Abstract
Background
Adherence to disease-modifying therapy is important in patients with Multiple Sclerosis (MS) to increase the positive outcomes and improve the quality of life. This study aimed to determine the effects of Continuous Care Model (CCM) using a smartphone application on adherence to treatment and self-efficacy among MS patients.
Methods
This quasi-experimental study with pre/posttest design was conducted on 72 MS patients in Shiraz, Iran from June 2020 to August 2021. The samples were randomly assigned to intervention (n = 36) and control (n = 36) groups. In the intervention group, the CCM using a smartphone application was implemented during two months. However, no intervention was performed for the control group. The data were collected using the self-report Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ) and MS Self-Efficacy Scale (MSSS) at baseline and two and four months after the intervention.
Results
The results showed an improvement in adherence to treatment and self-efficacy in the intervention group compared to the control group after implementing the virtual CCM and at the two-month follow-up (p < 0.001).
Conclusions
Implementing the CCM using a smartphone application resulted in improvements in the MS patients’ adherence to treatment and self-efficacy. It can be concluded that providing care using an interactive multimedia application can improve the outcomes as well as patients’ satisfaction, especially during the COVID-19 pandemic. Therefore, this approach is recommended to be used for nurses, healthcare providers, and clinicians.
Similar content being viewed by others
Background
Multiple Sclerosis (MS) is the most prevalent chronic, inflammatory, destructive, and progressive demyelinating disease of the central nervous system [1]. It has been estimated that 2–5 million people suffer from MS around the world. In Iran, averagely 80,000 individuals suffer from MS [2], with the highest prevalence rates being related to Tehran, Isfahan, and Fars provinces with 102, 106.46, and 78 individuals per 100,000 population, respectively [3, 4]. MS significantly influences patients’ daily activities and quality of life [5, 6].
Dealing with chronic diseases usually requires long-term treatment plans to empower patients [7], which include Disease-Modifying Therapies (DMTs) (supportive care, rehabilitation, and symptom management) [8]. Adherence to DMTs is essential to maximize the beneficial effects of MS treatment and reduce the number of clinical relapses [9]. On the other hand, failure to follow treatment and care regimens increases the risk of complications and mortality as well as the cost of healthcare [10]. According to the World Health Organization (WHO), compliance with treatment regimens is an important factor in the success of treatment. Poor compliance reduces the desired clinical effects and, consequently, reduces the effectiveness of health systems [11]. Various factors are associated with adherence to treatment including age, gender, socioeconomic status, limited effectiveness of treatments, type of MS, patient’s attitude, side effects of drugs, amnesia, depression, anxiety, and cognitive problems. Identification and elimination of these factors increase adherence to treatment and help choose the effective treatment for MS [9]. Erbay et al. [12] reported an adherence rate of 59.6% in MS patients.
Decreased self-efficacy is another problem faced by these patients [13]. Self-efficacy is the main prerequisite for behavior change including health behaviors [14]. Sikes et al. [15] reported that MS could reduce self-efficacy by up to 48%. Considering the chronic nature of MS, self-efficacy is an important internal factor for long-term control and management of this disease [16].
Despite the recent therapeutic advances, there is still no known cure for MS [17]. Consequently, continuous care seems to be necessary to prevent the complications and recurrence of the disease [18]. The Continuous Care Model (CCM) aims to establish an effective, interactive, and consistent relationship between the client and the nurse as a provider of healthcare services to evaluate the clients’ needs and problems and sensitize them to accept their continuous health behaviors and help maintain their recovery process and health promotion, which is compatible with the characteristics of chronic diseases and the dynamics of their problems [19]. The recommended treatment regimen often takes place at home and outside the scope of medical care in patients with MS [20]. Thus, providing remote healthcare services increases the access of patients with mobility or geographical limitations [21]. Nowadays, access to health services can be greatly facilitated due to the advances in computer and network technologies [22].
