s ability to correctly identify delayed elimination, and the lower the missed diagnosis rate. The comparison process for selecting the optimal model can be found in Additional File 1. We apply the optimal model to predict external validation sets. It was found that AUROC = 0.788 (0.753–0.822) in external validation, indicating good discrimination ability. We apply the optimal model to predict external validation sets. We used the optimal model to predict the external validation set, and the model demonstrated good performance. Among them, AUROC = 0.788 (0.753–0.822), AUPR = 0.648, specificity = 0.813 (0.780–0.840), sensitivity = 0.680 (0.625–0.735).
As illustrated in Fig. 6, the summary graph of SHAP elucidates the prediction of all samples. The SHAP values of each sample’s variable were plotted by scatter plot, and the relationship between SHAP values and outcomes was analyzed. In the XGBoost model, the SHAP summary plot ranked the importance of delayed MTX elimination variables as co-medication with omeprazole, Cr, UA, WBC, HGB, Age, HGB, ALB, immunological classification, weight, PT and TBIL. Additionally, a dependence plot was generated to assess the relationship between the variables and the predicted influence (Additional Figs. 1–11). The dependency graph lucidly portrays how individual variables affect the model’s predictions.
We constructed a Logistic regression nomogram using the 11 screened indicators. Figure 7 shows an example of using nomogram to predict MTX delayed elimination. The total score corresponds to the probability value on the risk axis, and a higher total score indicates a higher risk of MTX delayed elimination. We evaluated the nomogram with an AUROC of 0.886(0.844–0.929) as shown in Additional Fig. 12.
Discussion
Several research studies have illustrated that prolonged elimination after administering HD-MTX to children with ALL may result in serious adverse effects, particularly in those with atypical renal function [2, 5,6,7,8, 10]. We formulated a risk assessment algorithm for predicting delayed MTX elimination based on pre-medication information. This can facilitate healthcare professionals in recognizing the possibility of delayed MTX elimination in children with ALL.
In this study, age, weight, Cr, UA, TBIL, ALB, WBC, HGB, PT, immunological classification, and concurrent use of omeprazole were recognized as risk factors for delayed MTX elimination. Most of these autonomous risk factors have been reported in preceding research [8, 13,14,15, 17,18,19,20,21,22,23,24,25]. For instance, Nakano T discovered that age, MTX dosage, and TBIL were independent risk factors for delayed MTX elimination [8]. Xu’s research revealed that scrutinizing serum Cr concentration can proficiently anticipate the delay of MTX elimination, and that patients with delayed metabolism have elevated serum Cr levels [22]. A Japanese study found that serum UA levels were correlated with nephrotoxicity prompted by delayed MTX elimination [23]. Another analysis indicated that MTX toxicity could be engendered by combining proton pump inhibitors (such as omeprazole), penicillin family antibiotics, and specific antimicrobial agents [24,25,26,27]. We have retained most of the previous studies on influencing factors, while additionally incorporating FIB, PT, chloride in cerebrospinal fluid, and cerebrospinal fluid transparency. These parameters are easily obtainable in medical facilities, and the multifarious possibilities of causing MTX metabolism delay are exhaustively contemplated. For instance, HD-MTX therapy will prolong thrombin time and diminish FIB [28]. Additionally, distinct dosages of MTX exhibit notable drug concentrations in serum and cerebrospinal fluid [29]. The predictors WBC, HGB, and PT are seldom mentioned in preceding studies and require further validation.
Recently, ML techniques have garnered increasing attention in clinical research and emerged as a powerful instrument for addressing numerous healthcare problems [30,31,32]. In this investigation, we compared the performance of different ML models in different sampling methods for imbalanced data. Among these models’ evaluation, we found that the XGBoost in SMOTE and LightGBM in oversampling were comparable in performance. However, XGBoost demonstrated the better AUPR value and sensitivity. Nitesh Chawla et al. described that smote works by selecting the nearest instances in the feature space, drawing a line between the instances in the feature space, and drawing a new sample along a point of the line [33]. Consequently, we ultimately opted for XGBoost in SMOTE to construct the final prediction model. XGBoost is extensively utilized by data scientists and delivers the most cutting-edge outcomes on a plethora of issues. For instance, XGBoost forestalls overfitting and has the ability to handle voluminous data [34]. Luu Ho Thanh Lam et al. selected XGBoost as the optimal model after SMOTE, to classify the molecular subtypes of low-grade glioma [35]. Nwanosike EM et al. evaluated the advancements of ML algorithms in clinical applications, and the XGBoost algorithm exhibited the highest potential for clinical implementation [36]. We have also implemented the optimal prediction model on the web page to provide a reliable tool for clinical medical professionals and researchers. The web page address is https://cqmugj.shinyapps.io/mtx_jc/.
