Abstract
Background
To improve consistency and streamline development and publication of clinical guidelines (GL), there is a need for appropriate software support. We have found few specific tools for the actual authoring and maintaining of GLs, and correspondingly few analyses or reviews of GL development tool functionality. In order to assist GL developers in selecting and evaluating tools, this study tries to address the perceived gap by pursuing four goals: 1) identifying available tools, 2) reviewing a representative group of tools and their supported functionalities, 3) uncovering themes of features that the studied tools support, and 4) compare the selected tools with respect to the themes.
Methods
We conducted a literature search using PubMed and Google Scholar in order to find GL development tools (GDT). We also explored tools and Content Management Systems (CMS) used in representative organisations and international communities that develop and maintain GLs. By reading a selected representative group of five GL tool manuals, exploring tools hands-on, we uncovered 8 themes of features. All found tools were compared according to these themes in order to identify the level of functionality they offer to support the GL development and publishing process. In order to limit the scope, tools for designing computer-interpretable/executable GL are excluded.
Results
After finding 1552 published papers, contacting 7 organizations and international communities, we identified a total of 19 unique tools, of which 5 tools were selected as representative in this paper. We uncovered a total of 8 themes of features according to the identified functionalities that each tool provides. Four features were common among tools: Collaborative authoring process support, user access control, GL repository management, electronic publishing. We found that the GRADE methodology was supported by three of the reviewed tools, while only two tools support annotating GL with MeSH terms. We also identified that monitoring progress, reference management, Managing versions (version control), and Change control (tracking) were often the missing features.
Conclusion
The results can promote sector discussion and eventual agreement on important tool functionality. It may aid tool and GL developers towards more efficient, and effective, GL authoring.
Similar content being viewed by others
Background
Clinical practice guidelines (GL) are developed to provide evidence-based advice on diagnosis and treatment to clinicians at the point of care [1]. During the last two decades, major advances have been made in developing, disseminating and implementing GLs to improve healthcare outcomes [2]. Various guidelines on how to develop guidelines have been published, and standards are suggested for the development of trustworthy guidelines (guideline for guidelines) [3, 4]. The process of authoring GLs is complex, time consuming, and usually involves large, often geographically distributed, multidisciplinary teams. It is common to develop a guideline as an academic text, by means of a document editor, and most often the final version, with summary recommendations, is published in print and guideline web portals and services.
Organizations responsible for developing and maintaining GLs adopt Content Management Systems (CMS) in order to manage concurrent authoring, revision, review and publishing. Our previous case study on information systems support for maintaining national GLs in Norway showed that the currently used information systems or CMS are neither fulfilling the authors’ needs in terms of functionality, nor facilitating communication between authors developing guidelines collaboratively [5]. Different groups of researchers have proposed specialized software tools to ease the guideline development process while accommodating guideline authors’ needs. Collectively, we describe these tools as ‘guideline development tools’ (GDT) [6,7,8,9].
Developers of GDTs have their own assumptions and points of views, which results in software with different functionalities and features. In addition, the proposed GDTs have different foci in the guideline development process. To the best of our knowledge, there is no peer-reviewed paper analysing or comparing GDTs in detail. Therefore, characterising features supported by the GDTs, comparing them and presenting their area of focus were the main motivations behind this research. Consequently, this study has four main goals: 1) identifying available GDTs, 2) reviewing a representative group of GDTs and their supported functionalities, 3) uncovering themes of features that the studied GDTs support, and 4) compare the selected tools with respect to the themes.
We note that the scope of this study is limited to the GDTs for authoring GLs; therefore tools for guideline adaption and implementation are omitted. Furthermore, tools for authoring formalized (computer-interpretable) guidelines are also omitted, since they represent a distinct later step in GL development, overlapping with general software design and development tools that are well described in the research literature.
The rest of the article is organized as follows: The ‘Method’ section presents the search strategy to find GDTs and related CMSs, tool selection criteria, review method, and methods for uncovering themes. The ‘Results’ section presents the found GDTs, selected representative GDTs and themes of features. ‘Discussion’ tries to learn from the results, while ‘Conclusion’ acknowledges that tools must be tested in practice.