Educational software programs create a participatory platform and provide valuable information, thereby creating an opportunity to improve the disease process that allows patients to access the required information upon request [23]. Therefore, it is important to educate clients through multimedia, as an appropriate method to address the educational needs of patients with MS [24]. The main advantages of this method include the multimedia feature or using a combination of texts and audios/videos, comprehensive activation, reproducibility, and feedback [25]. Evidence has also shown that multimedia software-based education can improve knowledge and compliance with treatment regimens [26].
As mentioned earlier, continuous care aims at designing and maintaining a flexible, dynamic and continuous care relationship between the nurse and the patient for improving patient outcomes. Thus, this model can lead to acceptance, increase of insight, and continuous engagement [27]. The introduction of this model using an Android-based smartphone application seems to be a good option for providing services to MS patients, particularly during the COVID-19 pandemic. Up to now, few studies have been published on the effect of this remote model on self-efficacy and adherence to treatment among MS patients. Therefore, the present study aimed to evaluate the effects of CCM using a smartphone application on adherence to treatment and self-efficacy among MS patients.
Methods
Study design
This quasi-experimental study with the pre/posttest design was conducted on 72 MS patients referred to the MS Association affiliated to Shiraz University of Medical Sciences, Shiraz, Iran from June 2020 to August 2021.
The study sample size was estimated according to a similar study [28] and considering α = 0.05, β = 0.2, and attrition rate of 15%. Patients were recruited via convenience sampling and were then randomly assigned to the intervention (n = 36) and control (n = 36) groups through block randomization with the block size of four using the Random Allocation Software. The inclusion criteria were suffering from relapsing–remitting MS, aging 18–45 years, having mild to moderate disability (EDSS 0-5-5), not being in the recurrence phase, being able to use smartphones, having an Android smartphone, and having the history of MS for at least six months. The exclusion criteria were suffering from other types of MS (primary progressive, secondary progressive and progressive relapsing), unwillingness to continue cooperation, incomplete attendance in educational interventions, severe disease complications, and known psychophysical disorders.
Data collection
Data collection tools consisted of a demographic questionnaire, the MS-Treatment Adherence Questionnaire (MS-TAQ), and MS Self-Efficacy Scale (MSSS).
Measurement
Demographic information form
The demographic information form included two parts, namely demographic characteristics (age, gender, marital status, education level, and occupation) and disease-related information (duration of the disease, frequency of recurrence, and number of hospitalizations during the past year).
MS-Treatment Adherence Questionnaire
The MS-TAQ is a self-report tool for identifying barriers to adherence amongst MS patients taking DMTs. The MS-TAQ was developed by Wicks et al. [29] in the United States. It contained 30 items divided into three subscales, namely DMT-Barriers, DMT-Side Effects, and DMT-Coping Strategies. In the DMT-Barriers subscale, the patients who had missed at least one dose in the previous 28 days were asked 13 four-point questions pertaining to the barriers to adherence (“not important at all” to “extremely important”). In the DMT-Side Effects subscale, 10 treatment side effects that might have a negative effect on adherence to treatment were listed in form of five-point questions from “never” to “all or nearly all the time.” The DMT-Coping Strategies included seven yes/no questions regarding the coping strategies that might be effective in reducing the treatment side effects. The reliability of this tool was confirmed by Cronbach’s alpha coefficients ranging from 0.40 to 0.86. In addition, the convergent validity of this questionnaire was reported as the relationship between Missed Dose Ratio (MDR) and the treatment adherence subscale (r = 0.5). Its divergent validity was also reported as the relationship between coping strategies and MDR (r = 0.3). The Persian version of the scale was validated by Maghsoud Puryousef [30] with a Cronbach’s alpha coefficient of 0.87. In the present study also, the Cronbach’s alpha of this instrument was computed as 0.90.
