Abstract
Objective
Diabetic kidney disease affects approximately 40% of diabetic patients and is the leading cause of chronic kidney disease (CKD) worldwide. As a result, preventing renal complications in diabetic patients is critical. Ginger (Zingiber Officinale Rosco) is a popular spice and natral medicine. The present study was a systematic review focused on the existing evidence of the renoprotective effect of ginger extract on some features of diabetic kidney disease.
Methods
The literature was searched in online databases such as PubMed, Scopus, EMBASE, ProQuest databases, and Google Scholar from inception to July 2022.
Results
This review included 41 articles that met the eligibility criteria. Ginger supplementation was found to be associated with a significant decrease in blood glucose in 28 studies. Nine studies showed a significant reduction in malondialdehyde (MDA) after supplementation. Also, seventeen studies showed decreased serum levels of creatinine. Fifteen studies reported a decrease in total cholesterol (TC) and fourteen studies showed a lowered triglycerides (TG) concentrations. In twenty-six studies, ginger reduced renal injuries due to diabetes.
Conclusion
Ginger may improve blood sugar indices, lipid profile, some inflammatory markers, oxidative stress, and pathologic injuries in diabetic kidney disease. However, future well-designed clinical trials and meta-analyses are required for a solid consensus.
Similar content being viewed by others
Introduction
Diabetic kidney disease (DKD) formerly known as diabetic nephropathy (DN), is a microvascular complication of diabetes, occurring in about one-third of people with diabetes [1,2,3]. The International Diabetes Federation estimates that the disease will increase from 463 million in 2019 to 700 million in 2045 [4]. DKD patients have a high incidence of cardiovascular morbidity and mortality [5]. The cause of the pathogenesis of DKD is multifactorial. Hyperglycemia is a key factor in the progression of pathologic alterations in the kidneys [6]. Similarly, dyslipidemia is a predictive factor in DKD progression [7]. In diabetes, increased oxidative stress plays a pivotal role in the development of DKD [8]. Also, inflammation has a crucial role in the onset and progression of DKD [9]. Today, the use of nutrition therapies and nutritional supplements along with treatment strategies to control the risk factors for cardiovascular disease in patients, as well as those with kidney diseases, has received much attention [10].
Zingiber Officinale Roscoe is the scientific name for ginger, which belongs to the Zingiberaceae family [11]. This spice has been used in Chinese and ayurvedic medicine for centuries [12]. The antioxidant properties of medicinal herbs are related to environmental conditions, weather, seasonal changes, geographical area, degree of ripe, growth, and many other factors during planting and harvesting [13]. The smell of fresh ginger is due to the presence of a group of phenolic compounds called gingerol, similarly, the smell of dried ginger is due to the presence of shogaols, which are dehydrated compounds of gingerols. Ginger has been declared to be safe by the US food and drug administration [14]. It has beneficial features due to bioactive compounds like gingerol, shogaol, paradol, and zingerone [15].
Although several animal studies have been conducted to assess the impact of ginger on metabolic indicators in DKD, a systematic review has not been initiated in association with this matter. Several systematic reviews showed the potential effects of ginger supplementation on glycemic control, lipid profile, inflammatory markers, and oxidative stress in patients with diabetes, hyperlipidemia, arthritis, neurological diseases, asthma, and stroke disease [16,17,18,19,20]. Some studies found that ginger intake could significantly increase fasting blood glucose (FBS), total cholesterol (TC), triglycerides (TG), urea, creatinine (Cr), and urine protein or no significant change in FBS, urea, and Cr levels [21,22,23,24]. The inconsistent results obtained in different studies could be attributed to various factors such as ginger form, dose, and duration of intervention. A systematic review is required to comprehensively integrate results from studies. The goal of the present systematic review is to investigate the literature on ginger’s influence on glycemic indices, dyslipidemia, inflammatory markers, oxidative stress markers, renal function, and structure. The mechanisms of the impact of ginger are presented in the discussion.
Methods
Search strategy
PubMed, Scopus, Embase, ProQuest, and Google Scholar were used as search engines, and keywords were chosen from MeSH and non-MeSH terms including: (“Ginger” OR “Zingiber” OR “Shogaols” OR “zingerone” OR “Gingerols”) AND (“kidney” OR “renal” OR “dialysis” OR “Hemodialysis” OR “ End Stage Renal Disease” OR “ESRD” OR “chronic kidney disease” OR “CKD” OR “acute renal failure” OR “ARF” OR “nephropathy” OR “diabetic nephropathy” OR “Glomerular Filtration Rate” OR “GFR” OR “Albuminuria” OR “Proteinuria” OR “Creatinine”) AND (“diabetes” OR “diabetes mellitus” OR “type 2 diabetes” OR “T2DM” OR “type 1 diabetes” OR “T1DM” OR “gestational diabetes mellitus” OR “GDM” OR “ Insulin Dependent Diabetes Mellitus” OR “IDDM” OR “Non-Inslin Dependent Diabetes Mellitus” OR “NIDDM” OR “fasting blood sugar” OR “fasting blood glucose” OR “glucose intolerance” OR “glucose tolerant”(. Preferred reporting items for systematic reviews (PRISMA) guidelines were followed when conducting this review.
