Abstract
Background
Suanzaoren-Wuweizi herb-pair (SWHP), composed of Zizyphi Spinosi Semen (Suanzaoren in Chinese) and Schisandrae Chinensis Fructus (Wuweizi in Chinese), is a traditional herbal formula that has been extensively used for the treatment of insomnia. The study aimed to explore the targets and signal pathways of Suanzaoren-Wuweizi (S-W) in the treatment of anxiety by network pharmacology, and to verify the pharmacodynamics and key targets of SWHP in mice.
Methods
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) as well as literature mining were used to obtain the main chemical ingredients of Suanzaoren and Wuweizi. The SwissTargetPrediction platform was used to predict drug-related targets. The GeneCards, TTD, DisGeNET and OMIM databases were used to obtain potential targets for the treatment of anxiety with the chemical components of S-W. Drug-disease intersection genes were selected, and a protein-protein interaction (PPI) network was constructed using STRING. The core targets of S-W in the treatment of anxiety were selected according to the topological parameters, and GO functional enrichment as well as KEGG pathways enrichment analyses were performed for potential targets. The relationship network of the “drug-active ingredient-disease-target-pathway” was constructed through Cytoscape 3.8.0. The pharmacodynamics of SWHP in the treatment of anxiety was evaluated by the elevated plus maze (EPM), the light/dark box test (LDB) and the open field test (OFT). The mechanisms were examined by measuring monoamine neurotransmitters in brain of mice.
Results
The results showed that there were 13 active ingredients for the treatment of anxiety in the network. This includes sanjoinenine, swertisin, daucosterol, schizandrer B, wuweizisu C and gomisin-A. Additionally, there were 148 targets, such as AKT1, TNF, SLC6A4, SLC6A3, EGFR, ESR1, HSP90AA1, CCND1, and DRD2, mainly involved in neuroactive ligand-receptor interactions, the Serotonergic synapse pathway and the cAMP signaling pathway. After 1 week of treatment, SWHP (2 and 3 g/kg) induced a significant increase on the percentage of entries into and time spent on the open arms of the EPM. In the LDB test, SWHP exerted anxiolytic-like effect at 2 g/kg. In the open-field test, SWHP (2 g/kg) increased the number of central entries and time spent in central areas. The levels of brain monoamines (5-HT and DA) and their metabolites (5-HIAA, DOPAC) were decreased after SWHP treatment.
Conclusions
The anti-anxiety effect of SWHP may be mediated by regulating 5-HT, DA and other signaling pathways. These findings demonstrated that SWHP produced an anxiolytic-like effect and the mechanism of action involves the serotonergic and dopaminergic systems, although underlying mechanism remains to be further elucidated.
Similar content being viewed by others
Background
Anxiety, an aversive emotional state and commonly occurring mental disorder of the central nervous system, is contributing to an ever-increasing health burden worldwide. It’s comprised of a powerful emotional component associated with fearful thoughts and a physiological response. Kessler et al. [1] have reported that anxiety disorders are the most frequently diagnosed mental disorders in the United States with lifetime prevalence in approximately 30% of the population. Benzodiazepines are commonly used in the psychotherapeutic treatment of depression, anxiety and insomnia disorders, and possess sedative, hypnotic, and anti-anxiety properties [2,3,4]. Long-term use of benzodiazepines, however, is associated with complications such as withdrawal symptoms, therapeutic dose dependence, relapse anxiety, falls and fractures, as well as impairment in long-term cognitive functioning (which can remain for several months after benzodiazepines have been withdrawn) [3,4,5,6,7]. Therefore, the development of other anxiolytic drugs without such adverse effects is important for the treatment of anxiety disorders.
Chinese therapeutic herbs are traditionally used in combination (combined according to their properties so as to extend their abilities) and always demonstrate better pharmacological effects combined than when used alone [8]. Herb-pairs (Yaodui in Chinese), which are unique combinations of two traditional Chinese medicine (TCM) herbs [9], are frequently used as basic composition units in Chinese herbal formulas. They aim to achieve mutual reinforcement, assistance, restraint, and detoxication [10], and are much simpler to make than other complicated formulations [11].
SWHP is a combination of Zizyphi Spinosae Semen (Rhamnaceae, the dried seeds of Ziziphus jujube Mill. var. spinosa (Bunge) Hu ex H.F.Chou, officially recognized in the Chinese Pharmacopoeia) and Schisandrae Chinensis Fructus (Magnoliaceae, the mature fruits of Schisandra chinensis (Turcz.) Baill., officially recognized in the Chinese Pharmacopoeia). Ziziphi Spinosae Semen and Schisandrae Chinensis Fructus are documented in the Divine Husbandman’s Classic of the Materia Medica (the earliest dictionary of Chinese Materia Medica). The former is widely applied in China, Japan, Korea and other oriental countries to treat insomnia and anxiety symptoms [12,13,14,15,16], while the lignan component of the latter also has significant anti-anxiety effects in its own right [17].
SWHP was originally recorded in Puji Fang (Prescriptions for Universal Relief) in the Ming Dynasty, a famous ancient medical manuscript, and was used to treat insomnia and to calm nerves. In TCM, SWHP is used to treat mental diseases in more than 262 prescriptions according to the Dictionary of Chinese Medicine Prescription (including all of the prescriptions of China from the Qin and Han dynasties to the modern times), such as Anshen Pill, Yangxin Anshen Pill and Suanzaoren Powder. They are also applied in Zaoren Anshen Capsules and Jiannao Capsules, to treat the patients suffering from insomnia, forgetfulness, upset, dizziness and neurasthenia [18]. Moreover, it was reported that the combination Suanzaoren with Wuweizi acts synergistically and is beneficial for the treatment of insomnia in rats, where the mechanisms were related to regulating the neurotransmitter of monoamine [19,20,21]. Our previous study showed that SWHP had an antianxiety effect in RS rat models, and that the treatment may have targeted the mechanism of the ECS-BDNF-ERK signaling pathway [22]. However, the target and signal pathway of SWHP were not predicted systematically and verified. Hence this study was conducted to fill this gap in knowledge and understanding.
Network pharmacology is a new discipline which reveals the regulatory network of drugs in the body from the system level. The mechanism of drug action can be predicted by constructing the complex network relationship among “drugs, active components, targets and diseases”, particularly useful for the mechanism prediction of multi-target drugs developed from Traditional Chinese medicine [23,24,25].
The monoaminergic system [serotonin (5-HT) and dopamine (DA)] in the brain has been postulated to play an important role in the pathophysiology of anxiety disorders [26,27,28]. Several preclinical and clinical reports provide evidence to support that a dysfunction of the monoaminergic system may be implicated in the pathophysiology of anxiety disorders [26,27,28,29,30]. Zhang et al. [26] have shown that the modulation of the monoaminergic system forms the basis for the action of anxiolytic drugs, and this hypothesis provides a framework in which the pathophysiology and pharmacotherapy for anxiety may congregate on the modulation of monoaminergic system.
