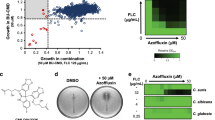
Abstract
Background
The development of multidrug resistance (MDR) associated with the overexpression of the efflux transporters Mdr1 and Cdr1 in Candida species impedes antifungal therapies. The urgent need for novel agents able to inhibit the function of both pumps, led us to evaluate this property in 137 extracts obtained from Argentinian plants.
Methods
The ability of the extracts to reverse efflux pump-mediated MDR was determined with an agar chemosensitization assay using fluconazole (FCZ) resistant Mdr1- and Cdr1-overexpressing clinical isolates of Candida albicans and Candida glabrata as well as Saccharomyces cerevisiae strains selectively expressing Mdr1 (AD/CaMDR1) or Cdr1 (AD/CaCDR1). The resistance-reversing activity of the most potent extracts was further confirmed using a Nile Red accumulation assay.
Results
Fifteen plant extracts overcame the FCZ resistance of Candida albicans 1114, which overexpresses CaMdr1 and CaCdr1, and AD/CaMDR1, with those from Acalypha communis and Solanum atriplicifolium being the most effective showing 4- to 16-fold reversal of resistance at concentrations ≥ 25 µg/mL. Both extracts, and to a lesser extent that from Pterocaulon alopecuroides, also restored FCZ sensitivity in CgCdr1-overexpressing C. glabrata 109 and in AD/CaCDR1 with fold reversal values ranging from 4 to 32 and therefore demonstrating a dual effect against Mdr1 and Cdr1. Both, A. communis and S. atriplicifolium extracts at concentrations ≥ 12.5 and ≥ 25 µg/mL, respectively, increased the intracellular Nile Red accumulation in all yeast strains overexpressing efflux pumps.
Conclusions
The non-toxic and highly active extracts from A. communis and S. atripicifolium, provide promising sources of compounds for potentiating the antifungal effect of FCZ by blocking the efflux function of Mdr1 and Cdr1 transporters.
Similar content being viewed by others
Background
Human health has been threatened by microbial infections since antiquity [1]. Among these, infections caused by fungal pathogens have had an enormous impact on public health [2, 3]. Approximately a billion people suffer from mild superficial fungal infections, while more than 150 million people, in particular immunocompromised patients, have severe and life-]. These serious systemic mycoses account for more than a million deaths per annum [threatening invasive diseases caused by fungi [45], with a 28–61% mortality rate attributed to candidiasis [6,7,8]. Although Candida albicans remains the main opportunistic fungus causing infections in hospitalized patients, other non-albicans species are emerging as prominent pathogens, with C. glabrata being the most common among these [9, 10]. A crucial factor that contributes to the severity of Candida infections is the development of multidrug resistance (MDR), in particul ar to one of the most relevant antifungal class, the triazoles and its first-line agent, fluconazole(FCZ) [11,12,13]. One of the most important MDR mechanisms leading to azole therapy failure is the overexpression of efflux pumps [12, 14]. These membrane proteins can extrude antifungal drugs from fungi, preventing their intracellular accumulation and thereby rendering these cells insensitive to their therapeutic effect [15]. The most common drug efflux transporters expressed by C. albicans are Mdr1 and Cdr1 [14, 16]. The drug/H+ antiporter Mdr1, belongs to the major facilitator superfamily (MFS) of proteins [17]. Mdr1 comprises 12 TMHs linked by hydrophilic loops, with the antiporter motif for the translocation of drugs across the membrane, present in TMH5 [18]. Cdr1 belongs to the pleiotropic drug resistance (PDR) subfamily of the ATP binding cassette (ABC) family of transporters, which hydrolyze ATP to provide the energy for the active drug efflux [19]. As an archetype ABC transporter, Cdr1 consists of two transmembrane domains (TMDs) each with six transmembrane helices (TMH) connected by extra and intracellular loops and two nucleotide binding domains (NBDs) [14, 20]. In C. glabrata, the C. albicans Cdr1 ortholog CgCdr1, is the most prominent efflux pump implicated in azole resistance [21, 22].
Given the important role of drug resistance in the failure of fungal therapy, there is a critical need for alternative antifungals or the development of novel strategies to overcome the MDR phenotype conferred by efflux transporters.
Plants are a great resource for the discovery of compounds with pharmacological potential [23,24,25,26,27,28,29], with some plant extracts or extract-derived components belonging to different chemical families, targeting Mdr1 or Cdr1 [30,31,32]. The immense chemical diversity of secondary metabolites from plants and the low number of species explored to date [33], encourage the scientific community and the pharmaceutical industry to include these products in efflux pump inhibitor (EPI) discovery pipelines.
With the aim of identifying promising reservoirs of novel compounds with the capacity to circumvent MDR by inhibiting C. albicans and C. glabrata efflux transporters Mdr1 and Cdr1, 137 extracts from plants of Argentina were evaluated.
Methods
Materials and reagents
Nile Red and betulin were purchased from Sigma-Aldrich (St. Louis, USA). The antifungal agent FCZ (purity 98.5%) was obtained from Parafarm (Buenos Aires, Argentina). FK506 (tacrolimus, purity ≥ 98%) was purchased from Carbosynth Ltd, UK. Sterile plastic laboratory products were purchased from Greiner Bio-One (Frickenhausen, Germany). All solvents were HPLC grade.