We constructed a nomogram, which was commonly used in previous studies to predict MTX delayed elimination. We found that the AUROC value using the nomogram was smaller than that of the optimal model (XGBoost). On the other hand, nomogram is a non-parametric model that requires the total score to obtain the probability. And it can’t automatically calculate the result, which is a bit inconvenient compared to ML. In addition, the model’s AUROC and specificity after external validation indicated that it had good discrimination and a low misdiagnosis rate. And the result also reflected the transportability and generalization ability of the model. On the other hand, it indicates that the model has good consistency in different time periods compared to the model development queue.
The current research is mainly to accurately diagnose the adverse reaction or MTX delayed elimination by using the post medication test index of methotrexate combined with ML. We summarize some similar studies and draw a Table 2. For example, Hu et al. created an ML-based model for predicting low-dose MTX-related hepatotoxicity with an AUC of 0.97 but only accuracy of 0.64 [37]. Zhan et al. employed an artificial intelligence algorithm to forecast neutropenia and fever caused by high-dose MTX in children with B-cell ALL, with an AUC of 0.870 [38]. The performance of our model is similar to that of Zhan M et al. [7], but inferior to Schmidt, D [13]. In addition, we summarized some researches on the analysis of MTX delayed elimination factors in recent years (see Additional Table 1). However, few studies have integrated the identified risk factors and applied them directly to the prediction of delayed MTX elimination. Zhan M et al. used hematocrit, risk classification, dose, SLC19A1 rs2838958, and sex indicators to develop a prediction model for delayed elimination of MTX. The highest AUC of the model was 0.807 (95% CI, 0.724–0.889) [7]. They used fewer variables and included genetic factors to build a prediction model with better performance. However, our predictors are easily obtainable and it is of great value in identifying MTX metabolic delay.
Nonetheless, the study has certain limitations. Firstly, the incidence, treatment, and individual differences in ALL across different regions may hinder the applicability of the model. Secondly, some variables, such as the genetic characteristics of the affected children and their living environment, have not been included. Thirdly, our study was retrospective research, the examination of some cases was done with inadequate equipment and training, and some indicators with missing values greater than 30% (e.g. urine volume) were not included in the model. Finally, the generalization ability of the model should be further confirmed through multi-center external validation in future studies.
Conclusions
In summary, this investigation illustrates that factor such as age, body weight, creatinine, uric acid, total bilirubin, albumin, white blood cell count, hemoglobin, prothrombin time, cellular morphological classification, and concomitant use of omeprazole could be served as predictors for delayed MTX elimination. Through the application of XGBoost after SMOTE, delayed MTX elimination can be effectively identified in children diagnosed with ALL. Our predictive model provides a reliable means for monitoring the metabolic delay of MTX, even in the absence of MTX plasma concentration monitoring. By utilizing this tool, medical professionals can take timely targeted measures to prevent the occurrence of MTX-related adverse drug events.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Abbreviations
- MTX:
-
Methotrexate
- HD-MTX:
-
High-dose methotrexate
- ALL:
-
Acute lymphoblastic leukemia
- CNS:
-
Central nervous system
- NHL:
-
Non-Hodgkin’s lymphoma
- AKI:
-
Acute kidney injury
- ML:
-
Machine learning
- LASSO:
-
Least absolute shrinkage and selection operator
- RFC:
-
Random forest classifier
- XGBoost:
-
eXtreme gradient boosting
- AdaBoost:
-
Adaptive boosting
- LightGBM:
-
Light gradient boosting machine
- CatBoost:
-
Categorical boosting
- GBDT:
-
Gradient Boosting Decision Tree
- TPOT:
-
Tree-based Pipeline Optimization Tool
- ANN:
-
Artificial Neural Network
- BMI:
-
Body Mass Index
- SMOTE:
-
Synthetic Minority Oversampling Technique
- AUROC:
-
Area under the receiver operating characteristic curve
- AUPR:
-
Area under PR curve
- SHAP:
-
Shapley Additive exPlanations
- Cr:
-
Creatinine
- UA:
-
Uric acid
- TBIL:
-
Total bilirubin
- ALB:
-
Albumin
- WBC:
-
White blood cell count
- HGB:
-
Hemoglobin
- PT:
-
Prothrombin time
- ALT:
-
Alanine aminotransferase
- PH:
-
Urine PH-value
- PCV:
-
Pressure controlled ventilator
- PLT:
-
Platelet count
- LDH:
-
Lactate dehydrogenase
- FIB:
-
Fibrinogen
- Cl:
-
Chloride ion
References
Lin LZL, Chen J, Huang C, Pharmacy DO, Hospital HG. Analysis of plasma concentration monitoring and adverse reactions of methotrexate in the pediatrics of our hospital. Chin J Clin Pharm. 2018;27:329–33.