The aim of this study is to explore the targets and signal pathways of Suanzaoren-Wuweizi in the treatment of anxiety by utilizing network pharmacology. As well, to verify the anxiolytic-like effect of SWHP by using the elevated plus maze (EPM), the light/dark box test (LDB) and the open field test (OFT). In order to explore the potential mechanisms on monoaminergic system, also examined was the level of monoamines serotonin (5-HT), dopamine (DA) and their metabolites 5-HIAA and DOPAC in brain of mice. The study of these issues not only offers better guidance for the clinical application of SWHP, but also provides foundations for new drug discovery. Figure 1 shows the flow chart of this whole analysis.
Material and methods
Data preparation
Therapeutic target database searching
The known therapeutic targets of anxiety drugs were obtained from the Genecards (https://www.genecards.org/) [31], Therapeutic Target Database (TTD) (https://db.idrblab.org/ttd/) [32] and OMIM (https://www.omim.org/) [33], with “anxiety” as the search term. By integrating the acquired target protein information and eliminating the repeated targets in the search results, the known targets responsible for the pathogenesis of anxiety were obtained. In the UniProt database (https://www.uniprot.org/) [34], the species was adjusted to “Homo sapiens”, while drug targets, protein names, and gene names, were uniformly standardized.
Prediction of the component targets for SWHP
The traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP; http://lsp.nwsuaf.edu.cn/tcmsp.php) [35] and Swiss Target Prediction database (http://www.swisstargetprediction.ch/) [36] were used to predict the candidate targets of Suanzaoren and Wuweizi, with the species set as “Homo sapiens.” Meanwhile, UniProt database (https://www.uniprot.org) was used to search for protein target IDs and gene IDs, to organize the target information of Suanzaoren and Wuweizi targets, and remove duplicates.
Protein-protein interaction (PPI) network analysis
STRING platform (https://string-db.org/) [37] was used to construct an interaction network among the target proteins. The protein type was set as “Homo sapiens”, the minimum interaction threshold was adjusted to “Medium confidence”, and other parameters were kept at default values. Cytoscape 3.8.0 software (http://www.cytoscape.org) [38] was used to visualize the PPI network between anxiety-related genes and suanzaoren-wuweizi-target encoding genes.
Bioinformatics analysis of SWHP-anxiety targets
To assess the biological significance of specific genes or proteins for Suanzaoren-Wuweizi-anxiety targets, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) [39,40,41] pathway enrichment analyses were performed using DAVID (https://david.ncifcrf.gov/) database. Construct the visualization network diagram of “drug-active ingredient-disease-target-pathway”.
Plant materials
The seeds of Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H.F. Chou were purchased from Sichuan Natural Pharmaceutical co., Ltd. (Sichuan, China). The fruits of Schisandra chinensis (Turcz.) Baill. were purchased from Liaoning Ludan Ltd. (Liaoning, China), and were identified by professor Shi Jin-Li, a botanist at the Beijing University of Chinese Medicine, China. The voucher specimens (No.20160122 of Semen Ziziphi spinosae and No.20150601 of Fructus Schisandrae) were maintained at the Institute of Traditional Chinese Medicine, Beijing University of Chinese Medicine, China. All the materials were dried in the drying room with active ventilation at room temperature (about 22–25 °C) until they achieved constant weight. The plant name was checked against www.theplantlist.org. And the study protocol complies with relevant Chinese institutional, national, and international guidelines and legislation.
Reagents and drugs
Diazepam (DZP) was obtained from Yimin Pharmaceutical Factory (Beijing, China, SFDA Approval No. H11020898). 5-HT, 5-hydroxy-3-indoleacetic acid (5-HIAA), dopamine, and 3,4-dihydroxyphenylacetic acid (DOPAC) were purchased from Sigma (St. Louis, MO, USA). Jujuboside A (PubChem CID: 51346169), Jujuboside B (PubChem CID: 24721031), Spinosin (PubChem CID: 155692), Schizandrin (PubChem CID: 23915), Deoxyschizandrin (PubChem CID: 43595) and Schisandrin B (PubChem CID: 158103) were purchased from Sichuan Vicky Biotechnology Co., Ltd. Formic acid, methanol and acetonitrile were purchased from Thermo Fisher Technology Co., Ltd. All the chemicals used in the study were of analytical grade.
Preparation of SWHP
SWHP (S:W, 2:1, w/w) were immersed in 80% ethanol (1:8, w/v) and boiled for 1 h. The SWHP was boiled in water (1:10, w/v) again for 1 h. The filtrates from each decoction were mixed and dried under reduced pressure at a temperature of < 60 °C to obtain the powder form of SWHP. The dried powder was then stored at 4 °C before use.
Alcohol-water extract analysis by UHPLC-LTQ Orbitrap MS [22]
We established the stable conditions of UHPLC-LTQ Orbitrap MS for the analysis of SWHP components in positive and negative ion mode, with good separation and comprehensive cracking information. This part is the chemical composition analysis of SWHP, which was completed and published by our research group. The results showed that a total of 30 compounds were identified including Zizyphusine, Angeloygomisin Q, Schisandrin A, B, C, Gomisin E, G, J, K1, K2, M1, M2, L2, Ceanothic acid, and Lignans (Fig.2, Table 1).
Animals
Male ICR mice (18–22 g) were purchased from the Chinese Academy of Military Medical Sciences and kept in cages (25 × 15 × 14 cm) at 22 ± 1 °C on a 12 h/12 h light/dark cycle (light on, 8:00 AM-8:00 PM). The mice were housed six per cage, and water and food were available ad libitum. All of the experiments were performed in a quiet room under dim red light between 8:00 AM and 12:00 PM. All efforts were made to minimize the number of animals used and their suffering. The experimental procedures were approved by the Animal Care and Use Committee of the Institute of Psychology of the Chinese Academy of Sciences (Protocol No. 20170327) and in accordance with the Guide for Care and Use of Laboratory Animals by National Institutes of Health (NIH Pub. No. 85–23, revised 1996).
Drug administration
The dry powdered extract of SWHP was dissolved in saline (0.05 g/mL). To evaluate the anxiolytic effect of SWHP, the mice were orally administered SWHP (1, 2, and 3 g/kg, the dosage was determined by previous studies [21]) 60 min prior to behavioral testing, or diazepam (2 mg/kg) 30 min before behavioral testing. Diazepam at a dose of 2 mg/kg was chosen as a positive control drug. The dose and administration route for diazepam were based on previous studies [42,43,44], which was sufficient to induce an anxiolytic effect. All of the drugs were prepared immediately before use and administered orally in a volume of 0.5 mL/25 g body weight for 7 days. All of the behavioral tests were performed on the 7th day of treatment (Fig. 3).
