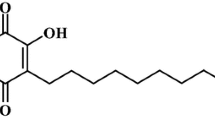
Abstract
Background
Bersama abyssinica is a common herb in Africa, with diverse medical uses in different areas. The plant is well-known in Tanzania for treating respiratory disorders such as TB, tonsillitis, bronchitis, and asthma, and it has lately been utilized to treat COVID-19 symptoms. Water extract of leaf and stem bark has been registered as an herbal medication known as 'Coviba Dawa' in Tanzania for the relief of bacterial respiratory infections. The extracts, however, have not been scientifically tested for their anti-viral activities. The aim of this work was to test for the cytotoxicity and antiviral effects of bioactive ingredients from B. abyssinica extracts against the Delta variant of the SARS-CoV-2 coronavirus.
Methods
B. abyssinica leaves and stem bark were dried under shade in room temperature and then pulverized to obtain small pieces before soaking into different solvents. One hundred grams of each, leaves and stem bark, were extracted in petroleum ether, dichloromethane, ethyl acetate and methanol. Water extract was obtained by decoction of stem bark and leaves into water. Phenols, flavonoids, tannins, and antioxidants were confirmed as components of the extracts. Analysis of polar extracts of bark stem bark and leaves was done. Antiviral screening and cytotoxicity experiments were conducted in a Biosafety Level 3 (BSL-3) Laboratory facility according to International Standard Operating Procedures (SOPs).
Results
By the use of LC–MS/MS analysis, this study confirmed the existence of four phenolic compounds in B. abyssinica water extract; 2,4-di-tert-butylphenol, 4-formyl-2-methoxyphenyl propionate, 7,8-Dihydroxy-4-methylcoumarin, and 2,3, 6-trimethoxyflavone with antioxidant activity. This study showed that, while the water extracts of B. abyssinica had significant antiviral activity against SARS Cov2 virus, it showed no cytotoxicity effect on Vero E6 cells. In particular, the water extract (Coviba dawa) showed 75% while ethylacetate fraction of B. abyssinica leaves showed a 50% in vitro viral inhibition, indicating that these substances may be useful for the development of future anti-viral agents.
Conclusion
We therefore recommend isolation of compounds for further profiling and development with a broader concentration range. We further recommend studies that determine the antiviral activity of extracts of B.abyssinica on other viral pathogens of clinical concern.
Similar content being viewed by others
Introduction
Coronavirus (SARS-CoV-2) causes Coronavirus disease (COVID-19), a serious viral infectious disease of global concern [1]. This disease has greatly impacted global economy, mobility, socio-economy, and health systems [2, 3].
Despite major advances in epidemic preparedness, Africa remains uniquely susceptible to COVID-19 [4]. According to the Infectious Disease Vulnerability Index, 22 out of the 25 African countries are most susceptible to an infectious disease outbreak [4]. This unique vulnerability to Africa needs local creative solutions to control infectious diseases. For example, while lock-down was one of the strategies adopted by most European and American countries, and least applicable in Africa due to several reasons including unavailability of basic services to support ‘Lock Downs’. In addition to allopathic treatment, the use of traditional medicines was one of the most popular strategies adopted in African countries to relieve severe symptoms of COVID-19.
According to recent investigations, traditional remedies have the ability to relieve COVID-19 symptoms and perhaps cure the disease [5]. India, China, and Nepal have reported to produce effective compounds derived from medicinal plants to cure a wide range of viral diseases, including SARS-CoV-2 infection [6,7,8,9]. Medicinal plants have been shown to be potential to prevent symptoms related to COVID-19 though more pharmacological studies are required to prove their activities [10, 11]. A number of approaches varying from social to biological have been adopted to combat COVID-19 in Sub Saharan Africa [12]. One of these promising approaches has been the use of medicinal plants and spice mixtures with unknown active components to alleviate the severe symptoms usually associated with COVID-19 [13].
A wide range of active compounds that treat microbial disorders, including viral infections have been discovered in variety of medicinal plants including Bersama abyssinica [14, 15]. Herbal extracts with antiviral activity have been identified throughout West Africa, particularly in Benin and the Ivory Coast [16]. Despite the wide use of natural medicinal herbs to treat a wide range of diseases in Africa, only a few scientific studies have objectively established and validated the effectiveness of the active ingredients in these plants against the broad spectrum of infectious agents endemic to Africa [17].