MS self-efficacy scale
MSSS was developed by Rigby et al. [31] in England. The MSSS included 14 items divided into four dimensions, namely independence and activity (five items), concerns and interests (four items), personal control (three items), and social confidence (two items). The items could be scored via a six-point Likert scale ranging from strongly disagree (1) to strongly agree (6). Thus, the total score could range from 6 to 84, with higher scores representing higher self-efficacy. The total score of the scale could be calculated through summing up the scores of the subscales. The reliability of the scale was approved by Cronbach’s alpha coefficient of 0.81. Moreover, the results of Exploratory Factor Analysis (EFA) indicated that the four-factor structure with 14 items explained 57.9% of the variance, which revealed the acceptable validity of the instrument. In Iran, the psychometric properties of MSSS were measured by Reshvanloo [32]. The validity of the scale was explored via construct validity (EFA) and divergent validity. Divergent validity was measured with a depression scale (r = − 0.74). Additionally, the reliability of the whole scale was confirmed by Cronbach’s alpha = 0.9. In the present study, the Cronbach’s alpha was calculated as 0.92.
Development of the smartphone application
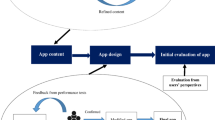
At first, educational materials on MS were extracted from authentic resources (i.e., textbooks, literature, etc.) and were evaluated and revised by two experts in the field of nursing and one neurologist. The educational content included the description of MS (i.e., pathophysiology, clinical manifestations, assessment and diagnosis, pharmacologic therapies, management of the disease, nutrition, pregnancy, exercise, etc.), COVID-19 and its prevention among MS patients, and self-management programs. Then, based on educational materials, electronic contents, images, animations, and audio and video clips were produced and designed in form of an installable application on electronic devices using an Android Studio program on the Android platform. Finally, the developed Persian multimedia software named the “MS App” was evaluated and tested by electronic content production engineers.
The “MS App” consisted of various sections including educational sections about the disease, injection training (i.e., interferon beta-1a and interferon beta-1a), entertainment section (i.e., motivational contents, music, etc.), night section (patients' night rest including audio podcasts, e-books, and instrumental music), chat room (for the patients to communicate with each other), and a notification system status bar. Besides, a section of the software was designed to interact with the researcher, so that the patients could communicate with the researcher whenever they needed. The software could be easily installed on Android smartphones and the contents were updated daily without the need to reinstall a new application (Additional file 1).
Intervention
Initially, the written informed consent form and the questionnaires were completed by both groups in the MS Association before the intervention. Then, the CCM using an Android-based interactive smartphone application was performed for the patients in the intervention group for 4 months. However, the control group only received the routine care. The CCM is a nursing care model that was developed and assessed psychometrically in patients with chronic coronary artery disease by Ahmadi et al. in Iran in 2001. This model consisted of four steps including orientation, sensitization, control, and evaluation [27].
The first stage (orientation) included explaining the aims, making a relationship with the clients, explaining the study protocol, collaboration to take part in the study, and involvement of the clients and their families in care. For this purpose, a 30–45-min session was held with the presence of the patients and their families at the clinic to identify the patients’ problems accurately motivate them to participate, explain the importance of continuous care, determine their expectations from each other, and express the need for continuation of the cooperation until the end of the study. At the end of the orientation session, the demographic information form, MS-TAQ, and MSSS were completed.
Sensitization stage Interventions were performed in this stage during two mouths. In doing so, sensitizing the clients to accept responsibility for their health was emphasized by evaluating their educational needs. Overall, the patients became familiar with the process of the disease, its complications, DMTs, adherence to treatment, and self-management programs and their possible questions were addressed through the MS App. This multimedia app could be easily installed on Android mobile phones.
Control stage This phase consisted of the assessment and continuity of care. Continuous care consultations and care needs were followed daily and weekly through a part of the MS App that was designed for this purpose. The telephone number of the researcher was given to the patients, as well.
Evaluation stage In the fourth stage, the effects of the interventions and follow-ups were evaluated using the scales for measuring and comparing adherence to treatment and self-efficacy.
Immediately after the intervention and at the two-month follow-up, the MSSS and MS-TAQ were completed by the two groups in an online platform due to the COVID-19 pandemic. In addition, the patients in the intervention group were requested to take part in an online survey and express their satisfaction with the implementation of the virtual CCM. At the end of the study, the MS App was made available to all the patients in the MS Association, Shiraz, Iran.