Eligibility criteria
Studies on the effect of ginger supplementation on DKD were included in this study. The PICO strategy for the research question of the study was patient/ population (P): animals mice or rats); Intervention (I): supplementation with ginger; Comparison (C): placebo group; and outcome (O): changed glycemic indices, lipid profile, inflammatory markers, oxidative stress, and renal function indicator.
Included studies include animal studies, English-language journals, and studies examining the effects of ginger on DKD. Excluded studies include studies in which ginger is supplemented in combination with other substances, studies in which we did not have access to the full text, and studies in vitro.
Data extraction
The first and third authors (PV and HR) screened the titles and abstracts of the qualifying studies separately. The relevant data including the first author’s name, year of publication, country, study population, sample size, gender of subjects, ginger dosage, duration of intervention, diabetes induction method, and outcome data were extracted. Eligible papers were assessed based on the goal checklist, the question of the study, and inclusion/ exclusion criteria. Articles not meeting the criteria for data collection were eliminated. Any discrepancies among reviewers were resolved through consultation with the authors. The quality of the selected studies was evaluated via a first author. Quality assessment studies used the syrcle’s tool.
Quality assessment
To assess the quality of studies the SYRCLE’s RoB tool evaluated studies based on ten criteria: random allocation sequence, animals similar at baseline, allocation concealment, random housing, blinded investigators, random outcome assessment, blinded outcome collection, incomplete data justification, unbiased conclusions and other. Each study could ultimately have a total score of 10 points.
Results
Selected articles
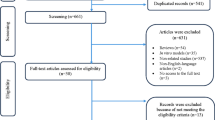
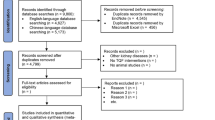
Figure 1 depicts a flowchart of the research selection. The initial search resulted in a total of 567 articles, resulting in 541 non-duplicated publications after removing 26 articles. Following a review of titles and abstracts, 492 articles were eliminated. 6 studies were excluded due to the lack of inclusion criteria. Finally, the present review found 41 articles that meet the eligibility criteria. Table 1 summarizes the characteristics of chosen studies.
Characteristics of the included studies
In total, after screening and deleting duplicate articles, forty-one studies were selected for this systematic review. All studies assessed diabetic mice or rats. Ginger was used in different shapes in this study, including ginger powder, ginger oil, aqueous ginger extract, ethanolic ginger extract, and bioactive compounds such as zingerone and shogaol. Ginger and ginger extract treatment dosages ranged from 80 to 1000 mg/kg and bioactive compounds treatment dosages ranged from 5 to 100 mg/kg. Intervention duration ranged from 2 to 16 weeks. Location of studies performed as follows: 9 in Egypt [29, 30, 35, 37, 38, 47, 48, 54, 59], 8 in India [26, 36, 39, 41, 42, 49, 51, 56], 6 in Saudi Arabia[22, 23, 44, 45, 52, 61], 4 in Iran [31,32,33, 53], 3 in Kuwait [34, 57, 58], 3 in Pakistan [28, 46, 55], 2 in Nigeria [24, 43], 2 in China [21, 27], 1 in South Korea [25], Jordan [50], Iraq [60] and Malaysia [40]. Studies were done from 2006 to 2021.
Quality assessment
A summary of the results of the quality assessment is demonstrated in Fig. 2. In the majority of studies, performance bias, detection bias, and allocation concealment were found to be unclear risks of bias.
Ginger and glycemic control in DKD
Twenty-eight of 31 studies showed that ginger intake lowers blood glucose levels [24, 25, 27, 29, 30, 34,35,36,37,38,39,40,41,42,43, 47,48,49, 51,52,53, 55,56,57,58,59,60,61]. On the contrary, in 2 studies, blood glucose levels increased [22, 23]. One study did not show any meaningful changes [21]. 6 out of 7 studies reported that ginger increases serum insulin levels [24, 37, 38, 57, 59, 60], whereas in another study the result was reversed [27]. Ginger reduced hemoglobin A1c (HbA1c) and C peptide in all studies that examined these biomarkers [21, 26, 27, 29, 59]. Finally, to assess the impact of ginger consumption, a subgroup analysis was performed for ginger forms. In the bioactive compound subgroup of ginger, 6-shogaol had a better effect on blood sugar than zingerone. In the ginger powder subgroup, hypoglycemia’s effect increases with increasing dose intake. In the ginger extract subgroup with a dose of 500 mg/kg or less, hypoglycemia increases with increasing dose. However, by a dose of more than 500 mg/kg of ginger extract, hypoglycemia was reduced.
Ginger and dyslipidemia in DKD
Sixteen out of 41 articles examined the effect of ginger on the lipid profile. The reduction of TC and TG were also reported by 15 [21, 26, 29, 30, 34, 35, 38, 41, 44, 49, 52, 54, 56, 59, 60] and 14 [21, 26, 29, 30, 34, 35, 38, 41, 44, 49, 52, 56, 59, 60] studies respectively. Ginger has been shown to improve low-density lipoprotein-cholesterol (LDL-C) [26, 29, 30, 35, 38, 52, 54, 56, 59] and high-density lipoprotein-cholesterol (HDL-C) [26, 29, 30, 35, 38, 49, 56, 59, 60] levels in nine studies. One study reported contradictory results for TC, TG, and LDL-C, as well as two studies for HDL-C [23, 44]. In the subgroup of bioactive compounds of ginger, by increasing the received dose, improving dyslipidemia increased. In the subgroup of ginger extract, higher doses had a better effect on TC, TG, LDL-C, and HDL-C levels. However, with a dose of more than 800 mg/kg of ginger extract, HDL-C was reduced.