Bersama abyssinica is among the well-studied plants in Africa, with diverse antimicrobial activities against bacteria and viruses [12, 13]. Recent study by Sinan et al. 2020 has revealed B. abyssinica to possess several active secondary metabolites with antioxidant, anti-respiratory and antimicrobial activities [18]. A previous study on local knowledge from southern Tanzania on the herbs for medicinal purposes found widespread usage of B. abyssinica for COVID-19 treatment [12]. Many other investigations have shown that the claimed bioactivity of phenolic compounds and gallic from B. abyssinica against viral infections, including COVID-19, is due to interference with viral RNA transcription and protein biosynthesis processes [19,20,21]. Despite the reported effectiveness and use of B. abyssinica against abroad range of pathogenic microbes, there is an apparent paucity of data specifically on its cytotoxic and antiviral effects against COVID-19. Therefore, this study was designed to evaluate the phytochemical, in-vitro cytotoxicity of B. abyssinica stem and leaf extracts on host cells and their antiviral activity against -SARS- CoV-2 virus.
Material and methods
All methods were carried in accordance with relevant guidelines and regulations.
Study site and collection of plant materials
Bersama abyssinica plant materials were obtained during the dry season of 2021 in the Isongole area, at a river line forest patch in the Rungwe District, Mbeya region (9o34′60.9 s 33o62′84 e). B. abyssinica plant materials were collected through collection permit # FMM 4052 and the plant was identified by Dr. Ester Mvungi from the University of Dar es salaam with the Voucher specimen number: ND.Zekeya Nos.01 which was deposited in the herbarium at the University of Dar es salaam. According to national (Tanzania) and international (IUCN) regulations and standards, the plant is of Least conservation concern. However, only aerial parts; leaves and stem bark were collected and dried in the shade at room temperature before being crushed into small pieces and soaked in various solvents. Extraction and phytochemical analysis were carried out at the Institute of Traditional Medicine of Muhimbili Institute of Health and Allied Sciences and the Government Chemist Laboratory Authority. Cytotoxicity and antiviral assays of the plant extracts were conducted at Basel University, Switzerland.
Extraction chemicals and materials
Absolute Methanol (Fluka Chemie GmbH, Zwijndrecht, NL), Dimethyl sulfoxide (DMSO)(RFCL Limited, Hayana, India), Dichololomethane, ethyl acetate and Methanol (Loba Chemie Pvt Ltd, Mumbai, India), Ferric Chloride (FeCl3), Ammonium hydroxide (NH4OH), Sulphuric acid (H2SO4) and 2–2-Diphenyl-1-picrylhydrazyl (DPPH) were used as extraction chemicals and for the analysis.
Preparation of plant materials and extraction
The plant components were chosen based on their recognized efficacy as components of the Coviba Dawa®, a herbal preparation registered in Tanzania. Furthermore, for conservation purposes, no roots were harvested for this investigation. Separately, the leaves and stem bark were air dried in the shade before being mashed into tiny particles with an electric blender (WESTPOINT M012) as described by Krakowska-Sieprawska et al., 2022 [22]. Extraction of active compounds from leaves and stem bark was conducted in accordance with the method described by Ong Es et al., 2006 [23] with minor modifications as per method described by Zekeya et al.,, 2022 [14] where the leaves and stem bark were then extracted separately in 1000 ml of petroleum ether, dichloromethane, ethyl acetate, and ethanol each, for 48 h twice. The extracts were filtered through muslin cloth on a plug of glass wool in a glass column, and solvents were evaporated in vacuum using a rotary evaporator. Water extracts were prepared by boiling 100 g of stem bark in 1L of water for 10 min and infusing 50 g of leaves in the stem bark decoction. Following that, the concoction was filtered with muslin cloth and lyophilized to yield dry extract. Before further usage, all extracts were refrigerated at 4 °C.
Determination of bioactive metabolites
The determination of active metabolites from extracts was performed according to the method described by Sinan et al. 2020 and John et al., 2014 [18, 24].
Determination of phenol
Two ml of Ferric Chloride (FeCl3) solution were added to the 2 ml of 100 mg/ml of each extract and fraction, the appearance of deep bluish-green solution indicated the presence of phenolic compounds.
Determination of flavonoid
The presence of flavonoid was determined by addition of 5 ml of dilute NH4OH into 2 ml of 100 mg/ml of extracts followed by addition of few drops of concentrated Sulphuric acid (H2SO4). Thereafter, a yellow coloration indicated the presence of flavonoid compounds.
Test for tannin
To test for tannin, 100 mg of each extract/fraction was boiled in 2 ml of water in a test tube and then filtered, followed by the addition of a few drops of 0.1% FeCl3 solution. The presence of tannin compounds was confirmed by the appearance of a brownish green, blue black color.
Determination of saponin
In a test tube, 100 mg of each extract/fraction was dissolved in 2 ml of distilled water and warmed before being violently shaken. The presence of saponin compounds was indicated by the production of froth lasting at least a minute.