Howard SC, McCormick J, Pui C-H, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471–82. https://doi.org/10.1145/2939672.2939785. .
Mantadakis E, Cole PD, Kamen BA. High-dose methotrexate in acute lymphoblastic leukemia: where is the evidence for its continued use? Pharmacotherapy. 2005;25(5):748–55. https://doi.org/10.1592/phco.25.5.748.63584.
Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. NAT REV CANCER. 2018;18(8):526. https://doi.org/10.1038/s41568-018-0015-6. vol 18, pg 471, 2018.
Leveque D, Santucci R, Gourieux B, Herbrecht R. Pharmacokinetic drug-drug interactions with methotrexate in oncology. Expert Rev Clin Pharmacol. 2011;4(6):743–50. https://doi.org/10.1586/ecp.11.57. .
Taylor ZL, Vang J, Lopez-Lopez E, Oosterom N, Mikkelsen T, Ramsey LB. Systematic review of pharmacogenetic factors that Influence High-Dose Methotrexate Pharmacokinetics in Pediatric Malignancies. Cancers. 2021;13(11). https://doi.org/10.3390/cancers13112837. .
Zhan M, Chen Z, Ding C, Qu Q, Wang G, Liu S, et al. Risk prediction for delayed clearance of high-dose methotrexate in pediatric hematological malignancies by machine learning. Int J Hemato. 2021;114(4):483–93. https://doi.org/10.1007/s12185-021-03184-w.
Nakano T, Kobayashi R, Matsushima S, Hori D, Yanagi M, Suzuki D, et al. Risk factors for delayed elimination of high-dose methotrexate in childhood acute lymphoblastic leukemia and lymphoma. Int J Hemato. 2021;113(5):744–50. https://doi.org/10.1007/s12185-020-03071-w. .
Xu W, Tang Y, Fang C, Song H, Shi S, Yang S, et al. Study on elimination delay in high dose methotrexate therapy in childhood acute lymphoblastic leukemia. Chin J Hematol. 2005;26(1):15–8.
Widemann BC, Balis FM, Kim A, Boron M, Jayaprakash N, Shalabi A, et al. Glucarpidase, leucovorin, and thymidine for High-Dose Methotrexate-Induced Renal Dysfunction: clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28(25):3979–86. https://doi.org/10.1200/JCO.2009.25.4540.
Kumar N, Sharma M, Singh VP, Madan C, Mehandia S. An empirical study of handcrafted and dense feature extraction techniques for lung and colon cancer classification from histopathological images. Biomed Signal Process Control. 2022;75:16. https://doi.org/10.1016/j.bspc.2022.103596.
Sharma M, Kumar N. Improved hepatocellular carcinoma fatality prognosis using ensemble learning approach. J Ambient Intell Humaniz Comput. 2021;15. https://doi.org/10.1007/s12652-021-03256-z.
Wang Y, Zhang H, Chen Y, Xu H, Gao P, Liu M, et al. Predictive analysis of methotrexate elimination delay based on logistic regression model and ROC curve. Chin J Hosp Pharm. 2015;35(07):618–22.
FANG Y, Ni W, Chen Z, Tang L, Shen A, Shi Y. Preliminary establishment of logistic regression prediction model for delayed excretion of high-dose chemotherapy based upon blood concentration monitoring of 7-hydroxymethotrexate. Chin J Hosp Pharm. 2021;41:886–92. https://kns.cnki.net/kcms/detail/42.R.20210317.0907.002.html.
