Abstract
Background
To assess the efficacy and safety of oral Guanxinshutong (GXST) capsules in Chinese patients with stable angina pectoris (SAP) in a prospective, multicenter, double-Blind, placebo-controlled, randomized clinical trial (clinicaltrials.gov Identifier: NCT02280850).
Methods
Eligible patients were randomized 1:1 to the GXST or placebo group. Current standard antianginal treatment except for nitrate drugs was continued in both groups, who received an additional 4-week treatment of GXST capsule or placebo. Primary endpoint was the change from baseline in angina attack frequency after the 4-week treatment. Secondary endpoints included the reduction of nitroglycerin dose, score of Seatntle Agina Questionnaire, exercise tolerance test defined as time to onset of chest pain and ST-segment depression at least 1 mm greater than the resting one.
Results
A total of 300 SAP patients from 12 centers in China were enrolled between January 2013 and October 2015, and they were randomly divided into the GXST group and the placebo group (150 patients in each group). Of whom, 287 patients completed the study (143 patients in the GXST group, 144 patients in the placebo group). The baseline characteristics of the two groups were comparable. After 4-week treatment with GXST capsules, the number of angina attacks and the consumption of short-acting nitrates were significantly reduced. In addition, the quality of life of patients were also substantially improved in the GXST group. No significant differences in the time of onset of angina and 1-mm ST segment depression were noted between the two groups. 7 patients (4.1%) in the GXST group and 3 patients (2.1%) in the placebo group reported at least one adverse event, respectively.
Conclusions
GXST capsules are beneficial for the treatment of SAP patients.
Similar content being viewed by others
Background
Stable angina pectoris (SAP) is one of the most common subtypes of coronary heart disease, and it affects approximately 54 million patients worldwide [1, 2]. According to guidelines published by the health authorities of the United States [3], Canada [4], Europe [5] and China [6], the current treatment for SAP relies on the use of anti-ischemic drugs, such as nitrates, β-blockers, calcium channel blockers (CCB), angiotensin converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARB), and is often combined with secondary prevention measures such as lipid lowering and anti-platelet drugs to prevent myocardial infarction and death, reduce the symptoms of ischemic attack and improve the quality of life (QOL) of patients [7, 8]. In addition, patients with symptomatic angina are often treated with both traditional Chinese medicine and western medicine in China.
Traditional Chinese medicine has been used in the treatment of chronic heart disease for more than 2000 years in China [9,10,11]. The components of the traditional herb and Mongolian medicine, Guanxinshutong (GXST) capsules, are Choerospondias axillaris, Salvia miltiorrhiza, Syzygium aromaticum, borneol and concretio silicea bambusae. Animal experiments in rats have shown that GXST capsules can increase coronary blood flow, improve myocardial oxygen supply, and regulate vascular compliance [12, 13]. Moreover, our previous studies have demonstrated that GXST capsules can limit myocardial infarct size through inhibition of inflammatory response and apoptosis in rats [12, 13]. Furthermore, a clinical observational study has shown that GXST capsules are effective and safe for the treatment of SAP patients [14]. However, the efficacy of the drug has not been established by well-designed randomized clinical trials in China.
To our knowledge, this is the first prospective, multicenter, double-blind, placebo-controlled, randomized clinical trial assessing the efficacy and safety of orally administered GXST capsules in SAP patients by comparing the effects of GXST capsules on antianginal effect, exercise tolerance and safety in SAP patients in China .
Methods
Study design
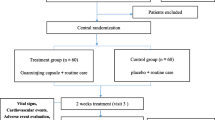
This was a phase IV double-blind, randomized, and placebo-controlled study conducted at 12 centers in China between January 2013 and October 2015. Neither the investigator nor the subject was aware of what treatment the subject received. The disposition of patients is summarized in Fig. 1. The study was designed and conducted in accordance with the requirements of Good Clinical Practice (GCP) and the Declaration of Helsinki. The study has been reviewed and approved by the Ethics Committee of General Hospital of Northern Therter Command (K (2013) 11). Then the study protocol was reviewed and approved by the Institutional Review Board of each medical center involved. All patients provided written informed consent prior to the study.