Determination for antioxidant
One hundred milligram grams (mg) of each sample was dissolved in 1 ml of extractor solvents, filtered, and divided equally between two test tubes. The mixture was agitated and left to stand for 1 min before adding 0.5 ml of pre-prepared 0.1 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) in one of the test tubes while DPPH was not added to the second test tube which was set as the control. The presence of antioxidant chemicals in the extract was shown by the production of discoloration in comparison to the control.
LC–MS/MS analysis of water extract of bark stems bark and leaves
The LC–MS/MS analysis of water extract was performed according to method described by John et al. 2014 [24]. The Q-orbitrap-Ultra High Performance (Thermo Fisher Scientific) was used for LC–MS/MS analysis of polar extract as per method described by Tyagi and Agarwal, 2017 and Pucot et al. 2021 [25, 26]. The extract was re-dried using Rotavap under reducing pressure with Nitrogen gas flowing at 15psi at 45 °C, and the Liquid Chromatography was eluted by mobile phases of 0.1% formic acid in water followed by 0.1% formic acid in Acetonitrile. The column conditions were 37 °C and 1.9µ of oven temperature and particle size, respectively. The linked MS was scanned in the 150–2000 m/z range with a resolution of 140,000 and an AGC Target1e6. The maximum IT setting was 200 ms with ionization mode (HESI) collision Energy of 45v.
Determination of antiviral and cytotoxicity activity
All antiviral screening and cytotoxicity experiments were conducted at Basel University in accordance with the method described by Klimkait et al. 1998 [27 and as per WHO Standard operating procedures (SOPs) for handling biohazardous specimens. Coronavirus SARS-CoV-2 –Delta B1 isolate was donated by Basel University.
All infections with live SARS-CoV-2 were strictly performed in a BSL-3 facility of the Basel University, Department of Biomedicine -Petersplatz in Molecular Virology Laboratory in accordance with the WHO and Federal Government of Switzerland (BAG) Laboratory Biosafety Guidance for working with SARS-CoV-2 with permit #A202850/3. The cells used are HeLa-based cells, which contain an LTR-driven lacZ reporter gene, termed as SX-R5 cells. The virus was subsequently grown in Vero E6 cells maintained at Basel University.
Antiviral activity
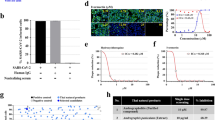
Antiviral activity was performed according to the method described by Klimkait et al. 1998 [27], with some minor modifications. Extracts/fractions were pre-diluted in a deep-well plate according to the dilution scheme in a way that afterwards the addition of a volume of 50µL would provide the final test concentration on the cells. Remdesivir (RDV) was included as an established and validated activity control. After extract/fraction addition, cultures were transferred to the BSL-3 facility. After about 30 min of preincubation of cells and extract, 100 pfu of the DELTA strain (BS-01) of SARS-CoV-2 virus were added to each culture well. Subsequently, after an adsorption period of 15–30 min, every well was overlayed with low-melting agarose according to the corresponding SOP. Cultures were incubated at 37 °C to allow virus-induced plaques to form. Because cytopathic changes (CPE) develop with time of incubation, the best time of harvest was established by microscopic inspection before the fixative paraformaldehyde (PFA) was added. Quantitative plaque formation was used to demonstrate viral replication in the infectivity range (number of plaques = around 100/well). Plaque reduction at a particular drug concentration was used to assess inhibitory potency. The RDV IC50 was at 2.5 µM, which corresponded to the reported activity. The plate on the right is a duplicate plate containing the same component concentrations but no virus. The fixed and stained culture plate is shown on the left plate (Fig. 1). Small white specks were used to represent viral plaques. Compound dilutions were performed from top to bottom, and red lines represent the corresponding compound concentration of the 50% -inhibition of plaque formation (IC50).
Cytotoxicity
The cytotoxicity assay was conducted in accordance with the method described by Klimkait et al. 1998 where all extracts and fractions (named compounds) were pre-diluted in DMSO (analytical grade) (Merck KGaA, Darmstadt, Germany) to obtain stock concentrations of 20 mg/mL. Further dilutions were done in culture medium (DMEM/2%FBS) at ratio of 1:3 until the final extract/fraction concentration as indicated. Cells were pre-seeded on day-1 as detailed in Fig. 1 to allow adherence to the culture plate. Extract/ fraction dilution and dispensing as described by Klimkait et al. 1998 (Fig. 2) to obtain serial dilution of each extract/fraction. This was to cover the entire anticipated biological activity range. DMSO concentrations on the cells were always below 0.5% final concentration to ensure full cell viability. A cell viability plate, using identical extract/fraction concentrations and cell count inhibiting coronavirus delta variant which was included for each extract/fraction as control. The cytotoxicity was also assessed for the extract exposure for 48 h.