Schmidt D, Kristensen K, Schroeder H, Wehner PS, Rosthoj S, Heldrup J, et al. Plasma creatinine as predictor of delayed elimination of high-dose methotrexate in childhood acute lymphoblastic leukemia: a danish population-based study. Pediatr Blood Cancer. 2019;66(6). https://doi.org/10.1002/pbc.27637. .
Tsurusawa M, Gosho M, Mori T, Mitsui T, Sunami S, Kobayashi R, et al. Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of Childhood NonHodgkin Lymphoma. Pediatr Blood Cancer. 2015;62(2):279–84. https://doi.org/10.1002/pbc.25305.
Qian X, Li L, Wang Q, Li L, Xie J. Research progress of high dose methotrexate-calcium folinate to rescue children with acute lymphoblastic leukemia. Chin J Hosp Pharm. 2016;36(18):1613–8.
Sun K, Tao HW, Ding TL, Li ZR, Qiu XY, Zhong MK, et al. Risk factors for high-dose methotrexate associated toxicities in patients with primary central nervous system lymphoma. J Clin Pharm Ther. 2022;47(12):2196–204. https://doi.org/10.1111/jcpt.13791. .
Kataoka T, Sakurashita H, Kajikawa K, Saeki Y, Taogoshi T, Matsuo H. Low serum albumin level is a risk factor for delayed Methotrexate Elimination in High-Dose Methotrexate Treatment. Ann Pharmacother. 2021;55(10):1195–202. https://doi.org/10.1177/1060028021992767. .
Pang L, Liu L, Zhao L. Research Progress in influence factors of Excretion Delay of high-dose methotrexate. Chin Pharm J. 2013;48(22):1892–6.
Fan F, Yu W, Xie R, Chen Q. Analysis of excretion delay of 24 hour high dose methotrexate by therapeutic drug monitoring. Chin J Hosp Pharm. 2016;36(2):115–9.
Xu W, Zhang L, Chen X, Pan B, Mao J, Song H, et al. Serum creatinine and creatinine clearance for predicting plasma methotrexate concentrations after high-dose methotrexate chemotherapy for the treatment for childhood lymphoblastic malignancies. CANCER CHEMOTH PHARM. 2014;73(1):79–86. https://doi.org/10.1007/s00280-013-2319-2. .
Kawaguchi S, Fujiwara S-I, Murahashi R, Nakashima H, Matsuoka S, Ikeda T, et al. Risk factors for high-dose methotrexate-induced nephrotoxicity. Int J Hematol. 2021;114(1):79–84. https://doi.org/10.1007/s12185-021-03132-8. .
Santucci R, Leveque D, Lescoute A, Kemmel V, Herbrecht R. Delayed elimination of Methotrexate Associated with Co-Administration of Proton Pump inhibitors. Anticancer Res. 2010;30(9):3807–10.
Schmutz JL. Drug interactions between methotrexate and antibiotics. Ann Dermatol Venereol. 2018;145(6–7):469–70. https://doi.org/10.1016/j.annder.2018.05.001. .
Narumi K, Sato Y, Kobayashi M, Furugen A, Kasashi K, Yamada T, et al. Effects of proton pump inhibitors and famotidine on elimination of plasma methotrexate: evaluation of drug-drug interactions mediated by organic anion transporter 3. Biopharm Drug Dispos. 2017;38(9):501–8. https://doi.org/10.1002/bdd.2091. .
Bolina JJ, Chatterley M, Jamali T. Interaction between Low-Dose Methotrexate and nonsteroidal anti-inflammatory drugs, penicillins, and Proton Pump inhibitors: a narrative review of the literature. Ann Pharmacother. 2017;51(2):163–78. https://doi.org/10.1177/1060028016672035. .
Flaxman AD, Vos T. Machine learning in population health: Opportunities and threats. PLoS Med. 2018;15(11):e1002702. https://doi.org/10.1371/journal.pmed.1002702. .
Totan M, Dagdemir A, Ak AR, Albayrak D, Kucukoduk S. Effects of high-dose methotrexate on the hemostatic system in childhood acute lymphoblastic leukemia. Med Oncol. 2001;36(4):429–33. https://doi.org/10.1002/mpo.1106. .