Methods
Searching for guideline development tools
We searched for peer-reviewed papers outlining relevant GDT or CMS in literature, contacted representative guideline development organisations and communities, and attended an international conference and a national meeting.
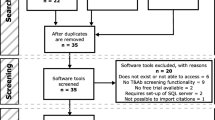
The primary literature search was performed using PubMed and Google Scholar in March 2015 and the last search was conducted in May 2016. The selection and review process was conducted according to PRISMA guidelines [10] and is presented in Fig. 1. Details of retrieved papers based on a combination of search keywords are presented in Table 1.
We reviewed the title and abstract of the retrieved papers. In case the title and abstract of the reviewed papers were not clear, we screened the full text. The two authors performed study selection in duplicate and discussed the inclusion or exclusion of papers until consensus was achieved.
In order to learn more about actual GDT in use, we contacted a sample of organizations and national/international communities with known GDT-related activity.
-
▪ Guideline International Network (G-I-N) [11].
-
▪ NICE (National Institute For Health and Clinical Excellence), United Kingdom
-
▪ Agency for Healthcare Research and Quality (AHRQ), USA
-
▪ National Health and Medical Research Council (NHMRC), Australia
-
▪ Norwegian Health Library (Helsebiblioteket)
-
▪ St. Olav’s University Hospital Trust (Norway)
-
▪ Innlandet Hospital Trust (Norway)
In addition, participation in two conferences was another source of identifying GDTs:
-
▪ DECIDE International Conference (http://www.cebhc.co.za/decide-conference), in Edinburgh, 2014, conference theme: evidence-based guideline development.
-
▪ The Norwegian Directorate of Health supplier conference, in Oslo, 2014, conference theme: procurement of potential GDT for authoring national clinical guidelines.
The selection criteria of GDTs and CMSs for analysis
We considered three main criteria:
-
1)
Some tools offer only partial support of the GL authoring process. In order to select a representative set of tools for comparison, we systematically ranked more general tools, covering more steps in the authoring process, higher than tools covering fewer steps. In the end, we omitted GDTs that were fully subsumed by more comprehensive tools.
-
2)
General-purpose CMS were not considered for review, only CMSs specifically supporting GL authoring.
-
3)
We excluded tools that only covered GL adaption, localization and/or implementation. GL or evidence profile repositories were excluded.
Method for analysing the GDTs and CMS
We explored the GDTs and CMSs that we found in literature and organisations hands-on. We also analysed the provided manuals for each tool in order to identify the functionalities and features they support. If we questioned certain functionalities, we contacted the developing team directly.
Method for uncovering themes
We uncovered themes of supported features and functionalities using the thematic synthesis method [12]. It allowed us to identify specific segments of text and recurring topics, label them, elicit high-level requirements and classify them into themes. Prose text descriptions were validated in seven brainstorming sessions between authors.
Results
The results according to the goals are: 1) identified GDTs and CMSs, 2) a selection of representative GDT, 3) a set of feature themes, and 4) a characterization of the selected tools according to the identified themes.
The identified GDTs and CMSs
The identified tools based on the searched sources are presented in the following sections.
Details of the literature search
Our literature search phrases and corresponding results were presented in Table 1. We generally found that publications describe the individual tools and their use, rather than their functionalities. In Table 1, MAGICapp is the subject of [13,14,15], GRADEPro is the subject of [16,17,18], and Internet Portal for guideline development (henceforth “Internet Portal”) is presented in [19, 20]. All the tools and methodologies reviewed by M. Peleg [21], except BRIDGE-Wiz [7], were aimed at computational representation, and require users to have programming knowledge or collaborate with knowledge engineers to encode guidelines; therefore, they are excluded from our study.