Enrollment criteria
Patients aged 18 to 75 years old were eligible to participate in the study if they had diagnosis of coronary artery disease (CAD) with SAP, as documented by at least one of the followings: 1) previous diagnosis of myocardial infarction (more than 6 months ago); 2) coronary angiography or computerized tomography coronary angiography showed at least one major coronary branch stenosis more than 50%; 3) underwent coronary revascularization (percutaneous coronary intervention (PCI) or coronary-artery bypass grafting) more than 3 months ago; 4) typical angina symptoms or positive exercise tolerance text (ETT). Major exclusion criteria included acute coronary syndrome, left main CAD regardless of whether revascularization was performed or not, aortic stenosis (moderate to severe), hypertrophic obstructive cardiomyopathy, congestive heart failure, echocardiographic left ventricle ejection fraction less than 40%, uncontrolled severe hypertension, complete left bundle branch block, Wolff-Parkinson-White syndrome, presence of ventricular paced rhythm, as well as liver and renal dysfunction.
Randomization and treatment
Patients were randomly assigned without stratification to receive GXST capsules or placebo in a 1:1 ratio using sealed envelopes. The physicians, patients, evaluators, and statistician were unaware of the random allocation. Treatment in the double-blind phase was continued until disease progression, adverse events (AEs), withdrawal of consent, or primary analysis. There were two times of unblinding during the study. For the first time of unblinding, the plan for the statistical analysis was completed before the database was locked and unblinded, then the second time of unblinding was carried out, and all patients were unblinded. All the patients included in this study received GXST capsule treatment or placebo treatment. In addition to nitrate drug treatment, the standard antianginal treatment was also allowed as a basic treatment, including aspirin, β-blockers, CCB, lipid-lowering statin drugs and ACEI/ARB. All of these treatments will be recorded in patients’ medical records as well as their case report forms (CRFs) in detail.
Each patient was asked to make a total of 3 study visits on Day − 7, 0 and 28 (±2) after randomization and treatment initiation. At the screening visit (Day − 7), demographic data, medical history, and concomitant medications were recorded, and physical examination was performed. Patients meeting the eligibility criteria entered the double-blind portion of the study. At baseline (Day 0), the number of average angina attacks per week were recorded, and the patients in each group underwent an ETT (modified Bruce Protocol) before being randomized to receive either 3 capsules of 0.3 g GXST or placebo three times daily for 4 weeks. The placebo was composed of Corn Starch, Silica, Caramel color, Sunset Yellow, Mixed Powder for GXST capsule and ethanol, which were then filled into the capsules. The appearance and smell of placebo are the same as that of GXST capsules. At the end of 4-week double-blind treatment, patients were instructed to return for clinic visit and all assessments including the number of average angina attacks per week, physical examination, resting electrocardiogram and ETT were repeated.
A nitroglycerin tablet (0.5 mg per tablet, manufactured by Beijing Yimin Pharmaceutical Group Co., Ltd.) is allowed if the patient has an attack of angina, and the patient will be asked to record the doses and times of administration in detail, and these informations will be collected by the investigator at the next follow-up visit. If the symptoms of angina are not relieved after 3 doses of nitroglycerin, the patient will be asked to go to the hospital for further treatment. If the patient has other underlying diseases, the concomitant drugs considered necessary for the treatment of these diseases are allowed to be used during the study period, and these drugs should be recorded in the patients’ medical records and CRFs in detail. However, no other Chinese herbal medicines or Chinese patent medicines with similar effects like GXST capsules are allowed to be used during the study period.
Endpoints and definitions
The primary outcome in this study is the change from baseline in angina attack frequency at 4 weeks of treatment. The primary endpoint will be measured 1 day before the inclusion and the 1st day after 4 weeks post-inclusion. The secondary outcomes include the following three items: 1) reduction of nitroglycerin dose; 2) score of Seatntle Agina Questionnaire (SAQ) [15, 16]; 3) positive ETT was defined as the time of onset of chest pain, and ST-segment depression at least 1 mm greater than the resting one, with or without limiting angina. For the overall efficacy assessment, the number of weekly anginal attacks were categorized according to the guideline for clinical trial of cardiovascular drugs in China [17]. A > 80% reduction in the number of angina attacks and nitroglycerin consumption after treatment was defined as highly effective, a 50–80% reduction was defined as effective, and a < 50% reduction was defined as no effically. QOL was assessed at baseline and 4 weeks after the procedure with the SAQ. The SAQ is a disease-specific instrument that quantifies five dimensions of QOL that are important to patients with angina and CAD, including physical limitations due to CAD, angina stability, angina frequency, satisfaction with treatment, and disease perception. All SAQ domains are scaled from 0 to 100 points, with higher scores indicating better QOL. All patients given double-blind drug with documented follow-up were included in the safety evaluation. Patients were observed and questioned at each visit about the occurrence of adverse events.