Data analysis
The data were analyzed by the SPSS 22 software at the significance level of p < 0.05. Kolmogorov–Smirnov test showed the normal distribution of the data related to adherence to treatment and self-efficacy (p > 0.05). Thus, the data were analyzed using descriptive indices such as mean and Standard Deviation (SD) and inferential statistics including chi-square, Fisher’s exact test, independent t-test, repeated measures ANOVA, and LSD post-hoc test.
Results
The results indicated that 66.7% of the patients were female and 48.6% were within the age range of 18–29 years. In addition, 62.5% of the patients were suffering from the disease for 1–5 years and 48.6% had a positive history of relapse. There were no significant differences between the intervention and control groups in terms of sociodemographic and clinical characteristics (p > 0.05) (Table 1).
The results of between-group and within-group comparisons of the mean scores of adherence to treatment before, after, and two months after the intervention have been presented in Table 2. The results of independent t-test showed no significant difference between the two groups regarding the mean score of adherence to treatment before the intervention (p = 0.83). However, this difference was significant immediately and two months after the intervention. Repeated measures ANOVA was used to compare the mean scores of adherence to treatment in each group before, immediately after, and two months after the intervention. In the intervention group, a significant difference was observed in this regard before, immediately after, and two months after the intervention (p < 0.0001) (Table 2).
The results of between-group and within-group comparisons of the mean scores of self-efficacy before, immediately after, and two months after the intervention have been presented in Table 3. The results of independent t-test showed no significant difference between the two groups concerning the mean score of self-efficacy before the intervention (p = 0.80). However, this difference was significant immediately and two months after the intervention. Repeated measures ANOVA was used to compare the mean scores of self-efficacy in each group before, immediately after, and two months after the intervention. In the intervention group, a significant difference was observed in this regard before, immediately after, and two months after the intervention (p < 0.0001) (Table 3). LSD post-hoc test was used to assess the changes in different stages of the research. Furthermore, the findings indicated that the majority of the patients in the intervention group were satisfied with this approach.
Discussion
The present study results indicated that applying the CCM using the MS App improved adherence to treatment and self-efficacy among the MS patients. Accordingly, the patients who received the MS App showed better levels of adherence to treatment compared to those in the control group immediately after the intervention and at the two-month follow-up. In fact, the mean score of adherence to treatment decreased by removing the barriers. Thus, obtaining a lower score indicated a better improvement in adherence to treatment. Previous studies demonstrated that the CCM was effective in treatment adherence among hemodialysis and myocardial infarction patients [33, 34] and affected lifestyle modification in patients with MS [35]. Similarly, Golan et al. [36] reported that an electronic notebook through smartphones could improve treatment adherence amongst MS patients.
In the present study, after applying the continuous care model and at the two-month follow-up, the mean score of self-efficacy was higher in the intervention group than in the control group. A recent study also concluded that the continuous care model of information-based hospital-family integration via online social media could improve self-efficacy, colostomy complications, and quality of life in colostomy patients [37]. In the same vein, Ehde et al. indicated the effectiveness of telephone-based self-management interventions in improving self-efficacy and reducing the complications of MS patients. They came to the conclusion that the telephone-delivered intervention was effective in engaging patients in care and improving their disabilities [38]. Another study also revealed that having access to smartphone apps enhanced women’s performance, self-efficacy, and health beliefs in breast self-examination [39]. Evidence has shown that during the COVID-19 outbreak, smartphones and apps have played a key role in several aspects of healthcare delivery and clinical practice among healthcare professionals [40, 41]. Therefore, alternative approaches can facilitate healthcare delivery during the pandemic.