Details of search in organizations
The G-I-N have published a list of suggested tools on their website [22]. Again, we found MAGICapp, GRADEPro, and Internet Portal. The other suggested tools on the Website [22] are designed to support only part of the guideline development process. We categorise them below:
-
1)
Adapt and implement existing guidelines: CAN-IMPLEMENT© [23]
-
2)
Make and publish summary of finding tables (SoF): DECIDE [24]
-
3)
Perform systematic review: Doctor Evidence platforms [25], RevMan 5 [26], Distiller [27], Rayyan [28], JBI-SUMARI [29], SRDR [30], EPPI-Reviewer 4 [31], CREBP SRA Systematic Review Creator [32] and Covidence [33]
-
4)
Maintain systematic review repository: Epistemonikos [34], and SRDR [30]
-
5)
Do semi-automated citation screening for systematic reviews: Abstrackr [35]
-
6)
Maintain guideline repository: GIN guideline library [36]
-
7)
Detect duplicates in systematic reviews: CREBP Systematic Review Assistant [37]
-
8)
Maintain evidence profile repository: GDTs database of evidence profiles [38]
By contacting to the other organizations, we found that NICE and NHMRC have their own in-house CMS; AHRQ does not use any tools or CMS. We found that the Norwegian Health Library, the St. Olav’s University Hospital Trust and the Innlandet Hospital Trust all used general-purpose CMS. The Norwegian Health Library piloted MAGICapp, and Innlandet Hospital Trust piloted purpose-built “Håndboka”.
Details of search in national/international communities
The Decide conference and the supplier conference uncovered, again, MAGICapp [15] and GRADEpro [39]. The national supplier conference furthermore uncovered Håndboka.
Selected tools for review
According to the tool selection criteria presented in Section 2.2, the selected tools were: MAGICapp, GRADEpro, Internet Portal, BRIDGE-Wiz, and Håndboka. BRIDGE-Wiz [7] is designed to be used in the panel meeting to systematically create more transparent recommendations. BRIDGE-Wiz enhances implementation by letting authors state the strength of the recommendation, the level of obligation, and the balance between the benefits and harms [7]. Since the implemented features in BRIDGE-Wiz are different from other GDTs that fully support the GL development process, we included it in our analysis. The next section describes themes of features uncovered in these five GDTs.
The uncovered themes of features
Based on hands-on experience and the thematic analysis method, we identified a total of 8 themes representing GDTs features: 1) Team and contribution management, 2) Project management, 3) Evidence management, 4) Guideline development, 5) Document management, 6) Guideline content enhancement, 7) Import, export and publication, 8) User experience enhancement. A list of identified themes, their description and intension are presented in Table 2.
Representative tools characterization
We reviewed the five representative tools (MAGICapp, GRADEpro, BRIDGE-Wiz, Håndboka, and Internet Portal) based on the uncovered themes and present the results in in Table 3. In case the functionality/feature is not supported by the tool, we used ‘-’ sign. Details of the results are presented in the see Additional file 1.
As shown in Table 3, four out of the five tools had common functionalities or features: collaboration, access control, guideline repository management, electronic publishing. The GRADE methodology is supported in three of the reviewed tools for rating of guideline recommendations. We found only two tools supporting MeSH annotation. We also identified that monitoring progress, reference management, managing versions (version control) and Change control (tracking) had least coverage. Results show that the identified themes and their features not only cover required functionalities and features for a GDT to support the guideline development process, but also include functionalities and features to improve guideline dissemination, guideline search, and integration of guideline recommendations to EHR. We note that we contacted the creators of the tools to confirm the results and our judgment.
Discussion
In this section we provide an analysis of the results with suggestions for improvement.
-
1)
Conflict of Interest and user access
Although GRADEpro and Internet portal can manage conflict of interest (COI) and have role based user access, they lack fine-grained edit access for COIs on specific guideline sections. We recommend fine-grained access control and administrator roles on section/recommendation level with different restriction level.