Plant materials and extract preparation
Aerial parts of plants (listed in Table S1, Supporting Information), were collected from December to March in the hills of Córdoba Province, Argentina, between -30.773428 to -31.797760 latitude and—64.109384 to—64.546803 longitude. Plants were chosen with regards to their availability and the accessibility, and scarce or absence of scientific information concerning their pharmacological and/or phytochemical profiles. Powdered material was extracted by maceration with 96% ethanol (3:1) for 48 h. The yield of each extract obtained after exhaustive solvent removal and expressed as percentage weight of plant material, is depicted in Table S1. Extract solutions were prepared in 96% ethanol just prior to use.
Strains and culture conditions
A clinical isolate of C. albicans, overexpressing CaMdr1 and CaCdr1 (strain 1114) [34] and the CgCdr1-overexpressing C. glabrata clinical isolate 109 [35, 36], both obtained from patients at the University Hospital of Universidade Federal de Juiz de Fora, Minas Gerais, were used. The strains were identified by MALDI-TOF mass spectroscopy [35, 36]. Minimum inhibitory concentration (MIC) values, determined with an agar dilution assay as described below, revealed that strain 1114 was 515-times more resistant to FCZ than the sensitive strain C. albicans ATCC 90028 (MICFCZ = 500 and 0.97 µg/mL, respectively) [37], whereas strain 109 displayed 64-fold more resistance to FCZ than C. glabrata ATCC 2001 (MICFCZ = 1,000 and 15.6 µg/mL, respectively) [37]. Both ATCC strains were used in the various assays for comparison purposes. In addition, a set of Saccharomyces cerevisiae strains overexpressing specific efflux pumps [38, 39], were included to relate effects to particular pumps. A sensitive null mutant (AD1-8u−) in which seven MDR efflux pump genes are deleted [MICFCZ = 2.0 µg/mL (agar dilution assay – see below)], and its FCZ-resistant derivatives: the AD/CaMDR1 strain that selectively overexpresses C. albicans Mdr1 (MICFCZ = 31.2 µg/mL by agar dilution assay) and the AD/CaCDR1 mutant strain that overexpresses C. albicans Cdr1 (MICFCZ = 250 µg/mL by agar dilution test) were used. Clinical isolates 1114 and 109 were kindly gifted by Dr. Antonio Ferreira-Pereira from the Universidade Federal do Rio de Janeiro while the S. cerevisiae strains were kindly gifted by Dr. Richard Cannon from the University of Otago. The mean fluorescence intensity (MFI) of Nile Red retained in untreated cells corresponded to 342, 416, 2,280 and 2,900 MFI units for strains 1114, 109, C. albicans ATCC 90028 and C. glabrata ATCC 2001, respectively and to 13,700, 4,730 and 52,900 MFI units for strains AD/CaMDR1, AD/CaCDR1 and AD1-8u−, respectively. The significantly lower MFI values (p < 0.05) for the resistant strains than for the control strains indicated an active efflux of Nile Red from resistant cells.
All yeast strains were stored at -20 °C in Sabouraud dextrose broth (SDB; Difco Laboratories, Detroit, MI, USA) containing 10% glycerol. Prior to use, strains were incubated on Sabouraud agar (Laboratorio Britania, Buenos Aires, Argentina), or in SDB, at 30 °C for 24 h. Cells from Sabouraud agar plates were used to prepare working suspensions in sterile saline.
Antifungal susceptibility assay
To assess if the extracts exerted antifungal activity per se, an agar dilution test was performed as described previously [28], with some modifications. Solid assays were preferred over liquid methods due to the characteristics of some extracts as they interfered with absorbance readings and/or caused precipitation. In short, molten Sabouraud agar medium was added to duplicate extract solutions to reach a final concentration of 200 µg/mL and poured into wells in 12-well microtiter plates. Suspensions of the target yeasts (2 µl of 6 × 105 cells/mL) were seeded on the surface of the solidified agar, and plates were incubated at 30 °C for 48 h. Negative control wells contained 5% final concentration of ethanol (the highest concentration of ethanol needed to completely solubilize the necessary mass of extract to achieve 200 µg/mL that at the same time did not affect yeast viability). The whole panel of extracts was screened using the four Candida strains and those extracts showing reversing properties on Mdr1- and Cdr1-expressing Candida strains, as determined by agar chemosensitization assay (see below), were further assayed using the S. cerevisiae mutants. The extracts showing a complete inhibition of microorganism growth in the primary screen, were then evaluated at decreasing concentrations to establish their MIC values. The MIC was defined as the minimum concentration of sample that completely inhibited the growth of the yeasts, as determined by visual observation.
To determine the MIC values for FCZ, which was also solubilized in ethanol, final concentrations of 125–2,000 and 0.48–125 µg/mL for the pump-expressing and pump-deficient yeasts, respectively, were used.
At least three separate replicates were performed for each assay.
Agar chemosensitization assay
The capability of the panel of extracts to sensitize the resistant clinical Candida strains to the toxic effect of FCZ, was determined using the antifungal susceptibility protocol except that the extracts at 200 µg/mL or at sub-MICs were added in combination with FCZ at 1/4 of the MIC (125 and 250 µg/mL in the assays with C. albicans 1114 and C. glabrata 109, respectively). The extracts showing sensitization activity at the initial maximum concentration were further evaluated against all the target strains, including resistant S. cerevisiae mutants, with serial dilutions to determine their minimum effective concentration (MEC) with FCZ at varying sub-MICs (1/2 to 1/64 of the MIC).