Result
The methanolic and water extracts showed the presence of diverse secondary metabolites (Table 1). However, B. abyssinica extracts and fractions possessed high amount of phenolic compounds in both leaves and stem bark fractions of ethyl acetate, methanol and water (Table 1). The results revealed the presence of tannins in stem bark extracts of petroleum ether, dichloromethane, ethanol and methanol whereas non of tannin was revealed in leaves. Flavonoids were also revealed in leaf and stem bark water extracts and fractions of ethyl acetate and methanol. Saponin was shown in petroleum stem bark extract, both leaves and stem bark of methanol and water. However, all fractions and extractsshowed positive antioxidant activity (Table 1).
The phytochemical analysis of water extracts by LC–MS/MS analysis revealed the presence of active compounds ranging from different phytochemical groups including phenols, coumarin and flavonoids. Compounds namely; 2,4-di-tert-butylphenol, 4-formyl-2-methoxyphenyl propionate 7,8-Dihydroxy-4-methylcoumarin and 2,3, 6-trimethoxyflavone were identified by this study (Table 2).
The antiviral activity screening revealed that B. abyssinica has active metabolites with a high potential for inhibiting coronavirus, including the Delta variant, which showed higher virulence than its predecessor variants. All fractions demonstrated anti-SARS-CoV-2 (Delta) activity in all concentrations except B. abyssinica dichloromethane stembark fraction (P3) and B. abyssinica dichloromethane leaf fraction (B2) where the later showed plaque reduction only at the highest concentration of 50 µg/mL, B. abyssinica methanolic stembark fraction and B. abyssinica methanolic leaf fraction (B1 and P4) exhibited moderate inhibition activity but in all concentrations and the inhibition was dose dependant, the highest dose exhibited high antiviral activity against delta variant.On other hand, P1 and P3 showed plaque reduction at lower dose of 16 µg/mL with exception of B. abyssinica petroleum ether stem bark extract (P2) and B. abyssinica petroleum ether leaf extract (P5) which did not show any viral inhibition. However, B. abyssinica water stem bark and leaf extract (B3) showed high antiviral activity reaching 75% and 50% viral inhibition at Concentration of 50 µg/mL and 16.8 µg/mL and no activity at 5 µg/mL. The activity of extracts and fraction were dose dependant and although most extracts exhibited activity at effective at concentration of 16 µg/mL (Fig. 1).
The water extract (B3) exhibited the highest inhibitory activity against Delta B1 by causing 75% viral death with no cytotoxicity effect on host cells at both 16 µg/mL and 50 µg/mL concentration. The methanolic leaf fraction (P4) also showed activity against SARS CoV -2, where 75% inhibitory activity was observed at concentration of 16 µg/mL and lowered to 50% at 50 µg/mL and to 25% at 5 µg/mL with no cytotoxicity effect on host cells in all. However, the slight cytotoxicity effect on host cells was observed in ethylacetate stem bark fraction (B4) at a concentration of 50 µg/mL (Fig. 2).
Discussion
Herbs including Bersama abyssinica have for long been used to treat infectious diseases in traditional treatment systems in various African countries [14, 18]. This plant has a diverse set of active metabolites, including antioxidants [28], and others with antiviral properties [29]. B. abyssinica concoction of stembark and leaves which are key components of Coviba Dawa, herbal preparation in use in Tanzania exhibit a high content of phenolic, tannin and flavonoid compounds in the dry water extract of stem bark and leaves. All extracts have shown remarkable antioxidant activity that could be responsible for viral inhibition as revealed by other studies [30]. Several studies revealed the presence of phenolic compounds which could be effective against viruses including SARS-CoV-2 [31, 32]. The phytochemical screening revealed high amount of phenolics in B. abyssinica stem bark and leaves water extracts, which could be associated with high inhibitory activity against SARS-CoV-2 Beta B1 compared to methanolic, ethyl acetate and petroleum ether extract with low phenolic content. The effectiveness of B. abyssinica water extract could be due high phenolic compounds that have been revealed by other studies to possess high antiviral activity [33, 34]. The presence of tannins in stem bark extracts seems to have antiviral activity on coronavirus [35, 36] where similar studies revealed similar activity of tannic acid on SARS-CoV-2 [37]. High amount of flavonoids were also revealed leaves extract of B. abyssinica which has been reported by several studies to have activity against SARS-CoV-2 [38]. This was revealed by high use of citrus fruits during Covid-19 eruption [39]. Saponin was also revealed in petroleum ether and ethyl acetate extracts of leaves which is also reported to have high inhibitory activity against viruses including SARS CoV-2 due to production of soap-like foaming responsible for antimicrobial activity [40, 41].