-
2)
Workflow support
The ‘development checklist’ and ‘setting milestones and deadline’ in MAGICapp are not integrated with workflow management. Similarly, GRADEpro does not track task completion based on the milestone manager. We recommend that GDTs interface with workflow management systems that can support the entire GL development process for distributed teams. In order to control and track how a guideline under development moves from one team member to another based on their contribution and track the steps, proper workflow management system support is necessary. A workflow model to manage content from initial draft to the publishable form would be an improvement. Plans milestones, checklists, progress of voting, review/approval should be considered. Progress monitoring at individual, group and project level is critically important. In addition, a workflow model should provide details about development/update, content changes, versions, feedback, task assignment, and active reminders. A customizable workflow management system can be used to tailor development to local practice.
-
3)
Collaborative literature search and evidence analysis
Evidence assessment is a collaborative process and authors may need to highlight or annotate the reviewed article. Our results show that only Internet Portal can import articles to a repository accessible to team members for further analysis, data extraction with the aim of making a systematic review. We recommend that GDTs supports annotation, annotation comments and storage of search results for each individual article.
-
4)
Automatic tracking of changes and version control
Developing guidelines is a collaborative process and change tracking is intertwined with development process and conflict resolution. We recommend that edit history, changes, forking, roll-backs and supporting user/role/group on the same granularity as COI-management and voting. Furthermore, we recommend that versioning may be automatic and milestone- or interval-based so as to simplify use of long-term, continuous development processes.
-
5)
Customizable guideline template
We recommend that the GDT support use and creation of guideline templates. This may significantly lower effort for developing new or local versions of a GL, and it will help less experienced developer teams. A customizable template can be used to tailor development to local practice and potentially increase tool adoption
-
6)
Usability of guideline development tools
Usability is a core concern for any software tool. BRIDGE-Wiz has been evaluated [7], but we did find few specific results for GDT usability. That is not surprising, since software engineering considers usability part of practice, and not research. However, we recommend that user interface design, wizard-based and template-based authoring, interactive user guides, and workflow management support be thoroughly evaluated with respect to utility and usability.
-
7)
Electronic GL publication and usability
In our previous studies about usability evaluation of GLs on the Web [40,41,42,43] we found that presentation format, layout, navigation and search functionality were important [44]. GDTs affect publication format and functionality and thus guideline usability. For example, as MAGICapp and GRADEpro allow publishing on various platforms, which may be both a challenge and boon in practice. As another example, in our previous study we found that guideline users are concerned with credibility of recommendations; hence providing additional information with the graded recommendation would increase their confidence in choosing recommendations [43]. We recommend that GDTs provide or enable easy publication of references, author justification as well as evidence in a comprehensive, yet unobtrusive way.
-
8)
Guideline annotation
EHR integration is very important, but relies on computational formalization, and domain modelling, which begins where we stopped. However, simple GL annotation may enhance search and retrieval related to specific patients. MAGICapp describes EHR content in the GL, enabling the GL recommendations to be instantiated with current patient data.
The analysed GDTs in this paper are developed by a joint collaboration of multidisciplinary and international teams (MAGICapp (Norway, Canada, Spain, Germany, Scotland, Lebanon, and USA), GRADEPro (Canada, Chile, Brazil, USA, American University of Beirut, Spain, Italy, Germany, Norway, USA, UK, World Health Organization (Switzerland), Finland, and Denmark), BRIDGE-Wiz (USA), internet Portal (Germany), and Håndboka (Norway)), hence the presented results and findings will be applicable to other guideline development organisations in other countries.
Much research and development have gone into formalizing GLs to make them computer interpretable. The accompanying software development tools, or integrated development environments [45] support the encoding of guidelines to facilitate content-based search, integration of electronic health records with guidelines and most importantly, executable decision and process support. [46]. As already stated, we regard this as a later step of GL deployment and implementation similar to software development, and not relevant for the initial GL authoring. Future work should explore the gaps authoring guideline content and for making them computer-interpretable. This will ease the flow of evidence to the clinic. The electronic development of clinical guidelines can facilitate the formalization step if they can communicate in terms of language, syntax, and semantic provided by the tools; therefore it reduces the challenges of the guideline maintenance process and therefore formalization process.