Statistical analysis
SPSS version 19.0 was used for statistical analysis. Results were presented as mean ± standard deviation (SD). All analyses were done by intention to treat. Missing data were not replaced. Outcomes data for the primary and secondary endpoints were compared as binary proportions. Categorical variables were compared using the Chi-squared test or Fisher’s exact test, and continuous data were compared using the t test or one-way analysis of variance. P < 0.05 was considered as the level of significance.
Results
Patient characteristics and baseline comparison
Signed informed consent was given by 300 SAP patients from 12 centers in China between January 2013 and October 2015. Of the 300 randomized patients there were 150 patients in the GXST group and 150 patients in the placebo group; 287 patients completed the study (143 patients in the GXST group, 144 patients in the placebo group) (Fig. 2). Before the primary endpoint analysis, 1 patient in the GXST group discontinued the study due to withdrawal of consent; 6 patients in the GXST group and 4 patients in the placebo group were lost to follow-up; 2 patients in the placebo group dropped out because of the onset of palpitation.
Baseline characteristics were comparable in age, gender, median body mass index, and smoking status between the two groups. At the time of enrolment, all the patients in the two groups were taking one or more types of standard anti-angina therapies such as ACEI/ARB, β-blocker, CCB, aspirin/anti-platelet drugs and lipid-lowering drugs. Detailed data of patient characteristics, medications and medical history were summarized in Table 1.
Primary endpoint
The number of anginal attacks at week 0 was defined as the baseline value. GXST capsules significantly decreased the number of attacks in comparison with placebo at week 4 (Table 2). The average number was 9.4 per week at the beginning of the study, but was significantly reduced to 2.5 attacks per week after the GXST therapy (P = 0.02). However, there was no difference between the baseline and treatment in the placebo group (P = 0.44). In the GXST group, GXST capsules were highly effective in 25.2% patients, effective in 51.0% patients, and there was no efficacy in 23.8% patients; in the placebo group, GXST capsules were only highly effective in 12.5% patients, effective in 33.3% patients, and there was no efficacy in most of the patients (54.2%).
Secondary endpoints
The nitroglycerin consumption at week 0 was defined as the baseline value. GXST capsules significantly decreased the nitroglycerin consumption in comparison with placebo at week 4 (p < 0.0001). In the GXST group, the nitroglycerin consumption was reduced by more than 80% in 39.9% patients after treatment, a 50–80% reduction was found in 41.3% patients, and a < 50% reduction was only found in 18.8% patients; in the placebo group, a > 80% reduction of nitroglycerin consumption was only found in 8.3% patients after treatment, a 50–80% reduction was in 41.7% patients, and a < 50% reduction was in most of the patients (50%).
At week 0, there were no significant differences in the SAQ score and subscales between the two groups. As shown in Table 3, highly significant improvements were found in the SAQ score, angina stability, angina frequency, treatment satisfaction, and disease perception (p < 0.0001 for all) in the GXST group, while there were no significant differences in physical limitations between the two groups (60.1 ± 10.5 vs. 58.0 ± 11.7, p = 0.11).
For the pretreatment ETT, patients in both the GXST and placebo groups had no difference in the time of onset of chest pain (430.24 ± 177.2 s vs. 456.42 ± 188.72 s, p = 0.71, Table 3) and 1 mm ST-depression (235.95 ± 153.11 s vs. 237.69 ± 174.34 s, p = 0.96, Table 3). After the 4-week treatment, there was also no significant difference in the time of onset of chest pain (GXST group vs. placebo group, 517.6 ± 165.2 s vs. 493.2 ± 152.9 s, p = 0.81, Table 3) and 1 mm ST-depression (GXST group vs. placebo group, 219.7 ± 172.2 s vs. 237.7 ± 174.3 s, p = 0.37, Table 3) between the two groups.