Betulin, is a triterpene isolated from Ligaria cuneifolia that showed potent inhibition of the human ABC transporter, P-glycoprotein (P-gp) at concentrations ≥ 0.39 µM [29]. This property encouraged us to evaluate the capacity of this compound to inhibit Mdr1 and Cdr1. As the concentration of betulin in the 200 µg/mL L. cuneifolia (parasitizing Vachellia sp.) extract was 8.6 µg/mL (19 µM), betulin was tested at the concentration range 6.25–50 µM.
FCZ sensitive Candida spp. and AD1-8u− were simultaneously assayed to determine if there were any synergistic effects other than that involving the transporters (extracts were used at 200 µg/mL and FCZ at 1/4 MIC: 0.24, 3.90 and 0.50 µg/mL in the assays with C. albicans 90028, C. glabrata 2001 and AD1-8u−, respectively). FK506, an inhibitor of Cdr1 [40], was used as a positive control, while ethanol at 5% was used as negative control. Fold reversal (FR) values were calculated as the ratio between the MIC with FCZ alone and the MIC of FCZ in the presence of the extract, from the results obtained in at least three independent experiments.
Nile Red accumulation assay
The capacity of the most active extracts to cause cells to accumulate the fluorescent cell-associated substrate of Mdr1 and Cdr1, Nile Red [41,42,43], was monitored by flow cytometry. Briefly, resistant and sensitive yeasts grown to exponential phase in SDB were centrifuged, washed two times with distilled water and kept on ice for 2 h [34]. Then, cells were incubated in 96-well microtitre plates (1 × 106 cells /well) containing SDB in the absence or presence of different concentrations of the extracts dissolved in DMSO at 30 °C for 2 h with shaking at 200 rpm. Then, Nile Red solution was added to each well (7 µM final concentration, with 2% glucose for assays with Cdr1) and cells were incubated at 30 °C for an additional 1.5 h with shaking at 200 rpm. Cells treated only with extracts, to rule out autofluorescence, or with Nile Red and FK506 (20 and 40 µM) as a positive control, were also run. Negative controls contained 1% DMSO instead of plant extract. Then, the fluorescence intensity of 25,000 cells was analyzed with a Life Technologies Attune-NxT flow cytometer with 96-well autosampler (Thermo Fisher Scientific, USA) using a 488 nm excitation laser and a 574/26 nm emission filter. The Nile Red fluorescence signal was analyzed using the Flowjo software (Tree Star, Inc. Ashland, OR). The results were expressed as fluorescence intensity ratio (FIR) values, calculated as the ratio of the MFI of the Nile Red in cells with the addition of the extract to the MFI of the Nile Red in the negative control cells [44, 45]. The MFI values of the negative control cells were comparable to that of Nile Red alone. Mean values were obtained from at least three independent experiments.
Cytotoxic effect on mammalian cells
To determine the potential cytotoxic effect of the extracts on peripheral blood mononuclear cells (PBMCs), used as a model of normal cells, an MTT assay was performed as previously reported [44, 46]. A hemolysis assay was carried out on red blood cells as described previously [47, 48]. The extracts, dissolved in DMSO, were evaluated at concentrations ranging from 12.5 to 200 µg/mL, DMSO at 1% was used as the negative control. The use of human blood, was approved by the Catholic University of Córdoba Research Ethics Board and all the participants gave written consent.
Results
Antifungal activity of extracts
Prior to evaluating the Mdr1/Cdr1 inhibitory activity of the plant extracts, their potential antifungal properties were determined, as the crude extracts likely contained several compounds with different bioactivities, including possible fungitoxic effects.
The screening of the 137 extracts from the plants described in Table S1 showed that they did not inhibit the growth of C. albicans 1114, C. glabrata 109, C. albicans 90028 or C. glabrata 2001 at the highest tested concentration (200 µg/mL). Extracts that showed anti-Mdr1 or anti-Mdr1/Cdr1 effect (see below) were further evaluated against the S. cerevisiae strains. Only extracts obtained from Acalypha communis, Argemone subfusiformis, Baccharis salicifolia, Lithrea molleoides and Pterocaulon alopecuroides showed antifungal effects with MIC values of 50, 200, 200, 25 and 100 µg/mL, respectively, against both AD/CaMDR1 and AD1-8u−. Therefore, sub-MIC values of these extracts were used in the chemosensitization and Nile Red accumulation assays using AD/CaMDR1 and AD1-8u−.
Chemosensitization of yeast to FCZ
In order to identify plant-derived extracts as sources of efflux pump inhibitors to circumvent MDR in pathogenic yeasts, 137 extracts (Table S1) were investigated. The screen for the ability of these extracts to overcome drug-efflux activity in the resistant C. albicans strain 1114, with increased expression of CaMdr1 and CaCdr1, showed that extracts obtained from A. communis, A. subfusiformis, B. salicifolia, Flourensia campestris, F. oolepis, L. cuneifolia, L. molleoides, Lorentzianthus viscidus, Monnina dictyocarpa, P. alopecuroides, Solanum atriplicifolium, S. palinacanthum and S. salicifolium improved the antifungal activity of FCZ, with fold-reversal (FR) of FCZ resistance values ranging from 2 to 16 (Table 1). All these extracts also decreased the MIC of FCZ for the resistant yeast AD/CaMDR1. While extracts from A. communis, L. cuneifolia, L. viscidus, S. atriplicifolium and S. salicifolium, showed similar level of activity against strain 1114 and AD/CaMDR1, the rest of the extracts gave higher FR and/or lower MEC values.