Behavioral test
Elevated plus maze (EPM)
Anxiolytic activity was measured using the EPM [45]. The maze was composed of two opposite open arms (30 cm × 5 cm × 0.2 cm) and two opposite closed arms (30 cm × 5 cm × 15 cm) in a cross configuration [46]. The arms extended from a central platform (5 cm × 5 cm), and the maze was elevated 45 cm above the floor. The entire maze was made of clear Plexiglas. Four 25-W red fluorescent lights were arranged as a cross 100 cm above the maze and provided 200 lx illumination. A video camera was suspended above the maze to record the movements of the mice [47]. The mice (n = 12 per group) [47,48,49,50] were randomly assigned to five experimental groups: vehicle control, 2 mg/kg diazepam, 1 g/kg SWHP, 2 g/kg SWHP, and 3 g/kg SWHP. The mice were individually placed in the center of the maze facing an open arm, and the number of entries into and the time spent on the closed and open arms were recorded during a 5 min observation period. Arm entries were defined as the placement of all four paws into an arm. The percentage of open-arm entries ([open arm entries/total arm entries] × 100) and the percentage of time spent on the open arms ([time spent on the open arms / total time spent on open arms and closed arms] × 100) were calculated for each animal. If a mouse fell from the apparatus, then it was removed from the study. SWHP and vehicle groups were orally administered 60 min before testing, and the positive control animals were treated with diazepam 30 min before evaluation in the maze. After each trial, the apparatus was cleaned with 70% alcohol.
Light/dark box test (LDB)
The LDB test was performed immediately after the EPM test. When the EPM test was completed, the mouse was immediately placed in the light/dark box. The apparatus (45 cm × 21 cm × 21 cm) consisted of two compartments, with one-third painted white and two-thirds painted black. These compartments were separated by a divider with a 3.5 cm × 3.5 cm opening at floor level [51, 52]. The white compartment was illuminated by two 60 W (300 lx) bulbs placed 30 cm above the box. Each mouse was gently placed in the corner of the white compartment away from the dark compartment and monitored for 5 min. The number of transfers from one compartment to the other and time spent in the white compartment were recorded. All of the sessions were recorded by a camera that was linked to a monitor in an adjacent room to avoid distractions [47]. The apparatus was thoroughly cleaned with 70% alcohol after each trial.
Open field test (OFT)
The apparatus of the open field was composed of a square arena (60 cm × 60 cm), with a white floor that was divided into 36 squares (10 cm × 10 cm), enclosed by 25-cm-high walls made of black Plexiglas. The arena was illuminated by two 60 W red lamps placed over the center. The lamps were close to each other, placed 120 cm above the floor and provided 100 lx illumination in the testing room [47]. The 16 squares in the center represented an exposed field. The other 20 squares that were adjacent to the walls represented a protected field (safe areas). The test was initiated by placing a single mouse in the middle of the arena and allowing it to move freely for 5 minutes [53]. The number of central entries, the time spent in the central area and the total distances were recorded by an automatic video tracking system. The OFT was performed 60 min after the final SWHP treatment and 30 min after diazepam treatment. After each trial, the apparatus was wiped clean with 70% alcohol to remove any traces left behind by previous animals.
Determination of monoamines and metabolites
The mice were decapitated by cervical dislocation immediately after the OFT. The brains were dissected and immediately placed on ice. The tissue samples were weighed and stored at − 80 °C until homogenization. The brain tissue was manually homogenized with three volumes (w/v) of ice-cold 0.1 M perchloric acid (100 𝜇L/mg wet weight) that contained 0.1 mM ethylenediaminetetra-acetic acid (EDTA). After homogenization and centrifugation at 12,000×g at 4 °C for 10 min, 20 𝜇L of the tissue homogenate supernatant was injected directly into a high-performance liquid chromatography (HPLC) system that was equipped with an electrochemical detector (Waters ECD 2465, Milford, Massachusetts, USA). The mixed standard was used as a reference. The levels of monoamines (5-HT, DA, 5- HIAA, and DOPAC) in the samples were expressed as nanograms per gram of fresh weight of tissue [54]. The HPLC system used a reversed-phase C18 column (2.1 mm × 150 mm, 3 𝜇m, Waters Atlantis). The mobile phase consisted of 50 mM citric acid-sodium citrate (pH 3.5), 0.3 mM Na2-EDTA, 1.8 mM dibutylamine, and 4% methanol. The flow rate was 0.35 mL/min, and the detector potential was + 0.75 V.
Statistical analysis
All data obtained is presented as mean ± standard error of the mean (SEM). First, check whether the data conforms to the normal distribution. When the data accorded with normal distribution, the statistical analysis was performed using one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls post hoc test and GraphPad Prism 5.0 software. When the data did not conform to the distribution of positive and negative, a nonparametric test was used to analyze the data. In cases of significant variation, the individual values were compared using Dunnett’s test. Values of p < 0.05 were considered statistically significant.
Results
Identification of anxiety-related targets
From Genecards, TTD, DisGeNET and OMIM platforms, a total of 1425, 57, 1048 and 52 anxiety-related targets were obtained respectively. After merging the targets predicted by the four platforms and deleting duplicates, a total of 1318 anxiety-related targets were identified.
Identification of S-W targets
From TCSMP and Swiss Target Prediction databases, a total of 423 and 449 targets (respectively) of Suanzaoren and Wuweizi were retrieved. After merging the targets predicted by the two databases and removing duplicates, a total of 282 targets were identified.
PPI network of anti-anxiety targets for S-W
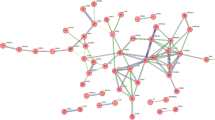
The targets of S-W and anxiety were screened, and 148 common targets were found between S-W and anxiety (Fig. 4). All interaction targets were imported into the STRING platform for PPI network analysis (Fig. 5). There were 148 nodes and 1068 edges in the network, and the average degree value was 14.4. A network analyzer tool was used for topology analysis, and genes with scores greater than average were selected as key targets by degree sorting. A total of 66 key targets were screened out. As can be seen from the figure, AKT Serine/Threonine Kinase 1 (AKT1), Tumor Necrosis Factor (TNF), Solute Carrier Family 6 Member 4 and 3 (SLC6A4, SLC6A3), epidermal growth factor receptor (EGFR), Estrogen receptor 1 (ESR1), Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1), Cyclin D1 (CCND1), Dopamine Receptor D2 (DRD2), and Mechanistic Target of Rapamycin Kinase (MTOR) may be the key targets of S-W in the treatment of anxiety disorders.
GO and KEGG pathway enrichment by S-W for potential anxiety targets
GO enrichment can be found in 1700 biological processes, 111 items related to cell composition and 162 items related to molecular function. The key targets were mainly involved in the biological process (BP) of the membrane potential regulation, G protein coupled receptor signaling pathway, blood circulation regulation, cellular component (CC) of presynaptic membrane, postsynaptic module, and has molecular function (MF) of processing neurotransmitter receptor activity, G protein-coupled receptor activity and ligand-gated ion channel activity (Fig. 6).