Conclusion
In this study we searched literature and contacted guideline development organisations to identify tools and CMS for development of GLs. We reviewed five academically reported GDTs (MAGICapp, GRADEpro, BRIDGE-Wiz, and Internet Portal) and Håndboka, as a representative of GL-specific CMSs. The results in this paper are useful for organisations and authors assessing and selecting a GDT.
By reading tool manuals, exploring the tools hands-on, comparing them against other, we uncovered, and proposed 8 themes that represent tool functionalities and features. Tools were selected according to their support of the guideline development process. Finally, the tools were characterized according to the 8 themes. Our purpose was not an overall GDT ranking, since GDTs are made for different purposes (i.e. only developing recommendations or a full guideline) and methodologies (i.e. developing recommendations based on GRADE). The identified themes can however be used for comparing tools, and in the future reconcile common features, and components. As guideline development manuals and methods are becoming more aligned, even standardized, this will drive alignment of GDTs. Furthermore, guideline representation and language standards encourage vendors to develop commercial applications for guideline implementations and EHR integration.
The tools may have limitations, and features, that are only possible to uncover after realistic use. Guideline author and editor requirements are also best captured when actually using the GDTs. Further study is required to understand if, and how, standard terminologies and ontologies impact the guideline encoding process. So, in summary:
What was already known?
-
▪ Guideline authors mainly use text editors or CMS for authoring and publishing clinical guidelines.
-
▪ The need for software support of GL authoring, maintenance and dissemination.
What this study added to our knowledge:
-
▪ Identification of research prototypes and tools from literature, meetings, professional networks and health organizations.
-
▪ Functionalities and features of representative GDTs.
-
▪ Eight themes representing the GDT features.
-
▪ Similarities and differences with regard to features among reviewed GDTs
-
▪ The GDT application area and supported development methodology.
Abbreviations
- CMS:
-
Content management systems
- GDT:
-
Guideline development tool
- G-I-N:
-
Guidelines International Network
- GLs:
-
Clinical guidelines
- Internet Portal:
-
Internet Portal for guideline development
References
Jaspers MW, Smeulers M, Vermeulen H, Peute LW. Effects of clinical decision-support systems on practitioner performance and patient outcomes: a synthesis of high-quality systematic review findings. J Am Med Inform Assoc. 2011;18(3):327–34.
Merritt TA, Palmer D, Bergman DA, Shiono PH. Clinical practice guidelines in pediatric and newborn medicine: implications for their use in practice. Pediatrics. 1997;99(1):100–14.
Steinberg E, Greenfield S, Mancher M, Wolman DM, Graham R. Clinical practice guidelines we can trust.1st edition. US National Academies Press; 2011.
WHO. WHO handbook for guideline development: World Health Organization (2nd edition); 2014. http://apps.who.int/iris/bitstream/10665/145714/1/9789241548960_eng.pdf.
Khodambashi S, Nytrø Ø, editors. Tool Support for Maintaining Clinical Guidelines: A Case Study. ECIME2015-9th European Conference on IS Management and Evaluation: ECIME 2015; 2015: Academic Conferences and publishing limited.
Kristiansen A, Brandt L, Alonso-Coello P, Agoritsas T, Akl EA, Conboy T, et al. Development of a novel multilayered presentation format for clinical practice guidelines. Chest. 2015;147:754–63
Shiffman RN, Michel G, Rosenfeld RM, Davidson C. Building better guidelines with BRIDGE-wiz: development and evaluation of a software assistant to promote clarity, transparency, and implementability. J Am Med Inform Assoc. 2012;19(1):94–101.
Brozek J, Oxman A, Schünemann H. GRADEpro, version 3.2 for windows. 2012.
Guyatt G, Vandvik PO. Creating clinical practice guidelines: problems and solutions. Chest. 2013;144(2):365–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
G-I-N. Guidelines International Network 2016 [cited 2016]. Available from: http://www.g-i-n.net.