Strengths and limitations
A key strength of the current study was the innovation in applying continuous care for the MS patients during the COVID-19 pandemic. Implementing the remote CCM during the pandemic minimized the patients’ face-to-face visits and consequently, reduced the risk of spread of the viral infection among patients, healthcare providers, and clinicians, and increased patient satisfaction. However, the study had several limitations including the selection of patients with a specific type of MS (relapsing–remitting), which might increase the likelihood of bias in the data. Another study limitation was selecting the samples within the age range of 18–45 years. With smartphone applications, technology is more likely to be embraced by the younger generation, thus limiting its applicability in older individuals. As another limitation, adherence to treatment was measured using self-report scale that did not directly assess adherence and had a weak correlation (r = 0.5) with the missed dose ratio. Other study limitations included the time-consuming design of the application, the outbreak of COVID-19 that prolonged the sampling process, and the short follow-up period (two months) due to the limited research time.
Conclusions
The results demonstrated that implementing the CCM using the MS App led to improvements in treatment adherence and self-efficacy among the MS patients. Thus, it can be concluded that providing care with an interactive multimedia application can enhance the outcomes as well as patient satisfaction, especially during the COVID-19 pandemic. Therefore, this approach is recommended to be used for nurses, healthcare providers, and clinicians. This approach can also be used to provide continuous care in other interventions for MS patients. However, its usefulness is required to be assessed in further studies.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CCM:
-
Continuous care model
- COVID-19:
-
Coronavirus disease of 2019
- MS:
-
Multiple sclerosis
- RR:
-
Relapsing–remitting
- MS-TAQ:
-
Multiple sclerosis treatment adherence questionnaire
- MSSS:
-
Multiple sclerosis self-efficacy scale
- MD:
-
Mean difference
- DMT:
-
Disease-modifying therapy
References
Oost W, Talma N, Meilof JF, Laman JD. Targeting senescence to delay progression of multiple sclerosis. J Mol Med. 2018;96(11):1153–66.
Feigin VL, Abajobir A, Abate K, Abd-Allah F, Abdulle A, Abera S, GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–97.
Azami M, YektaKooshali MH, Shohani M, Khorshidi A, Mahmudi L. Epidemiology of multiple sclerosis in Iran: a systematic review and meta-analysis. PLoS ONE. 2019;14(4):e0214738.
Abtahi S-H, Manavi S-P, Fereidan-Esfahani M. Updated systematic review on epidemiology of multiple sclerosis in Iran: central accumulation and possible role for industrial pollution. J Rev Med Sci. 2021;1(1):16–24.
Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, Khalil IA, Krohn KJ, Liang X, Naghavi M, et al. Global, regional, and national burden of multiple sclerosis 2013–2016. A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269–85.
Lorefice L, Fenu G, Frau J, Coghe G, Marrosu MG, Cocco E. The impact of visible and invisible symptoms on employment status, work and social functioning in multiple sclerosis. Work. 2018;60(2):263–70.
Reynolds R, Dennis S, Hasan I, Slewa J, Chen W, Tian D, Bobba S, Zwar N. A systematic review of chronic disease management interventions in primary care. BMC Fam Pract. 2018;19(1):1–13.
Kapica-Topczewska K, Collin F, Tarasiuk J, Chorąży M, Czarnowska A, Kwaśniewski M, Brola W, Bartosik-Psujek H, Adamczyk-Sowa M, Kochanowicz J. Clinical and epidemiological characteristics of multiple sclerosis patients receiving disease-modifying treatment in Poland. Neurol Neurochir Pol. 2020;54(2):161–8.
Verdugo RM, Herráiz ER, Fernández-Del Olmo R, Bonet MR, García MV. Adherence to disease-modifying treatments in patients with multiple sclerosis in Spain. Patient Prefer Adher. 2019;13:261.
Gerber B, Cowling T, Chen G, Yeung M, Duquette P, Haddad P. The impact of treatment adherence on clinical and economic outcomes in multiple sclerosis: real world evidence from Alberta. Canada Mult Scler Relat Disord. 2017;18:218–24.
World Health Organization. Adherence to long-term therapies: evidence for action. 2003.
Erbay Ö, Yesilbalkan ÖU, Yüceyar N. Factors affecting the adherence to disease-modifying therapy in patients with multiple sclerosis. J Neurosci Nurs. 2018;50(5):291–7.