KEGG pathway analysis revealed that there were eleven pathways associated with the anti-anxiety effects of S-W. These include: the neuroactive ligand-receptor interaction pathway, Serotonergic synapse, Cholinergic synapse, cAMP signaling pathway, cGMP-PKG signaling pathway, Calcium signaling pathway, Estrogen signaling pathway, GnRH secretion, Prolactin signaling pathway, Endocrine resistance and EGFR tyrosine kinase inhibitor resistance (Fig. 7).
Construction of “drug-active component-disease-target-pathway” network
Cytoscape 3.8.0 software was used to construct the visualization network diagram of “drug-active ingredient-disease-target-pathway”, as shown in Fig. 8. The network contains 13 chemical components, 148 action targets and 20 important pathways. Each chemical component in S-W corresponds to multiple targets and pathways, which further proves that S-W can treat anxiety disorders through multi-component and multi-target synergistic action.
Effects of SWHP in the EPM, LDB and OFT
We examined the effects of SWHP in the EPM in comparison with the vehicle group. As shown in Fig. 9A and B (Supplementary Materials), there were significant differences among the five groups in both the percentage of open-arm entries [F (4,55) = 5.275, P < 0.01] and the percentage of time spent on the open arms [F (4,55) = 3.747, P < 0.01]. After one-week of treatment, SWHP (2 and 3 g/kg) led to a significant increase in the number of entries into the open arms from the vehicle. From 32.83 ± 1.93% to 42.42 ± 1.68% and 39.58 ± 1.45% respectively for 2 and 3 g/kg treatments (n = 12, P < 0.01, P < 0.05; Fig. 9A) and the time spent in the open arms from the vehicle from 35.00 ± 1.75% to 44.25 ± 2.63% and 42.92 ± 1.75% respectively for 2 and 3 g/kg treatments (n = 12, P < 0.01, P < 0.01; Fig. 9B). SWHP at the lower dose (1 g/kg) did not generate significant effect on the percentage of open-arm entries or the percentage of time spent on the open arms. Meanwhile, DZP induced a significant increase in the percentage of open-arm entries from 32.83 ± 1.93% to 44.83 ± 2.90% (n = 12, P < 0.01; Fig. 9A) and the percentage of time spent on the open arms to 44.75 ± 2.15% (n = 12, P < 0.01; Fig. 9B) when compared with the vehicle group.
Effect of SWHP on the open arm entries (A) and the percentage of time spent in open arms (B) in the elevated plus maze, effect on the number of transitions (C) and the time spent in light compartment (D) in the light/dark box test, and effect on the number of center entries (E), time spent in central areas (F) and the total distances (G) in the open field test in mice
Figure 9C and D showd the effects of SWHP on the number of transitions and time spent in the light compartment in the LDB test in mice. Marked increases were observed in both the number of transitions [F (4,55) = 4.475, P < 0.01] and time spent in light compartment [F (4,55) = 2.526, P < 0.05]. After one-week of treatment, SWHP at the dose of 2 g/kg significantly increased the number of transitions from the vehicle from 9.75 ± 0.72 to 13.33 ± 0.88 (n = 12, P < 0.01; Fig. 9C) and time spent in light compartment from the vehicle from 95.35 ± 5.10 to 114.30 ± 4.72 (n = 12, P < 0.05; Fig. 9D). SWHP at the dose of 3 g/kg significantly increase the time spent in light compartment to 111.54 ± 4.21 (n = 12, P < 0.05; Fig. 9D). DZP also induced effects of increasing the number of transitions (14.92 ± 1.14, n = 12, P < 0.01; Fig. 9C) and time spent in light compartment (116.43 ± 7.66, n = 12, P < 0.05; Fig. 9D) when compared with the vehicle group.
The results for the OFT are shown in Fig. 9E-G. There were significant differences in the number of center entries [F (4,55) = 4.308, P < 0.01] and the time spent in central areas [F (4,55) = 5.339, 𝑃 < 0.01], but not in the total distances [F (4,55) = 0.941, P = 0.447; Fig. 9G]. Compared with the vehicle group, SWHP (2 g/kg) and DZP significantly increased the number of central entries from 16.08 ± 1.06 to 24.83 ± 1.82 (n = 12, P < 0.01; Fig. 9E) and 21.17 ± 1.65 (n = 12, P < 0.05; Fig. 9E) respectively as well, the time spent in central areas increased from 18.06 ± 0.98 to 27.96 ± 2.34 (n = 12, P < 0.01; Fig. 9F) and 27.35 ± 2.34 (n = 12, P < 0.01; Fig. 9F) for SWHP (2 g/kg) and DZP respectively. In addition, SWHP (3 g/kg) also significantly increased the number of central entries from 16.08 ± 1.06 to 23.75 ± 2.33 (n = 12, P < 0.01; Fig. 9E).
Mice were administered vehicle, SWHP (1,2,3 g/kg) or DZP for 7 days. Values were presented as mean ± S.E.M. (n = 12). * P < 0.05 and ** P < 0.01 as compared with the vehicle group. One-way ANOVA with Student-Newman-Keuls post hoc test.
Effects of SWHP on monoamine neurotransmitters and their metabolites
Table 2 illustrates the effects of SWHP on monoamine neurotransmitters and their metabolites. There were significant differences on the content of 5-HT, DA [F (4,54) = 2.94, P < 0.05, F (4,54) = 2.27, P < 0.05] and their metabolites 5-HIAA and DOPAC [F (4,54) = 2.60, P < 0.05, F (4,54) = 2.51, P < 0.05]. Compared with the vehicle group, SWHP (2 and 3 g/kg) and DZP significantly decreased the content of 5-HT from 123.33 ± 10.12 to 92.06 ± 9.58, 97.18 ± 4.61 and 92.58 ± 9.04 (n = 11–12, P < 0.05) respectively. As well, decreased the content of 5-HIAA from 341.91 ± 15.54 to 275.51 ± 20.37, 282.94 ± 23.05 and 278.04 ± 22.95 (n = 11–12, P < 0.05), and decreased the content of DA from 520.91 ± 36.21 to 415.40 ± 33.04, 420.43 ± 18.25 and 409.67 ± 35.12 (n = 11–12, P < 0.05). SWHP (2 g/kg) and DZP also significantly decreased the content of DOPAC from 166.30 ± 14.46 to 128.08 ± 8.91 and 124.86 ± 8.56 (n = 11–12, P < 0.05) when compared with the vehicle group (Supplementary materials are available online).
Mice were administered vehicle, SWHP (1, 2, 3 g/kg) or DZP for 7 days. Values were presented as mean ± S.E.M. (n = 11–12). *P < 0.05 and **P < 0.01 as compared with the vehicle group. One-way ANOVA with Student-Newman-Keuls post hoc test.
Discussion
Over time medical scientists have come to the realization that the pathogenesis and progression of diseases are often so complicated, that the therapeutic effect of one single drug may be modest, or hampered by various side effects and resistances in clinic [55, 56]. Herb pairs, a centralized representative of Chinese herbal compatibility, are the most fundamental and the simplest form of multi-herb formulae and are of great importance in the studies of herb compatibility considering their simplicity yielding the basic characteristics of complex formulae.