Safety
Safety population included all patients (n = 287) who received study drug (basic treatment plus GXST capsule or placebo). As seen in Table 4, similar rates of AEs were observed in the GXST (4.9%) and placebo (2.1%) groups (p = 0.165) during the study. Five AEs reported in the GXST group were considered to be related to the study drug with the exception of urinary tract infection in one patient and upper respiratory tract infection in one patient. In the GXST group, gastrointestinal discomfort was the most frequent adverse event (3.5%, 5/143).
Discussion
In this propective, multicenter, double-Blind, placebo-controlled, randomized clinical trial conducted in China, the results clearly showed that 4 weeks of adjunctive therapy with GXST capsules significantly reduced the number of anginal attacks in SAP patients. Moreover, the effects of GXST capsules were also assessed and demonstrated by secondary endpoints associated with the reduction of nitroglycerin consumption, as well as the improvement of SAQ score and subscales, although no significant differences in the time of onset of chest pain and 1 mm ST-depression in ETT were identified between the GXST and the placebo groups. Our results demonstrated that GXST capsules improve the clinical outcome of current standard therapies for SAP even with commonly used secondary prevention medicines.
The incidence of ischemic heart disease in China has increased despite aggressive management. SAP is a chronic disease condition that is treated to abolish or minimize symptoms, improve QOL, and reduce long-term morbidity and mortality [18,19,20]. Chinese herbal medicines have been widely used as an adjunct to western medicines, including nitrates, ACEI/ARB, β-blockers, CCB, aspirin/anti-platelet drugs and lipid-lowering drugs in treating angina in China [9,10,11]. However, there is still a knowledge gap to clearly establish evidence that Chinese herbal medicines are effective in improving the outcomes of angina patients.
The components of GXST capsule are Choerospondias axillaris, Salvia miltiorrhiza, Syzygium aromaticum, borneol and concretio silicea bambusae. In rat myocardial ischemia/reperfusion model, GXST capsules can reduce myocardial ischemia and infarct size, inhibit the increase of serum creatine phosphokinase activity, prevent cardiomyocyte apoptosis, decrease serum inflammatory cytokine levels following myocardial ischemia/reperfusion injury, increase coronary blood flow and myocardial oxygen supply [12, 13]. GXST capsules also inhibited the formation and progression of atherosclerotic plaque and stabilized the unstable plaque through down-regulating the MMP-9 expression in ApoE−/− mice model [21]. Moreover, GXST capsule had a potential inhibitory effect on platelet aggregation stimulated by ADP and Thrombin in rats, thereby having a definite therapeutic effect against thromboembolic disease [22]. Screening and analysis of key active constituents in GXST capsule using mass spectrum and integrative network pharmacology, 12 active compounds and 33 targets were found to have a role in the treatment of cardiovascular diseases, and 4 main active ingredients, including protocatechuic acid, cryptotanshinone, eugenol and borneol were selected to verify the effect on calcium signaling system in cardiomyocyte injury induced by hypoxia and reoxygenation, providing an understanding of the therapeutic effect of GXST capsules [23]. To further strengthen those data, we now decided to design a clinical trial as this might give a more realistic picture of the everyday use of the drug.
In the present study, we found that the number of weekly anginal attacks and nitroglycerin consumption with GXST capsule treatment were significantly reduced in comparison with placebo. The magnitude of antianginal benefit observed in the trial is similar to that observed in other antianginal trials using conventional agents [19], in which patients with minimal or moderate anginal symptoms receiving a maximum recommended therapeutic dose of a β-blocker, an additional reduction was observed when the β-blocker was combined with a CCB titrated to its maximal tolerated dose [24]. Of note, however, the benefits achieved by combining the β blocker and a CCB were associated with significant undesirable changes in hemodynamics. The use of GXST capsules may allow for more optimal anti-ischemic effect without excessive adverse effects on heart rate and blood pressure.
With regard to exercise capacity, there were no statistically significant differences in the time of onset of angina and 1-mm ST segment depression between the GXST and placebo groups. However, an increase in the duration of the two key efficacy parameters at peak and at trough was found in both GXST and placebo groups, indicating that both GXST and placebo generally had a favorable effect on ETT durations. These results may indicate a training effect, but are more likely a strong placebo effect which has been documented previously in SAP patients [25].