Generally, water extract exhibited varied types of active metabolites with two phenolics, coumari and flavonoid which could have synergetic activity against SARS-CoV-2. The increased activity of water extract could be attributed to a high concentration of polar molecules, particularly phenolic compounds with high antioxidant and antiviral properties [42]. Again, the presence of coumarin and flavonoids contributed to antiviral activity which havebeen reported by other studies to have high antiviral activity against SARS-CoV-2 [43, 44].
The presence of 2,4-di-tert-butylphenol in water extract could have contributed to activity against SARS-CoV-2, which is reported by other studies [45, 46] and presence of 7,8-Dihydroxy-4-methylcoumarin and 2,3, 6-trimethoxyflavone in the same extract have increased activity against SARS-CoV-2, which was reported to have antioxidant properties [47, 48], enhancing viral inhibition. In addition, 4-formyl-2-methoxyphenyl propionate was also identified in water extract which has various pharmacological uses including anticardiovasular, antioxidant and anti-inflammatory [49] that would have enhanced inhibition in SARS-CoV-2 B1. Other studies revealed that plants and foods with antioxidants are used for treatment of early stages of COVID-19 [50, 51]. It was also revealed that antioxidant combat viral infection through boosting immune system for protection against SARS-CoV-2 [52]. Recent study in Egypt revealed that medicinal plants have possessed high active metabolites for inhibition of Coronavirus [53]. The results from this study are supported by previous works that revealed the antiviral activity of herbal medicines though ant oxidation, anticoagulation and anti-inhibitory activity of natural phenolic compounds [54,55,56]. Several efforts toward discovery of SARS Cov-2 have been investigated though preclinical and clinical trials [57]. The findings showed high in vitro antiviral and, the cytotoxicity of B. abyssinica extracts and fractions that justifies the use of plant for medicinal purpose and could be potential agent for clinical trials in Tanzania.
Conclusion
This study discovered the presence of four active compounds in Bersama abysinica water extract which possessed the novel antiviral activity against SARS-CoV-2 Delta B1 by 75% viral inhibition with no cytotoxicity effect on cells. The ethylacetate fraction of B. abyssinica leaves also showed 50% inhibition of viral activity in vitro, indicating the high potential of these substances as future anti-viral/anti-microbial agents. We therefore recommend isolation of active compounds for further profiling and development with a broader concentration range with twofold dilutions. We further recommend studies that determine the antiviral activity of extracts of all extracts and compounds of B. abyssinica on other viral pathogens of clinical concern.
Availability of data and materials
Supplementary files; 1 B. abbyssinica phytochemical bioassay and 2. B. abyssinica antiviral bioassay, are provided with this submission.
Abbreviations
- MUHAS:
-
Muhimbili University of Health and Allied Sciences
- IUCN:
-
International Union for Conservation of Nature
- SARS-Cov-2:
-
Severe acute respiratory syndrome coronavirus-2
- B1:
-
Bersama abyssinica Methanolic stembark fraction
- P4:
-
Bersama abyssinica Methanolic leaf fraction
- B2:
-
Bersama abyssinica Dichloromethane leaf fraction
- P3:
-
Bersama abyssinica Dichloromethane stembark fraction t
- B3:
-
Bersama abyssinica Water stem bark and leaf extract
- B4:
-
Bersama abyssinica Ethylacetate stembark fraction
- P1:
-
Bersama abyssinica Ethylacetate leaf fraction
- P2:
-
Bersama abyssinica Petroleum ether extract
- P5:
-
Bersama abyssinica Petroleum ether leaf extract
References
Gangal N, Nagle V, Pawar Y, Dasgupta S. Reconsidering traditional medicinal plants to combat COVID-19. AIJR Preprints. 2020;34:1–6.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, y Piontti AP, Mu K, Rossi L, Sun K, Viboud C. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400.
Hadjidemetriou GM, Sasidharan M, Kouyialis G, Parlikad AK. The impact of government measures and human mobility trend on COVID-19 related deaths in the UK. Transp Res Interdiscip Perspect. 2020;1(6):100167.
Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155:104743 Ayebare RR, Flick R, Okware S, Bodo B, Lamorde M. Adoption of COVID-19 triage strategies for low-income settings. The Lancet. Respiratory medicine. 2020 ;8(4):e22.
Lim XY, Teh BP, Tan TY. Medicinal plants in COVID-19: potential and limitations. Front Pharmacol. 2021;12:355.
Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, Parveen R, Ahmad M. Indian medicinal plants and formulations and their potential against COVID-19–preclinical and clinical research. Front Pharmacol. 2021;11:2470.