During the study, network pharmacology was used to reveal the synergistic effect of multi-target, multi-component and multi-pathway herb-pair treatments at the molecular level. The components of SWHP were detected in the UHPLC-LTQ Orbitrap MS, including alkaloids (Zizyphusine), flavonoids (Ceanothic acid, Citric acid, Lancifodilactone C) in Ziziphi spinosae Semen, and lignans (Schisandrin A, B, C, Schisantherin B, C, Gomisin E, G, J, K1, K2, M1, M2, L2, Angeloygomisin Q) in Schisandrae chinensis Fructus, which is consistent with the types of chemical components predicted by network pharmacology of SWHP. This is also consistent with the literature, which report the components in Ziziphi spinosae Semen [57,58,59] and Schisandrae chinensis Fructus [60,61,62]. Through the prediction of drug targets and disease targets, 282 S-W targets and 1318 anxiety disorder targets were analyzed. The important targets of S-W in the treatment of anxiety disorder were identified. After PPI analysis, 66 key targets were screened out.
The target after the compatibility of S-W mainly focuses on AKT1, TNF, SLC6A4, SLC6A3, and DRD2. AKT/protein kinase B signaling pathway regulates cell growth and proliferation, and participates in cell processes, including apoptosis and glucose metabolism. Activation of AKT requires the activation of PI3K and PI3K-AKT pathway, which play an important role in improving anxiety and depression [63]. Related research shows that TNF- α is related to anxiety disorders, as it can promote the release of adrenocortical hormone through the hypothalamus pituitary adrenal axis system, thus causing neuroendocrine disorder and promoting the occurrence of anxiety and depression [64]. SLC6A4 gene can encode serotonin transporters, and SLC6A3 can encode dopamine transporters. In the stress response, the increased expression of the 5-HT2A receptor will enhance the anxiety and depression-like behavior of animals. When it is down regulated, the symptoms will be significantly reduced [65, 66]. Dopaminergic synapses are involved in the synthesis of dopamine (DA) in the brain. DA plays an important role in the generation and transmission of pleasant feelings and the storage of pleasant information. The activation of corresponding membrane receptors is the key to the role of DA. DA receptors have two subtypes, which can be divided into DRD1 and DRD2. When DRD2 is activated, the level of cAMP in cells decreases, and the expression of DRD2 increases significantly, which not only inhibits the production of cAMP but also affects the production of DA, thus leading to depression and anxiety [67].
Through the construction of network pathways, GO enrichment analysis, and KEGG pathway enrichment analysis, we found that the active components of S-W may play a role in the treatment of anxiety by participating in neuroactive ligand-receptor interaction, serotonin synapse and the cAMP signaling pathway. It is known from literature that the receptor biogenic imines contained in the neuroactive ligand-receptor interaction signal pathway is an essential stimulating nerve tissue molecule, which controls and regulates many important biological functions after binding to the corresponding receptors. For example, emotion, memory and the endocrine system. The disorder of this pathway or the down-regulation of the receptor will subsequently cause anxiety [68, 69]. The serotonin synaptic pathway was should also be considered; serotonin is a well-known molecule that produces a pleasant mood, and can participate in regulating mood, energy and memory. Serotonin is also related to the occurrence of anxiety disorders [70, 71]. The cAMP signaling pathway is involved in producing an anti-anxiety effect through the regulation of cAMP, which reduces the level of intracellular cAMP, resulting in a specific anxiolytic-like effect [72, 73].
In the present study we provided convincing evidence that SWHP extract administered by oral route produces a specific anxiolytic-like effect in EPM, LDB and OFT after one-week treatment in mice and the effect is involved with the monoaminergic system.
In the present study we provide convincing evidence that SWHP extract administered by oral route produces a specific anxiolytic-like effect in the EPM, LDB and OFT after one-week of treatment in mice, and the effect is involved with the monoaminergic system. The EPM is considered to be an etiologically valid animal model of anxiety because it uses natural stimuli (fear of a novel open space and fear of balancing on a relatively narrow, raised platform) that can induce anxiety in humans [74,75,76]. The time spent on the open arms and the number of entries into the open arms is used to assess a state of fear or anxiety [74, 77]. An anxiolytic agent increases the frequency of entries into the open arms and increases the time spent in open arms of the EPM. In the present study, diazepam (2 mg/kg) significantly increased time spent and number of entries into the open arms of the EPM. Moreover, the anxiolytic effect of SWHP (2 and 3 g/kg) was similar to that of DZP.
The light/dark test is based on the innate aversion rodents have to brightly illuminated areas and on the spontaneous exploratory behavior of rodents in response to mild stressors, that is, novel environment and light [78, 79]. Previous studies reported that this test is sensitive to benzodiazepines. Benzodiazepines may increase the number of visits and/or the time spent in the brightly lit area [80]. Our findings clearly suggested that DZP (2 mg/kg) and SWHP (2 g/kg) significantly increased the number of transitions between the compartments and the time spent in the light compartment of the LDB. SWHP at the dose of 3 g/kg also significantly increased the time spent in the light compartment. These results indicate that SWHP has anxiolytic activity.
The OFT is used in studies of the neurobiological basis of anxiety and screening for anxiolytic compounds [81]. The time spent in the central area and the number of central entries served as indices of anxiety and the distance was considered the index of locomotor activity [82, 83]. Activity in the central part of the open field is thought to be correlated with a degree of fear, while the behavior in the peripheral zone and along the walls of the field is thought to reflect general activity [83, 84]. All the data discussed up to here strongly suggests that the mice treated with SWHP (2 g/kg) and DZP (2 mg/kg) significantly increased the number of central entries and time spent in central areas. And the number of central entries was also significantly increased by SWHP at the dose of 3 g/kg. Therefore, SWHP showed a significant anxiolytic-like effect in this paradigm.
As a brain mechanism mediating effects of environmental factors, we focused on serotonin (5-HT) and DA systems in the present study. The current research on the etiology and pathogenesis of anxiety disorders has demonstrated that 5-HT is one of the key neurotransmitters modulating anxiety [85]. 5-HT and its metabolite (5-HIAA), as measured by HPLC, revealed a significant decrease in the SWHP (2 and 3 g/kg) and DZP (2 mg/kg) treated mice. The central dopaminergic system is considered a crucial factor in anxiety disorders. Foot-shock and anxiogenic drugs markedly increase cortical dopamine output in normal rats, and chronic treatment with imipramine completely inhibits these changes [86]. The present results are consistent with these reports. SWHP and DZP significantly reduced the tissue concentration of DA and DOPAC, the major metabolites of DA, in brain homogenates. Hence, these results further support the hypothesis that the anxiolytic effect of SWHP is mainly mediated via the monoaminergic system, including 5-HTergic and DAergic systems.
Notably, the neurotransmitter system predicted in network pharmacology not only included the 5-HT system and DA system, but also included the GABA system and cAMP signal pathway as predicted key pathways. Therefore, it is necessary to also determine GABA, which was reported to be one of the mechanisms of DZP, and the key proteins in the cAMP signal pathway, such as PKA, ERK, and RAP1a in the later stage. This is required in order to more comprehensively explain the anti-anxiety mechanism of SWHP.