Ginger and oxidative stress indices in DKD
Ginger reduced the malondialdehyde (MDA) levels in all 9 studies that examined it [21, 29, 31,32,33, 41, 42, 48, 52]. In all studies, the impact of ginger on the antioxidant defense system was evaluated, and positive results were found. In all studies, ginger elevated the level of glutathione (GSH) [21, 26, 27, 29, 38, 42, 48, 49, 52], catalase [26, 29, 42, 52, 59], superoxide dismutase (SOD) [26, 29, 42, 52, 59], glutathione reductase (GR) [26, 42], glutathione peroxidase (GPx) [26, 38, 42, 59] and total antioxidant capacity (TAC) [31,32,33, 60] factors. Similarly, 2 studies reported that administration of ginger decreased reactive oxygen species (ROS) levels [21, 26]. In the subgroup of the bioactive compounds of ginger, higher doses had a better effect on GSH levels. In the subgroup of ginger powder, higher doses had a better effect on MDA levels.
Ginger and inflammation biomarkers in DKD
Seven out of 41 studies investigated the influence of ginger on inflammatory markers. Ginger diminished tumor necrosis factorα (TNFα), interleukin6 (IL6), interleukin1β (IL1β), and nuclear factor kappa-light chain-enhancer of activated B cells (NFκB) serum levels in 7 [21, 26, 27, 29, 47, 52, 61], 5 [21, 26, 27, 29, 52], 3 [26, 29, 52], and 3 [26, 27, 61] studies, respectively. The studies did not show any adverse effects. Higher doses had a better effect on TNFα and IL6 levels in the subgroup of the bioactive compounds of ginger.
Ginger and renal function in DKD
Twenty-four studies evaluated the potential effect of ginger on kidney function indicators. In 17 of 21 studies ginger supplementation reduced serum creatinine levels [21, 23, 26,27,28,29,30, 35, 38, 44, 47,48,49, 52, 53, 57, 59]. Creatinine levels, on the other hand, increased in one study [22] and remained unchanged in three others [24, 37, 60]. Ginger decreased serum levels of urea, blood urea nitrogen (BUN), and uric acid in 13 [24, 29, 30, 35, 38, 44, 47,48,49, 51, 52, 54, 60], 6 [21, 26, 27, 29, 37, 59], and 6 [35, 48, 49, 51, 57, 59] studies, respectively. On the contrary, urea and uric acid levels were increased in one study [23]. Uric acid levels were not significantly changed in three studies [22, 37, 60]. One study showed no meaningful changes in urea and BUN levels [22]. Higher doses had a better effect on BUN and Cr levels in the subgroup of the bioactive compounds of ginger. By increasing ginger extract intake, the effect on uric acid increased. In the subgroup of ginger powder, higher doses had a better effect on urea levels.
Ginger and proteinuria in DKD
Urinary protein was decreased in 6 studies [34, 43, 53, 57, 58, 61] and increased in one study [23]. Moreover, urine albumin was decreased in 4 studies [21, 27, 29, 47].
Ginger and changes in histomorphology and structural renal in DKD
Among the studies, twenty-six evaluated the influence of ginger on histopathological changes in kidneys [21, 24,25,26,27, 29, 31,32,33, 37,38,39, 41, 42, 45,46,47, 50,51,52,53, 55, 56, 58, 59, 61]. All studies examining histomorphological changes showed beneficial effects. The beneficial impacts of ginger on bowman’s capsule atrophy, the surface area of bowman’s capsule, and bowman’s space were demonstrated in seven studies [21, 24, 25, 27, 33, 45, 46]. In 8 articles, necrosis of tubular and glomerular cells was reduced [21, 27, 29, 39, 41, 42, 58, 59], also hajhosseini et al. found that the number of apoptotic cells was reduced [31]. In 12 studies ginger reduced dilation and degeneration of tubules [21, 25, 27, 29, 32, 33, 39, 41, 42, 47, 59, 61]. Additionally, in four studies, the weight of the kidneys decreased at the end [21, 27, 28, 40], although, in one study, the weight of the kidneys did not change significantly [43].
Discussion
The present systematic review was conducted to discover the impact of different forms of ginger on metabolic indicators in DKD. The findings, to the best of our knowledge, show some positive effects of ginger in DKD. The result of the current systematic review exhibited that ginger has a beneficial effect on blood levels of glucose, insulin, C-peptide, and HbA1C. However, the results on blood glucose in the studies done by Abdulsalam et al. and Al-Attar et al. were contradictory [22, 23]. The conflicting results seem to be due to the fact that the dose of ginger was not clear because it was expressed as a percentage of the diet. However, in the studies with positive results, ginger was prescribed in mg/kg with specified dosages. Also, Xu Y et al. showed that 25 or 50 mg/kg of 6-shogaol reduced insulin serum levels [27]. Notably, in this study 6-shogaol was used, while other studies were based on ginger supplementation. There was insufficient evidence to draw conclusions about homeostatic model assessment of insulin resistance (HOMA-IR).