Extracts from A. communis and S. atriplicifolium displayed the most potent chemosensitizing effect, against both pump-expressing yeasts, with FR values ranging from 4 to 16 and MEC values as low as 25 µg/mL (Table 1). Both extracts, together with that from P. alopecuroides, were also effective in restoring FCZ susceptibility in the AD/CaCDR1 strain and in C. glabrata 109 that overexpresses CgCdr1 reversing the ABC/MDR resistant phenotype up to 32-fold and showing activity even at 25 µg/mL (Table 1). The rest of the extracts had no effect on the FCZ susceptibility of either AD/CaCDR1 strain or C. glabrata 109.
The chemosensitizing efficacy of A. communis and of S. atriplicifolium, extracts was comparable (p > 0.05) to that of the reference compound FK506 (Table 1).
The active extracts did not enhance the antifungal effect of FCZ on the sensitive strains C. albicans ATCC 90028, C. glabrata ATCC 2001 or S. cerevisiae AD1-8u−.
On the other hand, betulin at 50 µM did not restore FCZ activity on any of the Candida spp. or S. cerevisiae strains.
Nile Red accumulation
In view of the potent FCZ resistance reversal activity displayed by A. communis and S. atriplicifolium extracts, the ability of these to restore Nile Red accumulation in yeast cells was evaluated.
Both extracts strongly inhibited Mdr1- and Cdr1-mediated Nile Red efflux in strains C. albicans 1114, AD/CaMDR1, AD/CaCDR1 and C. glabrata 109, showing FIR values ranging from 1.54 to 3.68 at concentrations of 200 µg/mL (Table 2). As expected, the extracts showed no effect on Nile Red efflux in both sensitive Candida strains and in the null mutant of S. cerevisiae (Table 2). A. communis and S. atriplicifolium extracts were still able to enhance Nile Red retention (p < 0.05) at 50 and 25 µg/mL, respectively, in C. albicans 1114; at 50 µg/mL in AD/CaMDR1; at 12.5 and 50 µg/mL, respectively, in AD/CaCDR1; and at 25 and 50 µg/mL, respectively, in C. glabrata 109 (Table 2 and Figs. 1 and 2).
Effects of Acalypha communis extract on Nile Red accumulation in: (A) Candida albicans 1114; (B) Saccharomyces cerevisiae AD/CaMDR1; (C) S. cerevisiae AD/CaCDR1; and (D) C. glabrata 109. The intracellular Nile Red levels increased significantly in the cells treated with different concentrations of the extract. Significant differences from the negative control were determined by using paired one-tailed Student’s t test (***p < 0.001, **p < 0.01, *p < 0.05)
Effects of Solanum atriplicifolium extract on Nile Red accumulation in: (A) Candida albicans 1114; (B) Saccharomyces cerevisiae AD/CaMDR1; (C) S. cerevisiae AD/CaCDR1; and (D) C. glabrata 109. The intracellular Nile Red levels increased significantly in the cells treated with different concentrations of the extract. Significant differences from the negative control were determined by using paired one-tailed Student’s t test (***p < 0.001, **p < 0.01, *p < 0.05)
The Nile Red accumulation in assays performed with A. communis at 200 µg/mL in C. albicans 1114 and AD/CaCDR1 and with the same concentration of S. atriplicifolium in AD/CaCDR1 and C. glabrata 109 was similar (p > 0.05) to that in cells treated with FK506 at 40 µM (FIR values of 2.05 ± 0.06***, 1.80 ± 0.36**, 2.68 ± 0.06** against strains 1114, AD/CaCDR1 and 109, respectively). On the contrary, the accumulation of the dye was significantly lower (p < 0.01) in C. glabrata 109 treated with A. communis extract evaluated at 200 µg/mL and higher (p < 0.01) in C. albicans 1114 treated with S. atriplicifolium extract at the same concentration, respect to yeasts treated with FK506 tested at 40 µM. At the MEC values, both extracts were as active (p > 0.05) as FK506 at 20 µM (FIR = 1.46 ± 0.12*, 1.49 ± 0.12***, 1.58 ± 0.18* against strains 1114, AD/CaCDR1 and 109, respectively) in the strains evaluated, except for A. communis and S. atriplicifolium extracts assayed in AD/CaCDR1 and C. glabrata 109, respectively (p < 0.05).
It is important to note that the plant extracts did not exhibit auto-fluorescence.
Cytotoxic effect on mammalian cells
The toxic effect of the most active extracts on mammalian cells was investigated with MTT and hemolysis assays using PBMC and erythrocytes, respectively. The A. communis extract showed a half-maximal inhibitory concentration (IC50) value of 64.67 ± 0.99 µg/mL against PBMC and only showed hemolysis at > 100 µg/mL while the S. atripicifolium extract exhibited an IC50 value of 119.80 ± 0.95 µg/mL and no hemolysis at 200 µg/mL.
Discussion
In order to discover novel sources of compounds able to counteract the MDR linked to fungal ABC and MFS efflux pumps, a panel of 137 extracts from mostly native plants from Argentina, was screened.