Conclusions
In summary, the mechanism of SWHP for the treatment of anxiety was analyzed, and the reliability of the network pharmacology prediction was verified by animal experiments. Our findings open new perspectives for understanding SWHP for anxiety, and indicates that SWHP exerts an anxiolytic-like effect in the EPM, LDB and OFT, without affecting locomotor activity, in mice. In addition, this work provides evidence that the anxiolytic effect of SWHP appears to be mediated through the monoamine neurotransmitter levels. The findings of this study provide reference and a scientific basis for further study into the anti-anxiety effect of SWHP, as it provides an alternative safe and effective treatment with less side effects.
Availability of data and materials
All data and materials are described within the article. The corresponding author will provide if requested.
Abbreviations
- 5-HT:
-
5-hydroxytryptamine
- 5-HIAA:
-
5-hydroxy-3-indoleacetic acid
- AKT1:
-
AKT Serine/Threonine Kinase 1
- ANOVA:
-
Analysis of variance
- CCND1:
-
Cyclin D1
- DA:
-
Dopamine
- DOPAC:
-
3,4-dihydroxyphenylacetic acid
- DRD2:
-
Dopamine Receptor D2
- DZP:
-
Diazepam
- EDTA:
-
Ethylenediaminetetra-acetic acid
- EGFR:
-
Epidermal growth factor receptor
- EPM:
-
Elevated plus maze
- ERK:
-
Extracellular regulated protein kinases
- ESR1:
-
Estrogen receptor 1
- GABA:
-
γ-aminobutyric acid
- GO:
-
Gene ontology
- HSP90AA1:
-
Heat Shock Protein 90 Alpha Family Class A Member 1
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LDB:
-
Light/dark box
- MTOR:
-
Mechanistic Target of Rapamycin Kinase
- OFT:
-
Open field test
- PKA:
-
Protein Kinase A
- PPI:
-
Protein-protein interaction
- RAP1a:
-
Ras-associated protein 1a
- SEM:
-
Standard error of the mean
- SLC6A3:
-
Solute Carrier Family 6 Member 3
- SLC6A4:
-
Solute Carrier Family 6 Member 4
- SWHP:
-
Suanzaoren-Wuweizi herb-pair
- TCMSP:
-
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform
- TNF:
-
Tumor Necrosis Factor
References
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602.
Fang SY, Chen CY, Chang IS, Lin KM. Predictors of the incidence and discontinuation of long-term use of benzodiazepines: a population-based study. Drug Alcohol Depend. 2009;104(1–2):140–6.
Fassaert T, Dorn T, Spreeuwenberg PM, Dongen MC, Gool CAW, Yzermans CJ. Prescription of benzodiazepines in general practice in the context of a man-made disaster: a longitudinal study. Eur J Pub Health. 2007;17(6):612–7.
Rogers A, Pilgrim D, Brennan S, Sulaiman I, Watson G, Graham CC. Prescribing benzodiazepines in general practice: a new view of an old problem. Health. 2007;11(2):181–98.
Anthierens S, Habraken H, Petrovic M, Christiaens T. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25(4):214–9.
Cunningham CM, Hanley GE, Morgan S. Patterns in the use of benzodiazepines in British Columbia: examining the impact of increasing research and guideline cautions against long-term use. Health Policy. 2010;97(2–3):122–9.
Zandstra SM, Furer JW, Lisdonk EH, Van’t HM, Bor JH, Weel C, et al. Different study criteria affect the prevalence of benzodiazepine use. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):139–44.
Han CC, Guo JY. Antibacterial and anti-inflammatory activity of traditional Chinese herb pairs, Angelica sinensis and Sophora flavescens. Inflammation. 2012;35(3):913–9.
Wang S, Hu Y, Tan W, Wu X, Chen R, Cao J, et al. Compatibility art of traditional Chinese medicine: from the perspective of herb pairs. J Ethnopharmacol. 2012;143(2):412–23.
Liu ZR, Dong X, Ding X, Chen XF, Lv L, Li YY, et al. Comparative pharmacokinetics of timosaponin B-II and timosaponin A-III after oral administration of Zhimu–Baihe herb-pair, Zhimu extract, free timosaponin B-II and free timosaponin A-III to rats. J Chromatogr B. 2013;926(5):28–35.
Zhang ZQ. A review of the theory of prescription compatibility. Gansu J Tradit Chine Med. 2009;5:45–8.
Shou CH, Wang J, Zheng XX, Guo DW. Inhibitory effect of jujuboside a on penicillin sodium induced hyperactivity in rat hippocampal CA1 area in vitro. Acta Pharmacol Sin. 2001;22(11):986–90.
Wang C, You ZL, Xia Q, Xiong T, Xia Y, Yao DZ. Upregulation of Mark3 and Rpgrip1 mRNA expression by jujuboside a in mouse hippocampus. Acta Pharmacol Sin. 2007;28:334–8.
Peng ZC, Zhu JJ. Research advances in chemical constituents and pharmacological effects of semen Ziziphi Spinosae. Lishizhen Med Meteria Medica Res. 2001;12:86–7.
Han HS, Ma Y, Eun JS, Hong JT, Oh KW. Anxiolytic-like effect of methanol extract of Zizyphi Spinosi semen in mice. Biomolecules Ther. 2007;15(15):175–81.
Ahn NY, Jung JW, Oh HR, Shin JS, Hyeon SY. Anxiolytic-like effects of Sanjoin-Tang extracts and its ingredients in the elevated plus-maze in mice. Biomolecules Ther. 2004;12(3):151–6.
Liu X, Du RH, Yu CY, et al. Study on the anti-anxiety effect and mechanism of Lignans from Wuweizi [J]. J Beihua Univ (Nat Sci edition). 2015;05:609–12.
The State Pharmacopoeia Commission of PR China. The Pharmacopoeia of PR China, Part I. PR China, in Chinese: China Medical Science and Technology Press; 2020.
Chen JF, Gao JR, Ji WB, Jiang H. To explore the sedation and hypnosis effect and the mechanism of semen Ziziphi spinosae and Fructus schisandrae chinensis. Pharmacol Clin Chin Materia Med. 2013;29:128–31.
Gao JR, Ji WB, Jiang H, Chen JF. Effects of extracts from Ziziphi Spinosae semen and Schisandrae Chinensis Fructus on amino acid neurotransmitter in rats with insomnia induced by PCPA. Jo Chin Med Mater. 2013;36:1635–9.
Ji WB. Studies on the pharmacodynamics and mechanisms of sedative- hypnotic of extracts semen Ziziphi Spinosae and Fructus Schisandrae Chinensis. Anhui university of traditional. Chin Med. 2013;5:9–64.