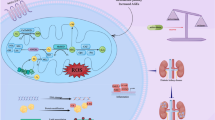
Several possible mechanisms have been suggested for ginger’s effect on glycemic indices. A mechanism was expressed in Fig. 3 to explain how ginger can improve blood glucose levels in liver cells. Ginger activates the AMP-activated protein kinase (AMPK) pathway [62]. Activation of this pathway inhibits forkhead box protein O1 (FOXO1), an important transcription factor in regulating the expression of genes involved in hepatic glucose production (gluconeogenesis) such as phosphoenolpyruvate carboxykinase PEPCK and glucose-6- phosphatase (G6pase), resulting in decreased hepatic glucose production [63]. Also, ginger inhibits the hepatic phosphorylase enzyme activity and suppresses glycogenolysis in liver cells, while increases the activity of glycogenesis enzymes [64]. According to a study, ginger can also increase the activity of hepatic glycolytic enzymes such as glucokinase, phosphofructokinase, and pyruvate kinase [29]. Another suggested mechanism is inhibition of the hepatic glucose 6 phosphatase enzyme activity, thereby reducing the conversion of glucose 6 phosphates to glucose, causes to decreasing blood glucose levels [65].
In one study, glucose uptake in rat muscle cells was increased due to translocation of glucose transporter4 (GLUT4) transporter to the plasma membrane, and the rise in GLUT 4 gene expression facilitated insulin-independent glucose uptake [66]. In addition, ginger activates the AMPK pathway [62]. Activation of AMPK by increasing the phosphorylation of insulin receptor substrate (IRS), phosphoinositide 3-kinase (PI3K), and protein kinase B (Akt) tyrosine roots improves insulin signaling and increases the translocation of GLUT4 transporter to the plasma membrane surface, and increases the entry of glucose into the cell [67]. Figure 4 shows how ginger may affect insulin sensitivity. Moreover, ginger can reduce Insulin resistance in skeletal muscle [68].
Dyslipidemia is one of the predictors of DKD progression [7, 69, 70]. In general, based on the present study, the lipid profile was improved due to ginger supplementation. Although the results of Attar’s study on lipid profile were quite the opposite, so that TC, TG, and LDL-C were increased and HDL-C was decreased. In Attar’s study, the dose of ginger oil was 2.5 and 5% of the diet and supplementation lasted for 2 weeks [23]. Also, Sangi S et al. showed that the application of 1000 mg/kg ginger aqueous extract for 3 weeks reduced serum HDL-C [44]. These results appear to be inconsistent due to the short duration of supplementation.
As shown in Fig. 3, ginger increases the expression of the peroxisome proliferator-activated receptor alpha (PPAR-α) gene by activating the AMPK-SIRT-PGC-1α pathway in the liver, which leads to the inhibition of the expression of regulatory genes such as sterol regulatory element binding protein1c (SREBP-1c) and acetyl-CoA carboxylase (ACC) in lipogenesis. As a result of the expression of ACC and SREBP1C genes, the synthesis of fatty acids and cholesterol is reduced [68]. Some other possible mechanisms were proposed for lowering lipid levels with ginger intake in two systematic reviews [20, 71]: (1) The reduction of the cholesterol biosynthesis by reducing farnesyl diphosphate liver production, (2) Induction of the conversion of cholesterol into bile acids and increased cholesterol excretion, (3) The liver uptake LDL-C from blood circulation and reduces cholesterol synthesis, (4) Increased pancreatic lipase, (5) Inhibition of lipid hydwrolysis in the intestine, (6) PPARδ pathway activation, (7) Decreased retinol-binding protein (RBP) expression, which is an indicator of hyperlipidemia, (8) The presence of niacin in ginger, which reduces TG and VLDL-C and uptake of LDL-C by liver, (9) The reduction of the conversion of excess carbohydrate to TG by reducing the expression of carbohydrate response element-binding protein (ChREBP) gene.
Inflammation and oxidative stress play an important role in the pathogenesis and progression of DKD [72, 73]. The results of the current systematic review support the beneficial effect of ginger on both inflammation and oxidative stress. Possible mechanisms for reducing inflammation by ginger are as follows: (1) NF-κB signaling pathway suppression [68], (2) Inhibition of cyclooxygenase2 (COX-2) and lipoxygenase, thus suppressing arachidonic acid (AA) metabolism, (3) Inhibition of prostaglandin synthesis, (4) Presence of some compounds in ginger that are serotonin blockers and reduce inflammation and prostaglandins production [17]. Hyperglycemia increases the production of reactive oxygen species )ROS(. Ginger reduces ROS directly or indirectly by lowering blood glucose [29]. Also ginger reduces oxidative stress and lipid peroxidation by scavenging free radicals [74]. Figure 5 shows the possible mechanisms for reducing oxidative stress, which are as follows: 1) preventing the formation of advanced glycation end products (AGEs) via nuclear factor erythroid 2-related factor2 (Nrf2) dependent pathway [3]. 2) inhibition of protein kinase C [75]. 3) inhibition of polyol pathway [76].
Overall, renal function indicators were improved based on the obtained results of the present study. However, urea and uric acid levels in Al-Attar AM et al. study and creatinine levels in Abdulsalam K et al. study were increased [22, 23]. The inconsistent results seem to be due to the fact that the exact effective dosage of ginger was not clear because in most studies, it was expressed as a percentage of the diet. As a matter of fact, in the studies showing the beneficial effects of ginger, it was prescribed in mg/kg with specified dosages.