The results indicated that 15 extracts rendered strains C. albicans 1114 and AD/CaMDR1 sensitive to FCZ by inhibiting the efflux linked to Mdr1 (Table 1). Among the effective extracts, those obtained from A. communis and S. atriplicifolium had the greatest ability to chemosensitize strains to FCZ and, together with the P. alopecuroides extract, also increased the antifungal effect of the azole in the Cdr1-overexpressing cells, AD/CaCDR1 and C. glabrata 109 (Table 1). According to these findings, A. communis, P. alopecuroides and S. atriplicifolium extracts behaved as dual inhibitors. The remaining extracts did not reverse the resistance to FCZ in the Cdr1-expressing yeasts, demonstrating a selective activity towards CaMdr1. These results would explain the lower chemosensitization observed for the clinical isolate C. albicans 1114 compared to S. cerevisiae AD/CaMDR1 for A. subfusiformis, B. salicifolia, F. campestris, F. oolepis, L. molleoides, M. dictyocarpa and S. palinacanthum extracts (Table 1), since although the MFS transporter Mdr1 may be targeted in, the ABC pump would be still active in C. albicans 1114, thus decreasing the intracellular concentration of FCZ and therefore evading partially its antifungal action.
The low MECs (25 µg/mL) that caused a reduction in the MICFCZ values of at least fourfold with the clinical isolates of Candida, highlighted A. communis and S. atriplicifolium as promising sources of Mdr1 and Cdr1 inhibitors to augment FCZ activity in the treatment for azole resistant candidiasis. None of the 15 active extracts increased the antifungal effect of FCZ in the sensitive C. albicans, C. glabrata and AD1-8u− strains, supporting the specific interference with the efflux function of the transporters.
L. cuneifolia is a hemiparasitic plant that grows in different hosts [49]. The activity of extracts obtained from this species from each of the host trees, L. molleoides, Vachellia sp. and Condalia buxifolia was similar (Table 1), suggesting that the parasitized plant did not influence the pump inhibitory effect.
Betulin, isolated from L. cuneifolia, has been found to strongly inhibit the human ABC transporter P-gp at micromolar concentrations by blocking the efflux of doxorubicin and consequently restoring the sensitivity of leukemia cells to its cytotoxic effect [29]. However, this triterpene did not reduce FCZ resistance in the target yeasts, even at the high concentration of 50 µM. This result suggests that betulin is a selective inhibitor of P-gp, at least with respect to Mdr1 and Cdr1. Although this result correlated with the lack of activity of L. cuneifolia extract against Cdr1, it is not in accordance with the effect of this plant against Mdr1, suggesting that another metabolite with Mdr1 blocking activity is present. The inability of betulin to inhibit the transport of FCZ mediated by yeast efflux pumps concurs with the observation that diterpenoid esters isolated from Euphorbia spp. were effective at inhibiting P-gp but were not active against C. albicans Mdr1 or Cdr1 [50].
Both A. communis and S. atriplicifolium extracts inhibited Nile Red transport at concentrations ≥ 12.5 µg/mL (Table 2 and Figs. 1 and 2) with FIR values ranging from 1.10 to 3.68 (Table 2). These results confirmed that the enhanced activity of FCZ was due to an increased intracellular accumulation. In these experiments, the Nile Red MFI values were lower for the untreated and treated Candida spp. than for the S. cerevisiae strains (Figs. 1 and 2). At the emission and excitation wavelengths used in this study (488 nm and a 574/26 nm, respectively), Nile Red labels neutral lipids within intracellular lipid droplets [51, 52]. The differences in the MFI values obtained, suggest that S. cerevisiae strains contain more neutral lipids than Candida strains.
Although the significantly lower MFI values (p < 0.05) for untreated AD/CaCDR1 (4,730) and AD/CaMDR1 (13,700) in comparison to AD1-8u− (MFI = 52,900), showed the effectiveness of the pumps at transporting Nile Red, the lower MFI value for AD/CaCDR1 than for AD/CaMDR1 (p < 0.05) indicated that Nile Red is more efficiently effluxed by CaCdr1 than by CaMdr1. Therefore, it is encouraging that A. communis, P. alopecuroides and S. atripicifolium extracts were able to inhibit this proficient ABC-type pump.
The same Nile Red-derived fluorescence in pump-deficient C. albicans, C. glabrata and AD1-8u− cells in the absence or presence of the extracts suggests that the increased FCZ susceptibility in MDR cells is likely achieved by inhibiting Mdr1 and Cdr1.
As far as we are aware, there have been few studies that have screened libraries of plant extracts for MDR reversal activity through interference with yeast transporters [32, 50]. In particular, no information was found in the literature with respect to this property in A. communis and S. atripicifolium extracts. The cycloartane triterpenes 16α-hydroxymollic acid, 15α-hydroxymollic acid, and 7β,16β-dihydroxy-1,23-dideoxyjessic acid have been isolated from A. communis [53], however there is no evidence that any of the compounds are Cdr1 and/or Mdr1 inhibitors. As far as we are aware, no compounds from S. atriplicifolium extracts have been identified. It is important to note that assays were performed with crude plant extracts likely to contain a mixture of compounds. The next step in this research will be to purify the active component(s) from the extracts. These compounds are likely to have a higher pump-inhibitory specific activity.
The A. communis and S. atripicifolium extracts were devoid of toxic effects on mammalian cells. The IC50 values obtained towards PBMC were higher than 20 µg/mL, which is the threshold established by the US National Cancer Institute (NCI) to consider an extract as cytotoxic [26]. In addition, neither extract caused hemolysis of human erythrocytes at concentrations of 100 µg/mL.