Young CA, Mills R, Rog D, Sharrack B, Majeed T, Constantinescu CS, Kalra S, Harrower T, Santander H, Courtald G. Quality of life in multiple sclerosis is dominated by fatigue, disability and self-efficacy. J Neurol Sci. 2021;426:117437.
Abraham C, Denford S. Design, implementation, and evaluation of behavior change interventions: a ten-task guide. In: The handbook of behavior change; 2020. p. 269–84.
Sikes EM, Cederberg KL, Baird JF, Sandroff BM, Motl RW. Self-efficacy and walking performance across the lifespan among adults with multiple sclerosis. Neurodegener Dis Manag. 2019;9(5):267–75.
Guicciardi M, Carta M, Pau M, Cocco E. The relationships between physical activity, self-efficacy, and quality of life in people with multiple sclerosis. Behav Sci (Basel). 2019;9(12):121.
Dumitrescu L, Papathanasiou A, Coclitu C, Constantinescu CS, Popescu BO, Tanasescu R. Beta interferons as immunotherapy in multiple sclerosis: a new outlook on a classic drug during the COVID-19 pandemic. QJM. 2021;114:691–7.
Cerqueira JJ, Compston DAS, Geraldes R, Rosa MM, Schmierer K, Thompson A, Tinelli M, Palace J. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2018;89(8):844–50.
Otaghi M, Bastami M, Borji M, Tayebi A, Azami M. The effect of continuous care model on the sleep quality of hemodialysis patients. Nephrourol Mon. 2016;8(3):67–78.
Bove R, Rowles W, Zhao C, Anderson A, Friedman S, Langdon D, Alexander A, Sacco S, Henry R, Gazzaley A. A novel in-home digital treatment to improve processing speed in people with multiple sclerosis: a pilot study. Mult Scler J. 2020;27(5):778–89.
Robb JF, Hyland MH, Goodman AD. Comparison of telemedicine versus in-person visits for persons with multiple sclerosis: a randomized crossover study of feasibility, cost, and satisfaction. Mult Scler Relat Disord. 2019;36:101258.
Ahluwalia SC, Friedman E, Siconolfi D, Saliba D, Phillips J, Shih R. Promises and pitfalls of health information technology for home-and community-based services. J Appl Gerontol. 2020;0733464820941364.
Sheikh A, Anderson M, Albala S, Casadei B, Franklin BD, Richards M, Taylor D, Tibble H, Mossialos E. Health information technology and digital innovation for national learning health and care systems. Lancet Digit Health. 2021;3:e383–96.
Kardan Barzoki E, Bakhshandeh H, Nikpajouh A, Elahi E, Haghjoo M. Comparison of the effect of education through lecture and multimedia methods on knowledge, attitude, and performance of cardiac care. Iran J Cardiovasc Nurs. 2016;4(4):6–13.
Malale K, Fu J, Nelson W, Gemuhay HM, Gan X, Mei Z. Potential benefits of multimedia-based home catheter management education in patients with peripherally inserted central catheters: systematic review. J Med Internet Res. 2020;22(12):e17899.
Xiao Q, Wang J, Chiang V, Choi T, Wang Y, Sun L, Wu Y. Effectiveness of mHealth interventions for asthma self-management: a systematic review and meta–analysis. Nurs Inform. 2018;2018:144–5.
Ahmadi F, Ghofranipour FA, Abedi HA, Arefi SH, Faghihzadeh S. The design of continous care model for the control of coronary artery disease. Modares J Med Sci (Pathobiol). 2002;4(2):97–104.
Khodaveisi M, Ashtarani F, Mohammadi N. Beikmoradi a, Mahjub H, Mazdeh M: The effect of continuous care on quality of life in multiple sclerosis patients. Avicenna J Nurs Midwifery Care. 2014;22(2):64–73.
Wicks P, Massagli M, Kulkarni A, Dastani H. Use of an online community to develop patient-reported outcome instruments: the Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ). J Med Internet Res. 2011;13(1):e12.
Pour Youssef Kaljahi M. The effectiveness of motivational interviewing on the treatment and quality of life of patients with MS. Tehran, Rashidin. 2013.