Cruzes DS, Dyba T, editors. Recommended steps for thematic synthesis in software engineering. Empirical Software Engineering and Measurement (ESEM), 2011 International Symposium on; 2011: IEEE.
Vandvik PO, Brandt L, Alonso-Coello P, Treweek S, Akl EA, Kristiansen A, et al. Creating clinical practice guidelines we can trust, use, and share: a new era is imminent. Chest. 2013;144(2):381–9.
Treweek S, Oxman AD, Alderson P, Bossuyt PM, Brandt L, Brożek J, et al. Developing and evaluating communication strategies to support informed decisions and practice based on evidence (DECIDE): protocol and preliminary results. Implement Sci. 2013;8:6.
Kristiansen A, Vandvik P, Alonso-Coello P, Rigau D, Brandt L, Guyatt G. 313WS electronic multilayered guideline format: a novel structure and presentation of trustworthy guidelines at the point of care. BMJ Qual Saf. 2013;22(Suppl 1):A9–A10.
Brozek J, Akl E, Falck-Ytter Y, Kunstman P, Meerpohl J, Mustafa R, et al. P307 guideline development tool (GDT)–web-based solution for guideline developers and authors of systematic reviews. BMJ Qual Saf. 2013;22(Suppl 1):82.
Brozek J, Akl E, Falck-Ytter Y, Kunstman P, Meerpohl J, Mustafa R, et al. 046 guideline development tool (GDT)–web-based solution for guideline developers and authors of systematic reviews. BMJ Qual Saf. 2013;22(Suppl 1):A26-A.
Schünemann H, Mustafa R, Brozek J, Carrasco-Labra A, Brignardello-Petersen R, Wiecioch W. Saudi Arabian: Saudi Arabian Manual for Healthcare Guideline Development. http://www.moh.gov.sa/depts/Proofs/Documents/SA%20Handbook%20_AB%2030-6-14%20v2.pdf.
Höhne W, Karge T, Siegmund B, Preiss J, Hoffmann J, Zeitz M, et al. An internet portal for the development of clinical practice guidelines. Appl Clin Inform. 2010;1(4):430.
Höhne W, Karge T, Siegmund B, Preiss J, Hoffmann J, Zeitz M, et al. An internet portal for the development of clinical practice guidelines. Appl Clin Inf. 2010;1:430–41. http://dx.doi.org/10.4338/ACI-2010-04-RA-0027. For personal or educational use only. aci-journal org. 2015:12-01
Peleg M. Computer-interpretable clinical guidelines: a methodological review. J Biomed Inform. 2013;46(4):744–63.
G-I-N. Guideline process - useful tools 2015 [cited 2016 May]. List of useful tools for the guideline process, their features and cross-compatibilities]. Available from: https://docs.google.com/spreadsheets/d/1XE-zbU-FqK08nFyfhvB17tXeq_r2j5BRAeZZ4Xa5Axg/edit#gid=0.
NursingCenter.com. CAN-IMPLEMENT©: Clinical practice guidelines tailored for local use [cited 2016 May]. Available from: http://www.nursingcenter.com/evidencebasedpracticenetwork/canimplement.aspx?id=1917711.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Evidence D. Doctor Evidence platforms [cited 2016 May]. Available from: http://drevidence.com/.
Cochrane. Review Manager (RevMan) [cited 2016 May]. Cochrane’s Informatics & Knowledge Management Department (IKMD)]. Available from: http://tech.cochrane.org/revman.
Evidence-Partners. Distiller software [cited 2016 May]. Available from: https://distillercer.com/.
Elmagarmid A FZ, Hammady H, Ilyas I, Khabsa M, Ouzzani M. Rayyan: a systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews. 2014 21–26 Sep.
Institute JB. Joanna Briggs Institute system for the unified management, assessment and review of information (JBI SUMARI). 2014.
AHRQ. SRDR: Systematic Review Data Repository: Agency for Healthcare Research and Quality (AHRQ) [cited 2016 May]. Available from: http://srdr.ahrq.gov/.