Conclusion
The increasing incidence of MDR in Candida species due to the overexpression of efflux pumps, led us to search new sources of compounds with the capacity to circumvent this worldwide problem. We found that 15 extracts, in particular those from A. communis and S. atriplicifolium, were able to reverse the resistance to FCZ by interfering with the efflux function of the MFS transporter Mdr1 and of the ABC pump, Cdr1. In the case of A. communis and S. atriplicifolium extracts, this property was observed at a concentration ≥ 25 µg/mL and decreased the resistance to FCZ up to 32-fold. Likewise, these extracts inhibited the efflux of Nile Red mediated by both Mdr1 and Cdr1. The modulation of efflux was not only observed with S. cerevisiae strains overexpressing Mdr1 or Cdr1 but also in MDR clinical Candida spp. strains, which highlights the therapeutic potential of the extract components. The traditional use of A. communis is as a purgative and for the treatment of skin wounds [27]. Although this study could not explain the above mentioned popular uses, it identifies a new biological activity of this plant species. No popular uses have been reported for S. atriplicifolium. The promising dual efflux pump inhibitory effect of A. communis and S. atriplicifolium extracts together with their low toxicity, highlight these as candidates to obtain active principles that can counteract antifungal resistance in Candida infections.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
References
Abers M, Schroeder S, Goelz L, Sulser A, St. Rose T, Puchalski K, Langland J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement Med Ther. 2021;21(1):124.
Rodrigues ML, Nosanchuk JD. Fungal diseases as neglected pathogens: a wake-up call to public health officials. PLoS Negl Trop Dis. 2020;14(2): e0007964.
Gnat S, Łagowski D, Nowakiewicz A, Dyląg M. A global view on fungal infections in humans and animals: infections caused by dimorphic fungi and dermatophytoses. J Appl Microbiol. 2021;131(6):2688–704.
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi. 2017;3(4):57.
Pathakumari B, Liang G, Liu W. Immune defence to invasive fungal infections: a comprehensive review. Biomed Pharmacother. 2020;130:110550.
Kato H, Yoshimura Y, Suido Y, Shimizu H, Ide K, Sugiyama Y, Matsuno K, Nakajima H. Mortality and risk factor analysis for Candida blood stream infection: a multicenter study. J Infect Chemother. 2019;25(5):341–5.
Colombo AL, Guimarães T, Sukienik T, Pasqualotto AC, Andreotti R, Queiroz-Telles F, Nouér SA, Nucci M. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med. 2014;40(10):1489–98.
Leroy O, Bailly S, Gangneux J-P, Mira J-P, Devos P, Dupont H, Montravers P, Perrigault P-F, Constantin J-M, Guillemot D. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care. 2016;6(1):1–11.
Ricotta EE, Lai YL, Babiker A, Strich JR, Kadri SS, Lionakis MS, Prevots DR, Adjemian J. Invasive candidiasis species distribution and trends, United States, 2009–2017. J Infect Dis. 2021;223(7):1295–302.
Teo JQ-M, Candra SR, Lee SJ-Y, Chia SY-H, Leck H, Tan A-L, Neo H-P, Leow KW-L, Cai Y, Ee RP-L. Candidemia in a major regional tertiary referral hospital–epidemiology, practice patterns and outcomes. Antimicrobial Resistance & Infection Control 2017;6(1):1–11.
Moudgal V, Sobel J. Antifungals to treat Candida albicans. Expert Opin Pharmacother. 2010;11(12):2037–48.
Lee Y, Puumala E, Robbins N, Cowen LE. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev. 2020;121(6):3390–411.
Tan J, Jiang S, Tan L, Shi H, Yang L, Sun Y, Wang X. Antifungal activity of minocycline and azoles against fluconazole-resistant Candida species. Front Microbiol. 2021;12:1185.
Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22(2):291–321.
Pristov K, Ghannoum M. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25(7):792–8.
Holmes AR, Lin Y-H, Niimi K, Lamping E, Keniya M, Niimi M, Tanabe K, Monk BC, Cannon RD. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob Agents Chemother. 2008;52(11):3851–62.
Redhu A. K, Shah AH, Prasad R. MFS transporters of Candida species and their role in clinical drug resistance. FEMS Yeast Res. 2016;16(4):fow043.
Banerjee A, Pata J, Sharma S, Monk BC, Falson P, Prasad R. Directed mutational strategies reveal drug binding and transport by the MDR transporters of Candida albicans. J Fungi. 2021;7(2):68.
Lamping E, Baret PV, Holmes AR, Monk BC, Goffeau A, Cannon RD. Fungal PDR transporters: phylogeny, topology, motifs and function. Fungal Genet Biol. 2010;47(2):127–42.
Prasad R, Balzi E, Banerjee A, Khandelwal NK. All about CDR transporters: Past, present, and future. Yeast. 2019;36(4):223–33.
Kumari S, Kumar M, Gaur NA, Prasad R. Multiple roles of ABC transporters in yeast. Fungal Genet Biol. 2021;150:103550.
Whaley SG, Zhang Q, Caudle KE, Rogers PD. Relative contribution of the ABC transporters Cdr1, Pdh1, and Snq2 to azole resistance in Candida glabrata. Antimicrob Agents Chemother. 2018;62(10):e01070-e1118.
Chiari ME, Joray MB, Ruiz G, Palacios SM, Carpinella MC. Tyrosinase inhibitory activity of native plants from central Argentina: isolation of an active principle from Lithrea molleoides. Food Chem. 2010;120(1):10–4.
Funes Chabán M, Karagianni C, Joray MB, Toumpa D, Sola C, Crespo MI, Palacios SM, Athanassopoulos CM, Carpinella MC. Antibacterial effects of extracts obtained from plants of Argentina: Bioguided isolation of compounds from the anti-infectious medicinal plant Lepechinia meyenii. J Ethnopharmacol. 2019;239:111930.