Khadka D, Dhamala MK, Li F, Aryal PC, Magar PR, Bhatta S, Thakur MS, Basnet A, Cui D, Shi S. The use of medicinal plants to prevent COVID-19 in Nepal. J Ethnobiol Ethnomed. 2021;17(1):1–7.
Tahir UI Qamar M, Alqahtani SM, Alamri MA, Chen LL. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020;10(4):313–9.
Vimalanathan S, Ignacimuthu S, Hudson JB. Medicinal plants of Tamil Nadu (Southern India) are a rich source of antiviral activities. Pharm Biol. 2009;47(5):422–9.
Aanouz I, Belhassan A, El-Khatabi K, Lakhlifi T, El-Ldrissi M, Bouachrine M. Moroccan Medicinal plants as inhibitors against SARS-CoV-2 main protease: Computational investigations. J Biomol Struct Dyn. 2021;39(8):2971–9.
Gyebi GA, Ogunro OB, Adegunloye AP, Ogunyemi OM, Afolabi SO. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CLpro): an in silico screening of alkaloids and terpenoids from African medicinal plants. J Biomol Struct Dyn. 2021;39(9):3396–408.
Amaechi UA, Sodipo BO, Nnaji CA, Owoyemi A, Omitiran K, Okedo-Alex IN, Eboreime E, Ajumobi O. Social approaches to COVID-19 pandemic response: effectiveness and practicality in sub-Saharan Africa. Pan Afr Med J. 2020;37(Suppl 1):2.
Dandara C, Dzobo K, Chirikure S. COVID-19 pandemic and Africa: from the situation in Zimbabwe to a case for precision herbal medicine. Omics. 2021;25(4):209–12 a journal of integrative biology.
Zekeya N, Ibrahim M, Mamiro B, Ndossi H, Kilonzo M, Mkangara M, Chacha M, Chilongola J, Kideghesho J. Potential of natural phenolic antioxidant compounds from Bersama abyssinica (Meliathacea) for treatment of chronic diseases. Saudi J Biol Sci. 2022;29(6):103273.
Zekeya N, Shahada F, Chacha M. In vitro antibacterial and antifungal activity of Tanzanian Bersama abyssinica. Int J Sci Res. 2014;3(7):1150–3.
Asian J. In-vitro antihelmentic evaluaion of leaf extract of bersama abyssinica (mellanthaceae) on Haemonchus contortus. Asian J Med Pharm Res. 2018;8(2):05–14.
Mbaveng AT, Zhao Q, Kuete V. Harmful and protective effects of phenolic compounds from African medicinal plants In Toxicological Survey of African Medicinal Plants. Elsevier. 2014;577–609.
Sinan KI, Chiavaroli A, Orlando G, Bene K, Zengin G, Cziáky Z, Jekő J, Fawzi Mahomoodally M, Picot-Allain MC, Menghini L, Recinella L. Biopotential of bersama abyssinica fresen stem bark extracts: UHPLC profiles, antioxidant, enzyme inhibitory, and antiproliferative propensities. Antioxidants. 2020;9(2):163.
Mansour MA, AboulMagd AM, Abdel-Rahman HM. Quinazoline-Schiff base conjugates: in silico study and ADMET predictions as multi-target inhibitors ofg coronavirus (SARS-CoV-2) proteins. RSC Adv. 2020;10(56):34033–45.
Genwali GR, Acharya PP, Rajbhandari M. Isolation of gallic acid and estimation of total phenolic content in some medicinal plants and their antioxidant activity. Nepal journal of science and technology. 2013;14(1):95–102.
Govea-Salas M, Rivas-Estilla AM, Rodríguez-Herrera R, Lozano-Sepúlveda SA, Aguilar-Gonzalez CN, Zugasti-Cruz A, Salas-Villalobos TB, Morlett-Chávez JA. Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp Ther Med. 2016;11(2):619–24.
Krakowska-Sieprawska A, Kiełbasa A, Rafińska K, Ligor M, Buszewski B. Modern methods of pre-treatment of plant material for the extraction of bioactive compounds. Molecules. 2022;27(3):730.
Ong ES, Cheong JS, Goh D. Pressurized hot water extraction of bioactive or marker compounds in botanicals and medicinal plant materials. J Chromatogr A. 2006;1112(1–2):92–102.
John BI, Sulaiman CT, George SA, Reddy VR. Spectrophotometric estimation of total alkaloids in selected Justicia species. Int J Pharm Pharm Sci. 2014;6(5):647–8.
Tyagi T, Agarwal M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J Pharm phytochemistry. 2017;6(1):195–206.