EPPI-Centre. EPPI-Reviewer 4 2010. Available from: http://eppi.ioe.ac.uk/CMS/Default.aspx?alias=eppi.ioe.ac.uk/cms/er4&.
CREBP-SRA. CREBP Systematic Review Creator. Available from: http://crebp-sra.com//. Accessed 9 May 2017.
Babineau J. Product review: covidence (systematic review software). J Can Health Libr Assoc. 2014;35(2):68–71.
Rada G, Pérez D, Capurro D, editors. Epistemonikos: a free, relational, collaborative, multilingual database of health evidence. Medinfo IMIA and IOS Press; 2013.
Rathbone J, Hoffmann T, Glasziou P. Faster title and abstract screening? Evaluating Abstrackr, a semi-automated online screening program for systematic reviewers. Syst Rev. 2015;4(1):1.
G-I-N. GIN guideline library- Guideline International Network [cited 2016 May]. Available from: http://www.g-i-n.net/library/international-guidelines-library.
Rathbone J, Carter M, Hoffmann T, Glasziou P. Better duplicate detection for systematic reviewers: evaluation of systematic review assistant-Deduplication module. Syst Rev. 2015;4(1):1–6. 10.1186/2046-4053-4-6.
Evidence-Prime. GDTs database of evidence profiles [cited 2016 May]. Available from: http://dbep.gradepro.org/search.
Brozek J, Oxman A, Schünemann H. GRADEpro.[computer program]. Version 32 for windows. 2008.
Khodambashi S, Gilstad H, Nytrø Ø. Usability evaluation of clinical guidelines on the web using eye-tracker. Stud Health Technol Inform. 2016;228:95.
Khodambashi S, Nytrø Ø, editors. Usability Evaluation of Published Clinical guidelines on the Web: A Case Study. 2016 IEEE 29th International Symposium on Computer-Based Medical Systems (CBMS); 2016: IEEE Conference Publicationsm. doi:10.1109/CBMS.2016.11.
Khodambashi S, Wang Z, Nytrø Ø. Reality versus user's perception in finding answer to clinical questions in published National Guidelines on the web: an empirical study. Procedia Comput Sci. 2015;63:268–75.
Khodambashi S, Perry A, Nytrø Ø. Comparing user experiences on the search-based and content-based recommendation ranking on stroke clinical guidelines-a case study. Procedia Comput Sci. 2015;63:260–7.
Khodambashi S, Gilstad H, Nytrø Ø. Usability evaluation of clinical guidelines on the web using eye-tracker. Medical informatics Europe (MIE 2016); 2016: Accepted, under publishing process.
Peleg M, Tu S, Bury J, Ciccarese P, Fox J, Greenes RA, et al. Comparing computer-interpretable guideline models: a case-study approach. J Am Med Inform Assoc. 2003;10(1):52–68.
De Clercq PA, Blom JA, Korsten HH, Hasman A. Approaches for creating computer-interpretable guidelines that facilitate decision support. Artif Intell Med. 2004;31(1):1–27.
World-Health-Organization. WHO Handbook for Guideline Development Process - World Health Organization 2010 [cited 2015 10.03.2015]. Available from: http://www.who.int/hiv/topics/mtct/grc_handbook_mar2010_1.pdf.
Editors ICoMJ. Conflict of Interest Disclosure Forms 2015 [cited 2015 22 July 2015]. Available from: http://www.icmje.org/about-icmje/faqs/conflict-of-interest-disclosure-forms/.
Qaseem A, Forland F, Macbeth F, OllenschlÃĪger G, Phillips S, van der Wees P. Guidelines international network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525–31.
Terrace L. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.
Guideline Development Checklist 2014 [cited 2015 22 July 2015]. Available from: http://cebgrade.mcmaster.ca/guidelinechecklistonline.html.
WHO. WHO Handbook for Guideline Development: World Health Organization; 2012 2012.