García Manzano MF, Joray MB, Laiolo J, Palacios SM, Carpinella MC. Cytotoxic activity of germacrane-type sesquiterpene lactones from Dimerostemma aspilioides. J Nat Prod. 2020;83(6):1909–18.
González ML, Joray MB, Laiolo J, Crespo MI, Palacios SM, Ruiz GM, Carpinella MC. Cytotoxic activity of extracts from plants of central Argentina on sensitive and multidrug-resistant leukemia cells: isolation of an active principle from Gaillardia megapotamica. Evid Based Complement Alternat Med 2018;13.
Joray MB, del Rollán MR, Ruiz GM, Palacios SM, Carpinella MC. Antibacterial activity of extracts from plants of central Argentina—isolation of an active principle from Achyrocline satureioides. Planta Med. 2011;77:95–100.
Joray MB, Trucco LD, González ML, Diaz Napal GN, Palacios SM, Bocco JL, Carpinella MC. Antibacterial and cytotoxic activity of compounds isolated from Flourensia oolepis. Evid Based Complement Alternat Med 2015;2015(11.
Laiolo J, Barbieri CL, Joray MB, Lanza PA, Palacios SM, Vera DMA, Carpinella MC. Plant extracts and betulin from Ligaria cuneifolia inhibit P-glycoprotein function in leukemia cells. Food Chem Toxicol. 2021;147:111922.
Tran-Nguyen V-K, Prasad R, Falson P, Boumendjel A. Modulators of the efflux pump Cdr1p of Candida albicans: mechanisms of action and chemical features. Curr Med Chem. 2017;24(30):3242–53.
Singh S, Hans S, Fatima Z, Hameed S. Overcoming fungal multidrug resistance by natural compounds targeting efflux pumps. Front Anti-Infect Drug Discov. 2017;7:1–12.
Kolaczkowski M, Kolaczkowska A, Środa K, Ramalhete C, Michalak K, Mulhovo S, Ferreira MJU. Substrates and modulators of the multidrug transporter Cdr1p of Candida albicans in antifungal extracts of medicinal plants. Mycoses. 2010;53(4):305–10.
Lautié E, Russo O, Ducrot P, Boutin JA. Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol. 2020;11:397.
Pinto ACC, Rocha DA, Moraes DC, Junqueira ML, Ferreira-Pereira A. Candida albicans clinical isolates from a southwest Brazilian tertiary hospital exhibit MFS-mediated azole resistance profile. An Acad Bras Cienc. 2019;91(3):e20180654.
Rocha DAS, Sa LFRd, Pinto ACC, Junqueira MdL, Silva EMd, Borges RM, Ferreira-Pereira A. Characterisation of an ABC transporter of a resistant Candida glabrata clinical isolate. Mem Inst Oswaldo Cruz 2018;113(4):e170484.
Neves-Junior A, Cartágenes-Pinto AC, Rocha DA, Sá LF, Junqueira MdL, Ferreira-Pereira A. Prevalence and fluconazole susceptibility profile of Candida spp. clinical isolates in a Brazilian tertiary hospital in Minas Gerais, Brazil. An Acad Bras Cienc 2015;87(2):1349–59.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antifungal susceptibility testing of yeasts [Internet]. 2nd ed. CLSI supplement M60. Wayne: CLSI; 2020. [https://clsi.org/standards/products/microbiology/documents/m60/]
Holmes AR, Keniya MV, Ivnitski-Steele I, Monk BC, Lamping E, Sklar LA, Cannon RD. The monoamine oxidase A inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS transporter efflux pump activities which reverses the azole resistance of Candida albicans and Candida glabrata clinical isolates. Antimicrob Agents Chemother. 2012;56(3):1508–15.
Lamping E, Monk BC, Niimi K, Holmes AR, Tsao S, Tanabe K, Niimi M, Uehara Y, Cannon RD. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6(7):1150–65.
Tanabe K, Bonus M, Tomiyama S, Miyoshi K, Nagi M, Niimi K, Chindamporn A, Gohlke H, Schmitt L, Cannon RD. FK506 resistance of Saccharomyces cerevisiae Pdr5 and Candida albicans Cdr1 involves mutations in the transmembrane domains and extracellular loops. Antimicrob Agents Chemother. 2018;63(1):e01146-e1218.
Reis de Sá LF, Toledo FT, Gonçalves AC, Sousa BA, dos Santos AA, Brasil PF, Duarte da Silva VA, Tessis AC, Ramos JA, Carvalho MA et al. Synthetic organotellurium compounds sensitize drug-resistant Candida albicans clinical isolates to fluconazole. Antimicrob Agents Chemother 2017;61(1):e01231–16.
Holmes AR, Cardno TS, Strouse JJ, Ivnitski-Steele I, Keniya MV, Lackovic K, Monk BC, Sklar LA, Cannon RD. Targeting efflux pumps to overcome antifungal drug resistance. Future Med Chem. 2016;8(12):1485–501.
Ivnitski-Steele I, Holmes AR, Lamping E, Monk BC, Cannon RD, Sklar LA. Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal Biochem. 2009;394(1):87–91.
Laiolo J, Lanza PA, Parravicini O, Barbieri C, Insuasty D, Cobo J, Vera D, Enriz RD, Carpinella MC. Structure activity relationships and the binding mode of quinolinone-pyrimidine hybrids as reversal agents of multidrug resistance mediated by P-gp. Sci Rep. 2021;11(1):1–18.