The SAQ, designed to be applicable across sex, race, and socio-economic status, is a 19-item self-administered questionnaire measuring health status in patients with ischemic heart disease across 5 domains: physical limitation, angina stability, angina frequency, treatment satisfaction, and QOL [15, 16]. One previous review on antianginal drugs, such as long-acting nitrates, β-blockers, and CCBs, and their impact on QOL failed to show a significant effect over placebo [26]. This study furthers the understanding of the benefits of GXST on QOL. We had documented the improvement in all SAQ domains. Interestingly, at 4-week follow-up, GXST treatment was associated with improvements in angina frequency, angina stability, treatment satisfaction, and QOL domains except for physical limitations when it was compared with placebo. Taken together, all these results suggested that GXST capsules are effective in symptom alleviation and disease modification in SAP patients.
In general, a few patients in the GXST group experienced treatment AEs. However the AEs observed in the GXST group were in line with the established safety profile of GXST capsules. Previous studies have reported that gastrointestinal discomfort is the most common adverse event. The safety data of GXST capsules in this study is comparable to previous reports and no new safety concern is generated.
Limitations of this study included a relatively short treatment duration-4 weeks. Longer term efficacies of GXST treatment still need to be assessed. In addition, there was only about 35% of the patients in the GXST and placebo groups taking one or more types of standard anti-angina therapies such as ACEI/ARB, β-blocker, aspirin/anti-platelet drug and lipid-lowering drugs. Whether GXST coadministered with other classic antianginal agents such as β-blockers and CCBs will be useful in very symptomatic SAP patients remains need to be confirmed. Well designed placebo-controlled trials are needed to address this issue.
Conclusions
GXST capsules were effective and well tolerated in SAP patients investigated in the trial. This resulted in a substantial reduction in the number of angina attacks and consumption of short-acting nitrates. Patients QOL was also substantially improved. Therefore, GXST capsules are beneficial for the treatment of SAP patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- ACEI:
-
angiotensin converting enzyme inhibitors
- AEs:
-
adverse events
- ARB:
-
angiotensin II receptor blockers
- CCB:
-
calcium channel blockers
- CRFs:
-
case report forms
- ETT:
-
exercise tolerance test
- GXST capsule:
-
Guanxinshutong capsule
- QOL:
-
quality of life
- RCT:
-
randomized clinical trial
- SAP:
-
stable angina pectoris
- SAQ:
-
Seatntle Agina Questionnaire
References
Bolayir HA, Kivrak T, Gunes H, Bolayir A, Karaca I. The association between serum serglycin level and coronary artery disease severity in patients with stable angina pectoris. Kardiol Pol. 2018;
Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the global burden of disease 2010 study. Circulation. 2014;129:1493–501.
Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929–49.
Mancini GB, Gosselin G, Chow B, Kostuk W, Stone J, Yvorchuk KJ, Abramson BL, Cartier R, Huckell V, Tardif JC, Connelly K, Ducas J, Farkouh ME, Gupta M, Juneau M, O'Neill B, Raggi P, Teo K, Verma S, Zimmermann R. Canadian cardiovascular society guidelines for the diagnosis and management of stable ischemic heart disease. Can J Cardiol. 2014;30:837–49.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003.
Cardiology, C.S.o. Guidelines on the diagnosis and treatment of stable coronary artery disease in China. Chin J Cardiol. 2018; in press
Ford TJ, Corcoran D, Berry C. Stable coronary syndromes: pathophysiology, diagnostic advances and therapeutic need. Heart. 2018;104:284–92.
Siama K, Tousoulis D, Papageorgiou N, Siasos G, Tsiamis E, Bakogiannis C, Briasoulis A, Androulakis E, Tentolouris K, Stefanadis C. Stable angina pectoris: current medical treatment. Curr Pharm Des. 2013;19:1569–80.
Gong P, Li Y, Yao C, Guo H, Hwang H, Liu X, Xu Y, Wang X. Traditional Chinese Medicine on the Treatment of Coronary Heart Disease in Recent 20 Years. J Altern Complement Med. 2017;23:659–66.
Luo J, Shang Q, Han M, Chen K, Xu H. Traditional Chinese medicine injection for angina pectoris: an overview of systematic reviews. Am J Chin Med. 2014;42:37–59.