Zhao C, Liu J, Shi JL, et al. Anxiolytic effect of alcohol-water extracted Suanzaoren-Wuweizi herb-pair by regulating ECS-BDNF-ERK signaling pathway expression in acute restraint stress male rats. Evid Based Complement Alternat Med. 2020;12:1–13.
Shixiu C, Jialei C, Ruifang X, Xin Z, Ling X, Changsheng D, et al. Action mechanisms for Jinfukang Oral liquid in treating non-small cell lung cancer based on network pharmacology. Chin Tradit Patent Med. 2019;41(7):1547–55.
Wenhua X, Jinghui Z, Yang Z, Xinwang Z, Yinru D, et al. Molecular biological mechanism of tanshinone IIA in treatment of coronary heart disease based on network pharmacology and bioinformatics. Chin Tradit Herb Drugs. 2019;50(5):1131–40.
Xia S, Dan R, Jing G, Gang Z, Yonghua W, Liang P, et al. The molecular mechanism of stroke treatment by Fufang Longmai Ningfang based on network pharmacology. Acta Pharm Sin. 2019;54(9):1588–96.
Zhang Y, Wang W, Zhang J. Effects of novel anxiolytic 4-butyl-alpha- agarofuran on levels of monoamine neurotransmitters in rats. Eur J Pharmacol. 2004;504(1–2):39–44.
Araújo J, Campos A, Ferreira M, Santos S, Damasceno M, Magalhães F, et al. Dioclea Altissima seed lectin (DAL) prevents anxiety-like behavioral responses in adult zebrafish (danio Rerio): involvement of GABAergic and 5-HT systems. CNS Neurol Disord Drug Targets. 2022;21(1):95–103.
Ahmed M, Azmat A. Decreased brain serotonin turnover rate following administration of Sharbat-e-Ahmed Shah produces antidepressant and anxiolytic effect in rats. Metab Brain Dis. 2017;32(6):1785–90.
Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K. Electrophysiology of the central serotonin system: receptor subtypes and transducer mechanisms. Ann N Y Acad Sci. 1990;600(1):93–103.
Revelli A, Tesarik J, Massobrio M. Nongenomic effects of neurosteroids. Gynecol Endocrinol. 1998;12(1):61–7.
Michael R, Vered CC, Jaime P, Doron L. GeneCards: integrating information about genes, proteins and diseases. Trends Genet. 1997;13(4):163.
Li YH, Yu CY, Li XX, Zhang P, Tang J, Yang QX, et al. Therapeutic target database update 2018: enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 2018;46:D1121–7.
Baxevanis A. Searching online mendelian inheritance in man (OMIM) for information on genetic loci involved in human disease. Curr Protocols Hum Genet Unit. 2012;9(13):1–10.
Dogan T, Saidi R, Mercier PL. Activities at the universal protein resource (UniProt). Nucleic Acids Res. 2013;42:D191–8.
Ru JL, Li P, Wang JN, Zhou W, Li BH, Huang C, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminformatics. 2014;6:1–13.
Boutet E, Lieberherr D, Tognolli M, Schneider M, Bansal P, Bridge AJ, et al. UniProtKB/Swiss-Prot, the manually annotated section of the UniProt knowledgeBase: how to use the entry view. Methods Mol Biol. 2016;1374:23–54.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):607–13.
Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: network analysis and visualization of proteomics data. J Proteome Res. 2019;18(2):623–32.
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–d361.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–51.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–51.
Han HS, Ma Y, Eun JS, Li RH, Hong JT, Lee MK, et al. Anxiolytic-like effects of sanjoinine Aisolated from Zizyphi Spinosi semen: possible involvement of GABAergic transmission. Pharmacol Biochem Behav. 2008;92(2):206–13.
Zou JB, Kong QL, Jiang LL. Study on the effect of antianxiety of Passiflora eduli. Chin Arch Tradit Chin Med. 2013;31(6):1332–3.
Mi XJ, Chen SW, Wang WJ, Wang R, Zhang YJ, Li WJ, et al. Anxiolytic-like effect of paeonol in mice. Pharmacol Biochem Behav. 2005;81(3):683–7.
Guo JY, Yuan XY, Sui F, Zhang WC, Wang JY, Luo F, et al. Placebo analgesia affects the behavioral despair tests and hormonal secretions in mice. Psychopharmacology. 2011;217(1):83–90.
Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp Jove. 2008;1088(22):1–4.
Liu J, Zhai WM, Yang YX, Shi JL, Liu QT, Liu GL, et al. GABA and 5-HT systems are implicated in the anxiolytic-like effect of spinosin in mice. Pharmacol Biochem Behav. 2015;128:41–9.
Jieshu Y, Min P, Jinli S, Huzhan Z, Yong L, Baosheng Z, et al. Evaluation of anxiolytic activity of compound Valeriana jatamansi Jones in mice. BMC Complement Altern Med. 2012;12:223.
Oliver G, Jun-Ichiro N, Shujiro S, Veronika B. Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J Ethnopharmacol. 2007;110:406–11.
Luis H, Margarita T, María TC, José LD. Assessing anxiety in C57BL/6J mice: a pharmacological characterization of the open-field and light/dark tests. J Pharmacol Toxicol Methods. 2014;69:108–14.
Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46(3):321–40.
Young R, Johnson DN. Comparison of routes of administration and time course effects of zacopride and buspirone in mice using an automated light/dark test. Pharmacol Biochem Behav. 1991;4(4):733–7.
Valeria C, Francesca D, Emiliano B, Franco M, Paolo R. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134(1):49–57.
Hu LL, Zhao XG, Yang J, Wang LM, Yang Y, Song TS, et al. Chronic scream sound exposure alters memory and monoamine levels in female rat brain. Physiol Behav. 2014;137:53–9.
Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4(1):71–8.
Zhao FR, Mao HP, Zhang H, Hu LM, Wang H, Wang YF, et al. Antagonistic effects of two herbs in Zuojin wan, a traditional Chinese medicine formula, on catecholamine secretion in bovine adrenal medullary cells. Phytomedicine. 2010;17(9):659–68.
Han RH, Park MH, Han YN. Cyclic peptide and peptide alkaloids from seeds of Zizyphus vulgaris. Phytochemistry. 1990;29(10):3315–9.
Fengxiang Z, Min L, Lirui Q, Zhihong Y, Chang L, Xiuyu S, et al. Rapid characterization of Ziziphi Spinosae semen by UPLC/Qtof MS with novel informatics platform and its application in evaluation of two seeds from Ziziphus species. J Pharm Biomed Anal. 2016;122:59–80.
Shenshen Y, Lanlan S, Houmin L, Xue S, Jun D, Yubo L. Rapid classification and identification of chemical components of Schisandra chinensis by UPLC-Q-TOF/MS combined with data post-processing. Molecules. 2017;22(10):E1778.
He XG, Lian LZ, Lin LZ. Analysis of lignan constituents from Schisandra chinensis by liquid chromatography-electrospray mass spectrometry. J Chromatogr A. 1997;757(1–2):81–7.