SIGN. Scottish Intercollegiate Guidelines Network Methodology checklist. SIGN. 2014. Available from: http://www.sign.ac.uk/checklists-and-notes.html.
Oxford. Oxford Centre for Evidence-Based Medicine. Levels of evidence [cited 2016 May]. Available from: http://www.sign.ac.uk/checklists-and-notes.html.
Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med. 2013;6(1):50–4.
West SL, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, et al. Systems to rate the strength of scientific evidence: Agency for Healthcare Research and Quality, US Department of Health and Human Services; AHRQ Publication No. 02-E016 April 2002.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174–81.
Yale University. Available Versions of BridgeWiz 2011 [cited 2016 May]. Available from: http://gem.med.yale.edu/BRIDGE-Wiz/.
Shiffman RN, Michel G, Krauthammer M, Fuchs NE, Kaljurand K, Kuhn T. Writing clinical practice guidelines in controlled natural language. Controlled Natural Language: Springer; 2010. p. 265-280.
Organization WH. International statistical classification of diseases and health related problems (The) ICD-10: World Health Organization; 2004.
Donnelly K. SNOMED-CT. the advanced terminology and coding system for eHealth. Stud Health Technol Inform. 2006;121:279. https://books.google.no/books?hl=en&lr=lang_en&id=Tw5eAtsatiUC&oi=fnd&pg=PA1&dq=International+statistical+classification+of+diseases+and+health+related+problems+(The)+ICD-10&ots=o37_mqFhJ&sig=SNAl2HuzMXLSWNFdzvcOqgoKyts&redir_esc=y#v=onepage&q=International%20statistical%20classification%20of%20diseases%20and10&f=false.
Miller G, Britt H. A new drug classification for computer systems: the ATC extension code. Int J Biomed Comput. 1995;40(2):121–4.
Liu S, Ma W, Moore R, Ganesan V, Nelson S. RxNorm: prescription for electronic drug information exchange. IT Prof. 2005;7(5):17–23.
Nelson SJ, Johnston WD, Humphreys BL. Relationships in medical subject headings (MeSH). Relationships in the Organization of Knowledge: Springer; 2001. p. 171-184.
Lamberts H, Okkes I. ICPC-2, international classification of primary care. 1998. https://link.springer.com/chapter/10.1007/978-94-015-9696-1_11.
McDonald CJ, Huff SM, Suico JG, Hill G, Leavelle D, Aller R, et al. LOINC, a universal standard for identifying laboratory observations: a 5-year update. Clin Chem. 2003;49(4):624–33.
Lindberg DA, Humphreys BL, McCray AT. The unified medical language system. Methods Inf Med. 1993;32(4):281–91.
About Epistemonikos [cited 2015 27 July 2015]. Available from: http://www.epistemonikos.org/en/about_us/who_we_are.
Beale T, Heard S, Kalra D, Lloyd D. OpenEHR architecture overview. The OpenEHR Foundation. 2006.
Acknowledgements
We are grateful for the support from project partners in the EviCare project: The Norwegian Knowledge Centre for the Health Services, DIPS ASA, Innlandet Hospital Trust and Oslo University Hospital.
Funding
We are grateful for the funding for the EviCare project (https://www.cristin.no/app/projects/show.jsf?id=337417), from Norwegian Research Council grant no 193022.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
SK and ØN developed the analysis design, SK performed the literature search and analysed the data under supervision of ØN. SK performed the analysis and wrote the manuscript. ØN supervised the analysis and co-wrote the manuscript. Both authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Details of the tools analysis. The additional file elaborated in details, when necessary, on the analysis of the reviewed tools (MAGICapp, GRADEpro, BRIDGE-Wiz, Håndboka, and Internet Portal) according to the presented results in Table 3. Each bold title represents the theme according to the uncovered themes in Table 2. (PDF 187 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Khodambashi, S., Nytrø, Ø. Reviewing clinical guideline development tools: features and characteristics. BMC Med Inform Decis Mak 17, 132 (2017). https://doi.org/10.1186/s12911-017-0530-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-017-0530-5