Pucot JR, Dapar ML, Demayo CG. Qualitative analysis of the antimicrobial, phytochemical and GC-MS profile of the stem ethanolic extract from Anodendron borneense (King and Gamble). J Complement Med Res. 2021;12(2):231–9.
Klimkait T, Stauffer F, Lupo E, Sonderegger-Rubli C. Dissecting the mode of action of various HIV-inhibitor classes in a stable cellular system. Adv Virol. 1998;143(11):2109–31.
Bene K, Camara D, Kanga Y, Zirihi GN. Antiscavenging potential of leaves extracts from Bersama abyssinica Fresen. (Melianthaceae). Int J Biol Chem Sci. 2017;11(6):2962–70.
Rotich W, Sadgrove NJ, Mas-Claret E, Padilla-González GF, Guantai A, Langat MK. IV-1 Reverse Transcriptase Inhibition by Major Compounds in a Kenyan Multi-Herbal Composition (CareVid™): In Vitro and In Silico Contrast. Pharmaceuticals. 2021;14(10):1009.
Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, Garofalo RP. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2011;183(11):1550–60.
Lanevski A, Yao R, Fenstad MH, Biza S, Zusinaite E, Reisberg T, Lysvand H, Løseth K, Landsem VM, Malmring JF, Oksenych V. Potential antiviral options against SARS-CoV-2 infection. Viruses. 2020;12(6):642.
Yang M, Wei J, Huang T, Lei L, Shen C, Lai J, Yang M, Liu L, Yang Y, Liu G, Liu Y. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in cultured Vero cells. Phytotherapy Research. 2021.
Illian DN, Siregar ES, Sumaiyah S, Utomo AR, Nuryawan A, Basyuni M. Potential compounds from several Indonesian plants to prevent SARS-CoV-2 infection: a mini-review of SARS-CoV-2 therapeutic targets. Heliyon. 2021;7(1):e06001.
Kiani AK, Dhuli K, Anpilogov K, Bressan S, Dautaj A, Dundar M, Beccari T, Ergoren MC, Bertelli M. Natural compounds as inhibitors of SARS-CoV-2 endocytosis: A promising approach against COVID-19. Acta Bio Medica: Atenei Parmensis. 2020;91:13.
Tazeen A, Deeba F, Alam A, Ali R, Ishrat R, Ahmed A, Ali S, Parveen S. Virtual screening of potential therapeutic inhibitors against spike, helicase, and polymerase of SARS-CoV-2 (COVID-19). Coronaviruses. 2021;2(1):89–105.
Wang SC, Chou IW, Hung MC. Natural tannins as anti-SARS-CoV-2 compounds. Int J Biol Sci. 2022;18(12):4669.
Haddad M, Gaudreault R, Sasseville G, Nguyen PT, Wiebe H, Van De Ven T, Bourgault S, Mousseau N, Ramassamy C. Molecular interactions of tannic acid with proteins associated with SARS-CoV-2 infectivity. Int J Mol Sci. 2022;23(5):2643.
Al-Karmalawy AA, Farid MM, Mostafa A, Ragheb AY, H. Mahmoud S, Shehata M, Shama NM, GabAllah M, Mostafa-Hedeab G, Marzouk MM. Naturally available flavonoid aglycones as potential antiviral drug candidates against SARS-CoV-2. Molecules. 2021;26(21):6559.
Gogoi N, Chowdhury P, Goswami AK, Das A, Chetia D, Gogoi B. Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease. Mol Diversity. 2021;25(3):1745–59.
Merarchi M, Dudha N, Das BC, Garg M. Natural products and phytochemicals as potential anti-SARS-CoV-2 drugs. Phytother Res. 2021;35(10):5384–96.
Ebob OT, Babiaka SB, Ntie-Kang F. Natural products as potential lead compounds for drug discovery against SARS-CoV-2. Natural Products and Bioprospecting. 2021;11(6):611–28.
Gligorijevic N, Radomirovic M, Nedic O, Stojadinovic M, Khulal U, Stanic-Vucinic D, Cirkovic VT. Molecular mechanisms of possible action of phenolic compounds in COVID-19 protection and prevention. Int J Mol Sci. 2021;22(22):12385.
El-Missiry MA, Fekri A, Kesar LA, Othman AI. Polyphenols are potential nutritional adjuvants for targeting COVID-19. Phytother Res. 2021;35(6):2879–89.
Özdemir M, Köksoy B, Ceyhan D, Sayın K, Erçağ E, Bulut M, Yalçın B. Design and in silico study of the novel coumarin derivatives against SARS-CoV-2 main enzymes. J Biomol Struct Dyn. 2022;40(11):4905–20.