Possible mechanisms for improving renal function by ginger are as follows: (1) Hyperglycemia induces free radicals that attribute to the activation of various downstream signaling cascades leading to structural and functional changes in the renal [77]. Ginger improves renal function through scavenging free radicals [74], (2) AGEs have a key role in the pathogenesis and the progression of DKD. AGEs accumulate in DKD as a result of decreased excretion and increased production resulting from oxidative stress [78]. Bioactive ginger components reduce protein glycation by trapping methylglyoxal [78]. Therefore, ginger inhibits the initiation and progression of DKD by reducing the glycation of proteins, (3) Urea induces free radical production and apoptosis that leads to functional changes in the kidney [79]. Ginger supplementation may reduce urea by inhibiting urea re-absorption in nephrons. Polyphenols and flavonoids present in ginger may play a role in renoprotective activities and lowering serum urea, creatinine, and uric acid levels [80], (4) In DKD lipid accumulation occurs in tubule epithelial cells, leading to kidney fibrosis. Xu Y et al. and Ramudu SK et al. showed that ginger reduced lipid content in kidney tissues [27, 41]. Therefore, ginger improves renal function and structure by reducing lipid accumulation.
Although proteinuria was decreased based on the current study, it was increased in the study done by Al-Attar AM et al. The ginger used in this study was in oil form, while in the other studies reviewed, powder, extract, or bioactive compounds of ginger were used, one of the probable reasons for different results. Notably, ginger reduces glomerular and tubular degeneration, reducing the thickening of glomerular basement membrane and restoring the integrity of kidney tissue membranes [26, 39, 52]. the possible mechanisms underlying the reduced proteinuria observed in the studies.
All studies that examined histomorphological changes of kidney showed beneficial effects of ginger. Several studies have shown that ginger improves pathological changes such as cytotoxicity caused by hyperglycemia [21], cell apoptosis [31], and bleeding in the cortical area of the kidney [32], repairs kidney damage, and restored membrane integrity in renal tissue and structural derangement [26].
Human studies
Recently, a clinical trial was published that investigated the effect of ginger on renal function in patients with T2DM [81]. Elsaadany et al. reported that 3000 mg/day of ginger reduced Cr, but not changed BUN significantly. Also, reported that FBS was reduced, but the reduction of HbA1c was not significant. HbA1c measures usually show the average blood glucose over the past 2 to 3 months. As the study duration was eight weeks, it seems that the lack of significant decrease in HbA1c is due to the short duration of supplementation. Also, reported that TG was reduced, but TC, LDL-C, and HDL-C did not meaningful changes. In agreement with these results, Pourmasoumi et al. demonstrated that low dose of ginger (≤ 2000 mg/day) had greater lowering impact on TG and TC [20].
Knowledge gaps and future direction
Due to the lack of human studies, future well-designed clinical trials with large sample sizes, various dosages, and long durations are required to reach definitive results about the use of ginger in the prevention and reduction of complications of DKD. Different dosages, supplementation duration, and diverse forms of ginger were the possible reasons for the inconsistency of the results. Not registering the study protocol was another study limitation.
Conclusion
As a whole, the results of the present systematic review indicated that ginger may have several beneficial effects on glycaemic indices, oxidative stress, inflammatory markers, lipid profile, and some renal function indicators. Although the results seem promising, further human trials are required to achieve more informative and conclusive results.
Availability of data and materials
The data is available for review.
References
Dounousi E, Duni A, Leivaditis K, Vaios V, Eleftheriadis T, Liakopoulos V. Improvements in the management of diabetic nephropathy. Rev Diabet Stud. 2015;12(1–2):119.
Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. 2015;2015:697010.
Sampath C, Rashid MR, Sang S, Ahmedna M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017;226:79–88.
Preguiça I, Alves A, Nunes S, Gomes P, Fernandes R, Viana SD, Reis F. Diet-induced rodent models of diabetic peripheral neuropathy, retinopathy and nephropathy. Nutrients. 2020;12(1):250.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–80.
Akhtar M, Taha NM, Nauman A, Mujeeb IB, Al-Nabet ADM. Diabetic kidney disease: Past and present. Adv Anat Pathol. 2020;27(2):87–97.
Radcliffe NJ, Seah Jm, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Invest. 2017;8(1):6–18.
Hakim FA, Pflueger A. Role of oxidative stress in diabetic kidney disease. Med Sci Monit. 2010;16(2):RA37–48.
García-García PM, Getino-Melián MA, Domínguez-Pimentel V, Navarro-González JF. Inflammation in diabetic kidney disease. World J Diabetes. 2014;5(4):431.
Escott-Stump S: Renal Disorders. Nutrition and Diagnosis-Related Care, 6th ed; Lippincott Williams & Wilkins; 2008. p. 785–820.
White B. Ginger: an overview. Am Family Phys. 2007;75(11):1689–91.
Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–32.
Škrovánková S, Mišurcová L, Machů L. Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res. 2012;67:75–139.
Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46(2):409–20.
Butt MS, Sultan MT. Ginger and its health claims: molecular aspects. Crit Rev Food Sci Nutr. 2011;51(5):383–93.