Zhang J, Meng H, Zhang Y, Zhang X, Shao M, Li C, Tu P. The therapeutical effect of Chinese medicine for the treatment of atherosclerotic coronary heart disease. Curr Pharm Des. 2017;
Liang Z, Liu LF, Yao TM, Huo Y, Han YL. Cardioprotective effects of Guanxinshutong (GXST) against myocardial ischemia/reperfusion injury in rats. J Geriatr Cardiol. 2012;9:130–6.
Liang Z, Yao TM, Huo Y, Han YL. The effects of Guanxinshutong on protection of left ventricular function after acute myocardial infarction in rats. Zhonghua nei ke za zhi. 2012;51:225–7.
Sun Z, Wuliji O. The observation on clinical effects of Guanxin Shutong capsule treating coronary heart disease and angina pectoris. J Inn Mong Univ National. 2006;21:422–4.
McGillion M, O'Keefe-McCarthy S, Carroll SL, Victor JC, Cosman T, Cook A, Hanlon JG, Jolicoeur EM, Jamal N, McKelvie R, Arthur HM. Impact of self-management interventions on stable angina symptoms and health-related quality of life: a meta-analysis. BMC Cardiovasc Disord. 2014;14:14.
Norris CM, Ghali WA, Saunders LD, Brant R, Galbraith PD. Systematic review of statistical methods used to analyze Seattle Angina Questionnaire scores. Can J Cardiol. 2004;20:187–93.
Administration, B.o.D. and Ministry of Health P.C. Chinese guidelines for the clinical trial of cardiovascular drug: antianginal drugs. In: Guidelines for clinical trial and non-clinical trial of new drug (Western medicine). The Bureau, Beijing, 1993.
Dabrowski R, Dobrowolski M. Stable coronary artery disease – medical treatment. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2017;43:228–31.
Henderson RA, O'Flynn N. Management of stable angina: summary of NICE guidance. Heart. 2012;98:500–7.
Long L, Anderson L, Dewhirst AM, He J, Bridges C, Gandhi M, Taylor RS. Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev. 2018;2:CD012786.
Huo Y, Liang Z, Han YL, Yao TM. The effect of Guanxinshutong capsule on the expression of matrix Metalloproteinases-9 and tissue matrix metalloproteinase Inhibitor-1 of atherosclerotic plaque in ApoE−/− mice. Chin J Arterioscler. 2014;22(5):463–6.
Wang WM, Xing XX, Gao H, Wang LY, Liu MN, FAN GW. Anti-platelet aggregation and anti-atrial contractile effect of Guanxinshutong capsule. J Tanjing Univ Tradit Chin Med. 2016;35(2):104–8.
Liu F, Du X, Liu PR, Sun YH, Zhang YM. Screening and analysis of key active constituents in Guanxinshutong capsule using mass spectrum and integrative network pharmacology. Chin J Nat Med. 2018;16(4):302–12.
Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (efficacy of Ranolazine in chronic angina) trial. J Am Coll Cardiol. 2006;48:566–75.
Thadani U, Ezekowitz M, Fenney L, Chiang YK. Double-blind efficacy and safety study of a novel anti-ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris. Ranolazine Study Group. Circulation. 1994;90:726–34.
Gandjour A, Lauterbach KW. Review of quality-of-life evaluations in patients with angina pectoris. PharmacoEconomics. 1999;16:141–52.
Acknowledgements
The authors appreciate Zhao Guiying for the dedicated efforts of testing patients’ serum samples.
Funding
No funding was received for the research reported.
Author information
Authors and Affiliations
Contributions
Conceived and designed the research: YLH. Performed the research: YL, LZ, SZL, XZW, JZ, XXT, YZ, BJC, DYL, JY, PKD, YZX, YMS, JLS, LL, XCW and YLH. Analyzed the data: YL, LZ, SZL, BJC and YLH. Wrote and edited the paper: YL, LZ and YLH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The trial protocol was reviewed and approved by the regulatory authorities in respective Institutional Review Boards of the medical centers involved. All patients provided written informed consent before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, Y., Zhang, L., Lv, S. et al. Efficacy and safety of oral Guanxinshutong capsules in patients with stable angina pectoris in China: a prospective, multicenter, double-blind, placebo-controlled, randomized clinical trial. BMC Complement Altern Med 19, 363 (2019). https://doi.org/10.1186/s12906-019-2778-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-019-2778-z