Zhao F, Wang P, Lucardi RD, Su Z, Li S. Natural sources and bioactivities of 2, 4-di-tert-butylphenol and its analogs. Toxins. 2020;12(1):35.
Leila A, Lamjed B, Roudaina B, Najla T, Taamalli A, Jellouli S, Mokhtar Z. Isolation of an antiviral compound from Tunisian olive twig cultivars. Microb Pathog. 2019;128:245–9.
Kabeya LM, Fuzissaki CN, Andrade MF, Azzolini AE, Taleb-Contini SH, Vermelho RB, Lopes JL, Lucisano-Valim YM. 4-methylcoumarin derivatives inhibit human neutrophil oxidative metabolism and elastase activity. J Med Food. 2013;16(8):692–700.
Mohamed AA, Rateb ME, Jasapars M. Potency of antioxidant properties of major secondary metabolite from Stevia rebaudiana in a comparison to synthetic antioxidants. Egyptian J Exp Biol (Botany). 2017;13:151–8.
Gabriele E. Synthesis of new sulfurated derivatives of natural and synthetic systems as multitarget anticancer agents and development of new drug discovery methodologies.2017.PhD Thesis-AIR Unimi
.Qin M, Cao Z, Wen J, Yu Q, Liu C, Wang F, Zhang J, Yang F, Li Y, Fishbein G, Yan S. An Antioxidant Enzyme Therapeutic for COVID‐19. Advanced Materials. 2020 Oct;32(43):2004901.
Lapenna D. Antioxidant therapy in COVID-19: The crucial role of early treatment and antioxidant typology. Clin Infect Dis: An. 2021;73(12):2370–1 (Official Publication of the Infectious Diseases Society of America).
Trujillo-Mayol I, Guerra-Valle M, Casas-Forero N, Sobral MM, Viegas O, Alarcón-Enos J, Ferreira IM, Pinho O. Western dietary pattern antioxidant intakes and oxidative stress: importance during the SARS-CoV-2/COVID-19 pandemic. Adv Nutr. 2021;12(3):670–81.
Abdo N, Moheyeldin O, Shehata MG, El Sohaimy S. Inhibition of COVID-19 RNA-Dependent RNA Polymerase by Natural Bioactive Compounds: Molecular Docking Analysis. Egypt J Chem. 2021;64(4):1989–2001.
.Davella R, Gurrapu S, Mamidala E. Phenolic compounds as promising drug candidates against COVID-19-an integrated molecular docking and dynamics simulation study. Materials Today: Proceedings. 2022;51:522–7.
Rathinavel T, Meganathan B, Kumarasamy S, Ammashi S, Thangaswamy S, Ragunathan Y, Palanisamy S. Potential COVID-19 drug from natural phenolic compounds through in silico virtual screening approach. Biointerface Res Appl Chem. 2020:10161–73.
Olas B. The antioxidant, anti-platelet and anti-coagulant properties of phenolic compounds, associated with modulation of hemostasis and cardiovascular disease, and their possible effect on COVID-19. Nutrients. 2022;14(7):1390.
Bimonte S, Crispo A, Amore A, Celentano E, Cuomo A, Cascella M. Potential antiviral drugs for SARS-Cov-2 treatment: preclinical findings and ongoing clinical research. In vivo. 2020;34(3):1597–602.
Acknowledgements
Authors are thankful to BioInnovate Africa for funding this study. We are also thankful to technicians at MUHAS and Prof. Thomas Klimkait from Basel University is acknowledged for accepting to conduct bioassay activities in his lab.
Funding
This study was funded by Bioinnovate Project through small Grant Evaluation and Validation of African Medicinal Plant: Bersama abyssinica for treatment of COVID-19 “BA CI2017 10.”
Author information
Authors and Affiliations
Contributions
NZ-wrote the proposal, designed the study and was the compiled data, HN and BM participated in extraction process and wrote first draft, AK and MK worked on data analysis and revised the first draft, RC and MM made critical reviews of all versions of the manuscript and advised on study design whereas JK and JC provided administrative support and revised the third draft. However, the final draft was revised by all authors. The author(s) read and approved the final manuscript.
Author’s information
Never Zekeya (PhD), an expert in botany and traditional medicine, developed and commercialized herbal medicine namely, Coviba Dawa for treatment of COVID-19 symptoms.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable in this study.
Consent for publication
Not applicable.
Competing interests
Authors declare that no competing interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zekeya, N., Mamiro, B., Ndossi, H. et al. Screening and evaluation of cytotoxicity and antiviral effects of secondary metabolites from water extracts of Bersama abyssinica against SARS-CoV-2 Delta. BMC Complement Med Ther 22, 280 (2022). https://doi.org/10.1186/s12906-022-03754-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03754-3