Morvaridzadeh M, Sadeghi E, Agah S, Fazelian S, Rahimlou M, Kern FG, Heshmati S, Omidi A, Persad E, Heshmati J. Effect of ginger (Zingiber officinale) supplementation on oxidative stress parameters: A systematic review and meta-analysis. J Food Biochem. 2021;45(2):e13612.
Jalali M, Mahmoodi M, Moosavian SP, Jalali R, Ferns G, Mosallanezhad A, Imanieh MH, Mosallanezhad Z. The effects of ginger supplementation on markers of inflammatory and oxidative stress: A systematic review and meta-analysis of clinical trials. Phytother Res. 2020;34(8):1723–33.
Jafarnejad S, Keshavarz SA, Mahbubi S, Saremi S, Arab A, Abbasi S, Djafarian K. Effect of ginger (Zingiber officinale) on blood glucose and lipid concentrations in diabetic and hyperlipidemic subjects: A meta-analysis of randomized controlled trials. J Funct Foods. 2017;29:127–34.
Aryaeian N. A review of the effect of Ginger in inflammation. Rahavard Salamat J. 2016;2(1):52–64.
Pourmasoumi M, Hadi A, Rafie N, Najafgholizadeh A, Mohammadi H, Rouhani MH. The effect of ginger supplementation on lipid profile: A systematic review and meta-analysis of clinical trials. Phytomedicine. 2018;43:28–36.
Cui Y, Shi Y, Bao Y, Wang S, Hua Q, Liu Y. Zingerone attenuates diabetic nephropathy through inhibition of nicotinamide adenine dinucleotide phosphate oxidase 4. Biomed Pharmacother. 2018;99:422–30.
Abdulsalam K, Alkalifa A. Effect of Ginger and its Extract on Blood Sugar and on Kidney Function of Type I Diabetic Rats. Middle East J Fam Med. 2016;7(10):12.
Al-Attar AM, Zari TA. Modulatory effects of ginger and clove oils on physiological responses in streptozotocin-induced diabetic rats. Int J Pharmacol. 2007;3:34–40.
Kazeem MI, Akanji MA, Yakubu MT. Amelioration of pancreatic and renal derangements in streptozotocin-induced diabetic rats by polyphenol extracts of Ginger (Zingiber officinale) rhizome. Pathophysiology. 2015;22(4):203–9.
Yi J-K, Ryoo Z-Y, Ha J-J, Oh D-Y, Kim M-O, Kim S-H. Beneficial effects of 6-shogaol on hyperglycemia, islet morphology and apoptosis in some tissues of streptozotocin-induced diabetic mice. Diabetol Metab Syndr. 2019;11(1):1–13.
Rehman MU, Rashid SM, Rasool S, Shakeel S, Ahmad B, Ahmad SB, Madkhali H, Ganaie MA, Majid S, Bhat SA. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) ameliorates renal function via controlling oxidative burst and inflammation in experimental diabetic nephropathy. Arch Physiol Biochem. 2019;125(3):201–209.
Xu Y, Bai L, Chen X, Li Y, Qin Y, Meng X, Zhang Q. 6-Shogaol ameliorates diabetic nephropathy through anti-inflammatory, hyperlipidemic, anti-oxidative activity in db/db mice. Biomed Pharmacother. 2018;97:633–41.
Irshad F, Toor RS, Hussain M. Diabetic nephropathy; effect of ginger extract on serum creatinine and paired kidney weight in alloxan induced diabetic nephropathy of albino rats. Prof Med J. 2018;25(7):1117–23.
Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother. 2018;106:381–9.
Hanna ET, Aniess W, Khalil AF, Abdalla ES, Hassanin EA, Nagib EW. The effect of ginger and thyme on some biochemical parameters in diabetic rats. IOSR J Pharm Biol Sci. 2014;9(3):54–61.
Hajhosieni L, Rostami FF, Khaki A. Bioflavonoids Effects of Ginger on Glomerular Podocyte Apoptosis in Streptozotocin-Induced Diabetic Rat. Crescent J Med Biol Sci. 2014;1(2):42–5.
Khaki A, Khaki A, Ahmadi-Ashtiani H, Rastegar H, Rezazadeh S, Babazadeh D, Zahedi A, Ghanbari Z. Treatment effects of ginger rhizome & extract of carrot seed on diabetic nephropathy in rat. J Med Plants. 2010;9(33):75–80.
Afshari AT, Shirpoor A, Farshid A, Saadatian R, Rasmi Y, Saboory E, Ilkhanizadeh B, Allameh A. The effect of ginger on diabetic nephropathy, plasma antioxidant capacity and lipid peroxidation in rats. Food Chem. 2007;101(1):148–53.
Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96(4):660–6.
Elshater A-EA, Salman M, Moussa M. Effect of ginger extract consumption on levels of blood glucose, lipid profile and kidney functions in alloxan induced-diabetic rats. Egypt Acad J Biol Sci Entomol. 2009;2(1):153–62.
Shanmugam K, Ramakrishana C, Mallikarjuna K, Reddy KS. The impact of ginger on kidney carbohydrate metabolic profiles in STZ induced diabetic rats. Asian J Exp Sci. 2009;23(1):127–34.