Rigby S, Domenech C, Thornton E, Tedman S, Young C. Development and validation of a self-efficacy measure for people with multiple sclerosis: the Multiple Sclerosis Self-efficacy Scale. Mult Scler J. 2003;9(1):73–81.
Reshvanlo AT, Soleimanian AA. Psychometric examination of Multiple Sclerosis self-efficacy scale. J Res Behav Sci. 2014;12(1):9–18.
Tayebi A, Rahimi A, Einollahi B, Mirsadeghi A, Hashemi S. The effect of continues care model on adherence to treatment in hemodialysis patients. J Crit Care Nurs. 2019;12(2):42–7.
Zakeri MA, Khoshnood Z, Dehghan M, Abazari F. The effect of the Continuous Care Model on treatment adherence in patients with myocardial infarction: a randomised controlled trial. J Res Nurs. 2020;25(1):54–65.
Khodaveisi M, Ashtarani F, Beikmoradi A, Mohammadi N, Mahjub H, Mazdeh M, Ashtarani E. The effect of continuous care on the lifestyle of patients with multiple sclerosis: a randomized clinical trial. Iran J Nurs Midwifery Res. 2017;22(3):225.
Golan D, Sagiv S, Glass-Marmor L, Miller A. Mobile phone-based e-diary for assessment and enhancement of medications adherence among patients with multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6(3):2055217320939309.
Xia L. The effects of continuous care model of information-based hospital-family integration on colostomy patients: a randomized controlled trial. J Cancer Educ. 2020;35(2):301–11.
Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945–58.
Shakery M, Mehrabi M, Khademian Z. The effect of a smartphone application on women’s performance and health beliefs about breast self-examination: a quasi-experimental study. BMC Med Inform Decis Mak. 2021;21(1):1–10.
Andrews JA, Craven MP, Lang A, Guo B, Morriss R, Hollis C. The impact of data from remote measurement technology on the clinical practice of healthcare professionals in depression, epilepsy and multiple sclerosis: survey. BMC Med Inform Decis Mak. 2021;21(1):1–17.
Buabbas AJ, Aldousari S, Ayed AK, Safar M, Alkandari O. Usefulness of smartphone use among surgeons in clinical practice during the pandemic of COVID-19: a cross-sectional study. BMC Med Inform Decis Mak. 2021;21(1):1–9.
Acknowledgements
This article was extracted from Seyed Mojtaba Kazemi’s MSc thesis in Medical-Surgical Nursing, which was approved and financially supported by the Vice-chancellor of Research and Technology of Shiraz University of Medical Sciences, Shiraz, Iran. Hereby, the authors would like to thank the staff of the MS Association as well as the participants for their cooperation. They would also like to appreciate Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for improving the use of English in the manuscript.
Funding
This study was financially supported by the Vice-chancellor for Research Affairs of Shiraz University of Medical Sciences, Shiraz, Iran (No. 21498). The VCR had no role in conducting the study and publication of the article.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception and design of the study. The data were collected by SMK. Data analysis and interpretation was done by MR, SMK, and MaR., SMK and MR conducted the intervention and participated in drafting the manuscript. All authors revised the manuscript critically for important intellectual content and approved the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Helsinki Declaration and was approved by the Research Ethics Committee of Shiraz University of Medical Sciences (Approval No. IR.SUMS.REC.1399.201). All the patients were fully informed about the aim of the research, anonymity, and confidentiality of their personal information and their written informed consent forms were obtained. Furthermore, the control group participants were provided with the designed programs and trainings at the end of the research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The Persian “MS App” consisted of various sections including main menu, MS introduction, beyond MS, entertainment section, and night episode.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kazemi, S.M., Rakhshan, M., Rivaz, M. et al. The effects of continuous care model using a smartphone application on adherence to treatment and self-efficacy among patients with multiple sclerosis. BMC Med Inform Decis Mak 22, 53 (2022). https://doi.org/10.1186/s12911-022-01785-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-022-01785-x