Yuan G, Swa B, Rc C, Jx C, Fm C. Characterization of lignans in Schisandra chinensis oil with a single analysis process by UPLC-Q/TOF-MS. Chem Phys Lipids. 2019;218:158–67.
Wang J, Jiang B, Shan Y, Wang X, Sun J. Metabolic mapping of Schisandra chinensis lignans and their metabolites in rats using a metabolomic approach based on HPLC with quadrupole time-of-flight MS/MS spectrometry. J Sep Sci. 2020;43(2):378–88.
Nan S, Zihu T, Bing Y, Xixi Y, Zhengling M. Xiaoyao powder modulates the myelin function of mPFC-BLA neural circuit through PI3K/ AKT/ mTOR pathway to alleviates the anxiety and depression phenotype in VaD mice. J Nanjing Univ Tradit Chin Medi. 2022;38(3):212–9.
Zhongye J, Na L, Hongbo Z, Xiaojuan L, Yueyun L, Jiaxu C. Effects of Xiao Yao san on the changes of inflammatory factor IL-1β, IL-6, TNF-α levels in rats blood serum with liver depression and spleen deficiency syndrome anxiety induced by chronic immobilization stress. J Tradit Chin Med. 2016;31(6):822–6.
Guochao L, Zhu Q. Experimental study of the effect of wuyou decoction on the expressions of hippocampal 5-HT1AR and 5-HT2AR in depression model rats. World J Integrated Tradit Western Medi. 2019;14(08):1099–104.
Mingjun X. Molecular mechanism of 5-HT2AR/5-HT1AR in Hippocampus involved in anxiety like behavior in posttraumatic stress disorder mice: Zhejiang University; 2018.
Lin L, Junbo Z, Xiaofei Z, Mei W, Dongyan G, Xiao Z, et al. Discussion on the mechanism of Xiaoyao wan in the treatment of depression and anxiety based on network pharmacology. J Hainan Med Univ. 2021;27(14):1091–7.
Lingzhen P, Zhiyong Y, Changying Z, Chong C, Shaohua L. Effects of long-term use of diazepam on neuroactive ligand-receptor interaction signaling pathway. J China Pharm Univ. 2011;42(5):443–6.
Su SY, Hsieh CL, Wu SL, Cheng WY, Li CC, Lo HY, et al. Transcriptomic analysis of EGb 761-regulated neuroactive receptor pathway in vivo. J Ethnopharmacol. 2009;123(1):68–73.
Xue JX, Xi GS, Qing QJ, Zhang JJ. Review of obsessive-compulsive disorder biological mechanism and treatment. Progress Modern Biomed. 2018;18(7):1387–91.
Long H, Liu B, Hou B, Wang C, Li J, Jiang T. Authors’ response to maternal age as a potential explanation of the role of the L allele of the serotonin transporter gene in anxiety and depression in Asians. Neurosci Bull. 2014;30(3):536–7.
Chunhui Z. Research progress of cyclic adenosine monophosphate signaling pathway in the treatment of anxiety disorder. China Pharm. 2015;26(28):4011–4.
Lundegaard PR, Anastasaki C, Grant NJ, Sillito RR, Zich J, Zeng Z, et al. MEK inhibitors reverse cAMP-mediated anxiety in zebrafish. Chem Biol. 2015;22(10):1335–46.
Ann-Katrin K, Paul CG, Zoltán S. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol Biol. 2019;1916:69–74.
Munekazu K, Keizo T, Tsuyoshi M. Elevated plus maze for mice. J Vis Exp. 2008;22(22):1088.
Bruijnzeel AW, Behnood-Rod A, Malphurs W, et al. Oxycodone decreases anxiety-like behavior in the elevated plus-maze test in male and female rats. Behav Pharmacol. 2022;33(6):418–26.
Cruz APM, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49(1):171–6.
Michel B, Martine H. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1–3):55–65.
Natalia K, Vootele V. Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure. Physiol Behav. 2014;133:30–8.
Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32(3):777–85.
Ann-Katrin K, Paul CG, Zoltán S. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019;1916:99–103.
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(3):3–33.
Rentesi G, Antoniou K, Marselos M, Fotopoulos A, Alboycharali J, Konstandi M. Long-term consequences of early maternal deprivation in serotonergic activity and HPA function in adult rat. Neurosci Lett. 2010;480(1):7–11.
Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125(1–2):141–9.
Jantarima P, Sutthasinee P, Sarinee KT. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behav Brain Res. 2009;198(1):142–8.
Dazzi L, Serra M, Spiga F, Pisu MG, Jentsch JD, Biggio G. Prevention of the stress-induced increase in frontal cortical dopamine efflux of freely moving rats by long-term treatment with antidepressant drugs. Eur Neuropsychopharmacol. 2001;11(5):343–9.
Acknowledgments
The authors would like to thank all of the colleagues who contributed to this study.
Funding
This work was supported by the Key New Drugs Innovation project from Ministry of Science and Technology (2012ZX09102201–018) and Graduate program of Beijing University of Chinese Medicine (2017-JYB-XS-066).
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. J. L. was responsible for the pharmacological activity and the manuscript writing. J.-L.S. suggested the research idea; J.-L.S. and J.-Y.G. conceived and designed the experiments, contributed to the discussion of results and reviewed the entire manuscript. Y. C. and X.-J.M. were responsible for the mechanism research; Z.-Q.Z. and S.-N.W. conducted the HPLC research and analyzed and interpreted the data; M.-X.L. and S.H. assisted to complete the pharmacodynamic experiment. The authors declare that all data were generated in-house and that no paper mill was used. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental procedures were approved by the Animal Care and Use Committee of the Institute of Psychology of the Chinese Academy of Sciences (Protocol No. 20170327) and in accordance with the Guide for Care and Use of Laboratory Animals by National Institutes of Health (NIH Pub. No. 85–23, revised 1996).
The seeds of Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H.F. Chou were purchased from Sichuan Natural Pharmaceutical co., Ltd. (Sichuan, China), the fruits of Schisandra chinensis (Turcz.) Baill. were purchased from Liaoning Ludan Ltd. (Liaoning, China), and they were identified by professor Shi Jin-Li, a botanist at the Beijing University of Chinese Medicine, China. The voucher specimens (No.20160122 of Semen Ziziphi spinosae and No.20150601 of Fructus Schisandrae) were maintained in Institute of Traditional Chinese Medicine, Beijing University of Chinese Medicine, China. The study protocol complies with relevant Chinese institutional, national, and international guidelines and legislation.
Furthermore, the study was conducted in agreement with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
Jie Liu, Jin-Li Shi, Jian-You Guo, Yi Chen, Xiao-Jie Ma, Sheng-Nan Wang, Zhi-Quan Zheng, Ming-Xuan Lin and Shuai He declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Shi, JL., Guo, JY. et al. Anxiolytic-like effect of Suanzaoren–Wuweizi herb-pair and evidence for the involvement of the monoaminergic system in mice based on network pharmacology. BMC Complement Med Ther 23, 7 (2023). https://doi.org/10.1186/s12906-022-03829-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03829-1