Laiolo J, Tomašič T, Vera DMA, González ML, Lanza PA, Gancedo SN, Hodnik Ž, Peterlin Mašič L, Kikelj D, Carpinella MC. Analogues of the lignan pinoresinol as novel lead compounds for P-glycoprotein (P-gp) inhibitors. ACS Med Chem Lett. 2018;9(12):1186–92.
González ML, Vera DMA, Laiolo J, Joray MB, Maccioni M, Palacios SM, Molina G, Lanza PA, Gancedo S, Rumjanek V et al. Mechanism underlying the reversal of drug resistance in P-glycoprotein-expressing leukemia cells by pinoresinol and the study of a derivative. Front Pharmacol 2017;8(205):
Funes Chabán M, Antoniou A, Karagianni C, Toumpa D, Belén Joray M, Bocco JL, Sola C, Athanassopoulos C, Carpinella MC. Synthesis and structure-activity relationships of novel abietane diterpenoids with activity against Staphylococcus aureus. Future Med Chem. 2019;11(24):3109–24.
MB Joray ML González SM Palacios MC Carpinella 2011 Antibacterial activity of the plant-derived compounds 23-methyl-6-O-desmethylauricepyrone and (Z, Z)-5-(trideca-4,7-dienyl)resorcinol and their synergy with antibiotics against methicillin-susceptible and -resistant Staphylococcus aureus J Agric Food Chem 59 21 11534 11542
Ricco MV, Bari ML, Bagnato F, Cornacchioli C, Laguia-Becher M, Spairani LU, Posadaz A, Dobrecky C, Ricco RA, Wagner ML et al. Establishment of callus-cultures of the Argentinean mistletoe, Ligaria cuneifolia (R. et P.) Tiegh (Loranthaceae) and screening of their polyphenolic content. Plant Cell, Tissue and Organ Culture (PCTOC) 2019;138(1):167–80.
Esposito Ml, Nim S, Nothias L-Fl, Gallard J-Fo, Rawal MK, Costa J, Roussi F, Prasad R, Di Pietro A, Paolini J. Evaluation of jatrophane esters from Euphorbia spp. as modulators of Candida albicans multidrug transporters. J Nat Prod 2017;80(2):479–87.
Ramírez-Castrillón M, Jaramillo-Garcia VP, Barros HL, Henriques JAP, Stefani V, Valente P. Nile red incubation time before reading fluorescence greatly influences the yeast neutral lipids quantification. Front Microbiol 2021;12(
Radulovic M, Knittelfelder O, Cristobal-Sarramian A, Kolb D, Wolinski H, Kohlwein SD. The emergence of lipid droplets in yeast: current status and experimental approaches. Curr Genet. 2013;59(4):231–42.
Gutierrez-Lugo M-T, Singh MP, Maiese WM, Timmermann BN. New antimicrobial cycloartane triterpenes from Acalypha communis. J Nat Prod. 2002;65(6):872–5.
Acknowledgements
F. Gil and J. Laiolo acknowledge receipt of a Scholarship from the National Research Council of Argentina (CONICET). M.C. Carpinella is staff member of CONICET.
Funding
This research was funded by the Catholic University of Córdoba, CONICET (PIP- 2021–2023) and FONCyT (PICT 2017–1381, PICT 2019–2019-00721, PICT-2020-SERIEA-01627).
Author information
Authors and Affiliations
Contributions
F.G. and J.L. made the experiments; B.B-P. contributed to the design of the experiments; F.G., J.L., R.D.C. and M.C.C. interpreted the data; R.D.C. contributed S. cerevisiae strains; A.F-P. contributed Candida spp.; M.C.C. and A.F-P. initiated the project; M.C.C received the financial support, design, supervised all experiments, and wrote the manuscript; M.C.C., R.D.C. and A.F-P. revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Permission for the collection of plant samples was obtained from the Secretaría de Ambiente, Gobierno de la Provincia de Córdoba (N° 12289005371220). Voucher specimens (Table S1) were made from each plant and, after being identified and authenticated by the botanist Eng. G. Ruiz, were deposited in the Marcelino Sayago Herbarium of the School of Agricultural Science, Catholic University of Córdoba.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Plants from central Argentina screened for Mdr1 and Cdr1 inhibitory effect. Fig. S1 Flow cytometric determinations of the effect of Acalypha communis extract on Nile Red accumulation in: (A) Candida albicans 1114; (B) Saccharomyces cerevisiae AD/CaMDR1; (C) Saccharomyces cerevisiae AD/CaCDR1; and (D) Candida glabrata 109. Fig. S2 Flow cytometric determinations of the effect of Solanum atriplicifolium extract on Nile Red accumulation in: (A) Candida albicans 1114; (B) Saccharomyces cerevisiae AD/CaMDR1; (C) Saccharomyces cerevisiae AD/CaCDR1; and (D) Candida glabrata 109. Fig. S3 Determination by flow cytometry of the effect of (A) Acalypha communis and (B) Solanum atriplicifolium extracts on Saccharomyces cerevisiae AD1-8u-, Candida albicans ATCC 90028 and Candida glabrata ATCC 2001. Histograms represent cells treated with Nile Red alone in red and cells treated with Nile Red and extracts in blue.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gil, F., Laiolo, J., Bayona-Pacheco, B. et al. Extracts from Argentinian native plants reverse fluconazole resistance in Candida species by inhibiting the efflux transporters Mdr1 and Cdr1. BMC Complement Med Ther 22, 264 (2022). https://doi.org/10.1186/s12906-022-03745-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03745-4