Thyss A, Milano G, Deville A, Manassero J, Renee N, Schneider M, EFFECT OF DOSE AND, REPEAT INTRAVENOUS 24 HR INFUSIONS OF METHOTREXATE ON CEREBROSPINAL-FLUID AVAILABILITY IN CHILDREN WITH HEMATOLOGICAL MALIGNANCIES. Eur J Cancer. 1987;23(6):843–7. https://doi.org/10.1016/0277-5379(87)90289-6. .
Kumaraswamy E, Kumar S, Sharma M. An invasive ductal carcinomas breast Cancer Grade classification using an ensemble of convolutional neural networks. Diagnostics. 2023;13(11):17. https://doi.org/10.3390/diagnostics13111977.
Lam LHT, Le NH, Tuan LV, Ban HT, Hung TNK, Nguyen NTK, et al. Machine learning model for identifying antioxidant proteins using features calculated from primary sequences. Biology-Basel. 2020;9(10):13. https://doi.org/10.3390/biology9100325.
Chawla NV, et al. Smote: synthetic minority over-sampling technique. J ARTIF INTELL RES. 2002;16(1):321–57. https://doi.org/10.1613/jair.953.
Chen T, Guestrin C, XGBoost:. A Scalable Tree Boosting System. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; San Francisco, California, USA: ACM; 2016. p. 785–94.https://doi.org/10.1145/2939672.2939785.
Lam LHT, Do DT, Diep DTN, Nguyet DLN, Truong QD, Tri TT, et al. Molecular subtype classification of low-grade gliomas using magnetic resonance imaging-based radiomics and machine learning. NMR Biomed. 2022;35(11):13. https://doi.org/10.1002/nbm.4792.
Nwanosike EM, Conway BR, Merchant HA, Hasan SS. Potential applications and performance of machine learning techniques and algorithms in clinical practice: a systematic review. Int J Med Inform. 2022;159. https://doi.org/10.1016/j.ijmedinf.2021.104679.
Hu Q, Wang H, Xu T. Predicting Hepatotoxicity Associated with Low-Dose Methotrexate using machine learning. J Clin Med. 2023;12(4). https://doi.org/10.3390/jcm12041599.
Zhan M, Chen Z, Ding C, Qu Q, Wang G, Liu S, et al. Machine learning to predict high-dose methotrexate-related neutropenia and fever in children with B-cell acute lymphoblastic leukemia. Leuk. 2021;62(10):2502–13. https://doi.org/10.1080/10428194.2021.1913140.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and Technology Research Project of Chongqing (Grant numbers: cstc2015shms-ztzx10011), the Intelligent medicine project of Chongqing Medical University (Grant numbers: ZHYX2019005, ZHYX202211, YJSZHYX202224 and YJSZHYX202215), Key project of Chongqing Science and Health Joint Medical Scientific Research Project (Grant numbers: 2022ZDXM020) and Future Medicine Youth Innovation Team project of Chongqing Medical University (Grant numbers: W0081).
Author information
Authors and Affiliations
Contributions
J.G., H.L.W. conceived and designed the study. C.J., S.Q.C., Y.Zu. developed the study design and drafted the manuscript. C.J., Z.Y.L., J.J. contributed to the collection of data. T.Y.X., X.W., T.T.W. contributed to constructive discussions. J.G., C. J, Y.Zg. contributed to model building. H.L.W., J.G., Y.Zu., C.J. provided funding resources. Z.C.W and Y.T.J provided the latest data and data processing technology for external validation. All authors reviewed and approved the manuscript prior to its submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by the Ethics Committee of Chongqing Medical University, and with its approval, this study required no informed consent. All methods were performed in accordance with the Declaration of Helsinki and the relevant guidelines. Due to all the data were anonymized data in this study, the Ethics Review Committee of Chongqing Medical University has waived the informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jian, C., Chen, S., Wang, Z. et al. Predicting delayed methotrexate elimination in pediatric acute lymphoblastic leukemia patients: an innovative web-based machine learning tool developed through a multicenter, retrospective analysis. BMC Med Inform Decis Mak 23, 148 (2023). https://doi.org/10.1186/s12911-023-02248-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-023-02248-7