Elkott AF, El-Sayed S, Abdel-Aziz AM. The effects of Ginger (Zingiber officinale) on histology and immunohistochemistry of liver and kidney and certain haematological parameters in Alloxan-induced Diabetic rats. Egypt J Exp Biol(zool). 2010;6(1):61–70.
Kadah M. Protective Effect of cinnamon, clove and ginger spicces or their essential oils on oxidative stress of streptozotocin-induced diabetic rats. Arab Univ J Agric Sci. 2010;18(1):137–54.
Ramudu SK, Korivi M, Kesireddy N, Lee L-C, Cheng I-S, Kuo C-H, Kesireddy SR. Nephro-protective effects of a ginger extract on cytosolic and mitochondrial enzymes against streptozotocin (STZ)-induced diabetic complications in rats. Chin J Physiol. 2011;54(2):79–86.
Abdulrazaq NB, Cho MM, Win NN, Zaman R, Rahman MT. Beneficial effects of ginger (Zingiber officinale) on carbohydrate metabolism in streptozotocin-induced diabetic rats. Br J Nutr. 2012;108(7):1194–201.
Ramudu SK, Mallikarjuna K, Kesireddy SR. Efficacy of ethanolic extract of ginger on kidney lipid metabolic profiles in diabetic rats. Int J Diabetes Developing Ctries. 2011;31(2):97–103.
Shanmugam KR, Mallikarjuna K, Nishanth K, Kuo CH, Reddy KS. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011;124(4):1436–42.
Eleazu C, Iroaganachi M, Okafor P, Ijeh I, Eleazu K. Ameliorative potentials of ginger (Z. officinale Roscoe) on relative organ weights in streptozotocin induced diabetic rats. Int J biomedical science: IJBS. 2013;9(2):82.
Sangi S. Effect of Nigella sativa, Zingiber officinale, Cinnamomum zeylanicum on serum lipid profile, glucose, weight, and kidney function tests in the animal model of STZ induced diabetes melitus in male rats. Int J Biology Pharm Allied Sci. 2018;7(5):877–905.
Sangi S, Elwahab M. Experimental evaluations of the nephroprotective properties of ginger (Zingiber officinale), Cinnamomum verum and Nigella sativa in STZ induced diabetic rats. Int J Biology Pharm Allied Sci. 2017;6(6):1195–209.
Irshad F, Munawar S, Rasheed A. Effects Of Ginger Extract On Glomerular Mesangial Matrix Of Kidneys In Alloxan Induced Diabetic Nephropathy Of Albino Rats. J Bahria Univ Med Dent Coll. 2018;8(2):87–91.
Abd Elwahab AH, Ali FI. Mitigation of alloxane-induced renal damage by zingiber officinale (ginger) root in rats: an impact on oxidative stress, inflammatory cytokines and tissue damage. Al-Azhar Assiut Med J. 2015;13(1):153–62.
Hassan DR. Antioxidative and Kidney Protective Effects of Ginger (Zingiber Officinale) in Hyperglycemic Male Rats. Egypt J Nutr. 2017;32(2):61–87.
Jiyil M, Luka C, Mafuyai C, Pamela N. Effect of Aqueous Extract of Zingiber officinale (Ginger) on Some Biochemical Parameters in Streptozotocin-induced Diabetes Rats. Asian J Res Med Pharm Sci. 2019;8(1–2):1–8.
Al-Qudah MM, Al-Ramamneh MA, Al-Abbadi A, Al-Salt J. The Histological Effect of Aqueous Ginger Extract on Kidneys and Lungs of Diabetic Rats. Int J Biol. 2018;10(4):23–8.
Kumari P, Choudhary SK, Ghosh A, Kumar A, Ali M, Kumar R. Comparative nephroprotective effect of cinnamomum cassia and zingiber officinale on diabetic mice. World J Pharm Res. 2020;9(3):1191–205.
Almatroodi SA, Alnuqaydan AM, Babiker AY, Almogbel MA, Khan AA, Husain Rahmani A. 6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation. Pharmaceutics. 2021;13(3):317.
Payami S-A, Babaahmadi-Rezaei H, Ghaffari M-A, Mansouri E, Mohammadzadeh G. Hydroalcoholic Extract of Zingiber officinale Improves STZ-Induced Diabetic Nephropathy in Rats by Reduction of NF-κB Activation. Jundishapur J Natural Pharmaceut Prod. 2019;14(2):1–6.
AM T, EM T, Abdelkareem E, Azouz T. Evaluation of pharmacodynamics of ginger (Zingiber officinale) compared with gliclazide in diabetic rats. Syst Reviews Pharm. 2020;11(11):970–4.
Irshad F, Ali U, Saleem S. Effects of ginger solvent in alloxan induced diabetic nephropathy in rats. Pak J Med Health Sci. 2018;12(2):768–71.
CS MJ. P, S V: Anti diabetic, hypolipidemic, and histopathological analysis of zingerone in streptozotocin-induced diabetic rats. Asian J Pharm Clin Res. 2016;9(3).
thomson M, Qattan kkA, Divya JS, Ali M. ameliorative action of garlic (Allium sativum) and ginger (zingiber officinale) on biomarkers of diabetes and diabetic nephropathy in rats: comparison to aspirin. Int J Pharmacol. 2013;9(8):501–12.
Al-Qattan KK, Thomson M, Alnaqeeb M, Ali M. Garlic and Ginger Attenuate Structural Nephropathy Progression in Diabetic Rats. In. 2007;21:62–71.
Yassin S, Mahran KMA, El-Baky AAA, Soliman AM. Modulatory Effects (Anti-diabetic and Anti-oxidant) of Ginger, Garlic, and their Mix on StreptozotocinNicotinamide Induced Diabetic Rats. J Innovative Sci Inform Serv Netw. 2019;16(3):3163–79.
Ghudhaib KK. Evaluation of Ginger Rhizomes Extract Effect on Glucose Level, Lipid Profile and Liver Function in Induced Alloxan Diabetic Mice. Res J Pharm Biol Chem Sci. 2018;9(3):435–41.
Al Malki WH, Abdel-Raheem IT, Dawoud MZ, Abdou RF. 6-shogaol protects against diabetic nephropathy and cardiomyopathy via modulation of oxidative stress/NF-κB pathway. Pak J Pharm Sci. 2018;31(5):2109–17.
Deng X, Zhang S, Wu J, Sun X, Shen Z, Dong J, Huang J. Promotion of mitochondrial biogenesis via activation of AMPK-PGC1ɑ signaling pathway by Ginger (Zingiber officinale Roscoe) extract, and its major active component 6‐Gingerol. J Food Sci. 2019;84(8):2101–11.
Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1α interaction. Nature. 2003;423(6939):550–5.
Mozaffari-Khosravi H, Talaei B, Jalali B-A, Najarzadeh A, Mozayan MR. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2014;22(1):9–16.
Khandouzi N, Shidfar F, Rajab A, Rahideh T, Hosseini P, Taheri MM. The effects of ginger on fasting blood sugar, hemoglobin A1c, apolipoprotein B, apolipoprotein AI and malondialdehyde in type 2 diabetic patients. Iran J Pharm Res. 2015;14(1):131.
Li Y, Tran VH, Duke CC, Roufogalis BD. Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes. Planta Med. 2012;78(14):1549–55.
Son MJ, Miura Y, Yagasaki K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology. 2015;67(4):641–52.
Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann N Y Acad Sci. 2017;1398(1):83–98.
Jitraknatee J, Ruengorn C, Nochaiwong S. Prevalence and risk factors of chronic kidney disease among type 2 diabetes patients: a cross-sectional study in primary care practice. Sci Rep. 2020;10(1):1–10.
Kawanami D, Matoba K, Utsunomiya K. Dyslipidemia in diabetic nephropathy. Ren Replace Therapy. 2016;2(1):1–9.
Zhu J, Chen H, Song Z, Wang X, Sun Z. Effects of ginger (Zingiber officinale Roscoe) on type 2 diabetes mellitus and components of the metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Evid-based Complementary Alternative Med. 2018;2018:5692962.
Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediat Inflamm. 2012;2012:1–12.
Vasavada N, Agarwal R. Role of oxidative stress in diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12(2):146–54.
Hamed MA, Ali SA, Saba El-Rigal N. Therapeutic potential of ginger against renal injury induced by carbon tetrachloride in rats. Sci World J. 2012;2012:840421.
Lee T-Y, Lee K-C, Chen S-Y, Chang H-H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382(1):134–9.
Saraswat M, Suryanarayana P, Reddy PY, Patil MA, Balakrishna N, Reddy GB. Antiglycating potential of Zingiber officinalis and delay of diabetic cataract in rats. Mol Vis. 2010;16:1525.
Mahmoodnia L, Aghadavod E, Beigrezaei S, Rafieian-Kopaei M. An update on diabetic kidney disease, oxidative stress and antioxidant agents. J Ren injury Prev. 2017;6(2):153.
Zhu Y, Zhao Y, Wang P, Ahmedna M, Sang S. Bioactive ginger constituents alleviate protein glycation by trapping methylglyoxal. Chem Res Toxicol. 2015;28(9):1842–9.
Vanholder R, Gryp T, Glorieux G. Urea and chronic kidney disease: the comeback of the century?(in uraemia research). Nephrol Dialysis Transplantation. 2018;33(1):4–12.
Hussein MAA. The effect of ginger (Zingibar officinale) aqueous extract on some biochemical parameters and kidney function in male mice. Sc Kufa Med J. 2012;15(1):273–8.
Elsaadany MA, AlTwejry HM, Zabran RA, AlShuraim SA, AlShaia WaA, Abuzaid OI, AlBaker WI. Antihyperglycemic Effect of Fenugreek and Ginger in Patients with Type 2 Diabetes: A Double-Blind, Placebo-controlled Study. Curr Nutr Food Sci. 2022;18(2):231–7.
Acknowledgements
The authors thank Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran.
Financial support
This study recived no fund.
Author information
Authors and Affiliations
Contributions
All aouthors (PV, MZ, HR and ZG) had equal contribution in drafting, searching, analysis and reviewing the manuscript. All the author read and confirmed the manuscript and ZG accepted corresponding the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicabl.
Consent for publication
Not applicabl.
Competing interests
The authors didn’t report conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Veisi, P., Zarezade, M., Rostamkhani, H. et al. Renoprotective effects of the ginger (Zingiber officinale) on Diabetic kidney disease, current knowledge and future direction: a systematic review of animal studies. BMC Complement Med Ther 22, 291 (2022). https://doi.org/10.1186/s12906-022-03768-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03768-x