Abstract
Background
Astilbe rivularis L. is an indigenous medicinal plant growing in high altitude of Darjeeling Himalayan region of India and Nepal. The plant rhizome has been used traditionally as medicine by local tribes to treat various ailments including infectious and other diseases. The present study aims to evaluate the plant rhizome for chemical composition and in vitro antioxidant, antibacterial and cytotoxic bioactivities.
Methods
The methanolic extract of rhizome was analyzed for phytochemical constituents by biochemical and GC-MS methods. The antibacterial property of the extract was monitored by agar well diffusion assay. Antioxidant potential was assessed by in vitro DPPH and ABTS scavenging assays and scavenging of induced ROS in normal cell line using fluorescent probe 2′, 7′- dichlorofluorescin diacetate. Cytotoxic effect of the extract in cancer and normal cell lines was determined by MTT assay.
Results
Rhizome methanolic extract contained terpenoids, flavonoids, tannins, phenols, alkaloids, saponins and reducing sugars. Further analysis of extract by GC-MS showed the presence of nine major constituents belonging to terpenoids and fatty acid groups. The extract had marked in vitro ROS scavenging activity and moderate antibacterial activity against gram positive and gram negative bacteria. It showed cytotoxicity to neuroblastoma (SHSY5Y) cell line with IC50 value < 100 μg ml− 1 but had least damaging effect on normal cells, like human embryonic kidney (HEK-293) and liver (WRL-68) cell lines.
Conclusion
The study suggests that Astilbe rivularis has potential as source of new potent antibacterial, antioxidant and anticancer agents. Further studies on purification and characterization of active compounds from Astilbe rivularis and their biological evaluation are highly recommended.
Similar content being viewed by others
Background
Respiration is an important metabolic process of biological combustion to generate energy; however, the process also produces harmful intermediates called reactive oxygen species (ROS). The excessive accumulation of ROS leads to cumulative damage of biomolecules, such as proteins, lipids and nucleic acid, resulting in oxidative stress. Oxidative stress has been associated with several disease conditions such as diabetes, stroke, cancer, arteriosclerosis, alzheimer’s disease, cardiovascular diseases and ageing [1, 2].
Plants are being used as therapeutics from ancient time without the prior knowledge of their active components. They have been the major source of phytochemicals with therapeutic potential. These phytochemicals also act as reducing agents to reverse oxidation by donating electrons and/or hydrogen ions. Moreover, natural compounds as drugs have reduced side effects due to their regular intake as components of vegetable food. Scientific studies on ethnomedicinal plants have resulted in discovery of several therapeutic drugs [3, 4]. Previous research works on anticancer potential of plants resulted in the development of valuable anticancer drugs, such as taxol, camptothecin, vincristine and vinblastine [5]. These drugs are reported to target mitotic spindle assembly, chromosome segregation, cell division and apoptosis [6]. In recent years, natural anticancer compounds have been reported with various targeting mechanisms, like up-regulation of p16INK4A, preventing MRCK- kinase that targeting multiple gene products and targeting mitotic processes in different types of cancer, such as human mouth epidermal carcinoma, murine leukemia, human colorectal cancer and prostate cancer [7].
Astilbe, a genus with 18 species of rhizomatous flowering plants of the family Saxifragaceae, is native to mountain ravines and woodland in Asia and North America [8]. It is a traditional medicinal plant used by ethnic people of Eastern Himalayan region of India and Nepal. Almost all parts of the plant are being used as medicine with most preferential use of the rhizome part. Although the plant has shown antiviral, antidiabetic and antiulcer properties, little scientific information are available on its efficacy as drug source. The plant extract has shown antiviral effect against Herpes Simplex Virus [9]. Pentacyclic triterpenoids isolated from the Astilbe plant enhanced glucose uptake via the activation of Akt and Erk1/2 in C2C12 myotubes [10]. Moreover, a compound (AR-I) isolated from the plant leaves showed antiulcer activity against ethanol induced gastric ulcer and cysteamine induced duodenal ulcer in albino rats [11].
Our survey on the use of medicinal plants by tribes and local inhabitants of hilly area of Darjeeling revealed the therapeutic importance of rhizome of Astilbe rivularis (AR) in reducing various types of infections as well as diabetes. Therefore, in the present study, the methanolic extract of the rhizome was investigated for phytochemical classes followed by identification of major phytoconstituents by GC-MS. AR rhizome extract showed in vitro antioxidant and antibacterial activities. The extract had considerably greater cytotoxic effect against Neuroblastoma cell line (SHSY5Y) compared to normal cells, like Human Embryonic Kidney (HEK-293) and Liver (WRL-68) cell lines. To our knowledge, this is the first study of its kind on AR where the plant showed multiple bioactivities and thus can be a potential candidate for pharmaceutical industries.
Materials and methods
Plant material, human cell lines and chemicals
Astilbe rivularis rhizome was collected from Jalapahar Cantonment Forest situated at high altitude of Darjeeling Himalayan region (27o 2′9.6252 N and 88o 15′ 45.6192 E) of West Bengal, India, during the month of February, 2016. The plant was authenticated by Dr. Monoranjan Chowdhury, Assistant Professor, Department of Botany, University of North Bengal, Siliguri, India. A voucher specimen (NBU-Bot/10045/2016) has been deposited in the institutional herbarium for future reference. Neuroblastoma (SHSY5Y), Human Embryonic Kidney (HEK-293) and Liver (WRL-68) cell lines were obtained from National Centre for Cell Science, Pune, India. All other chemicals of analytical grade were purchased from Sigma-Aldrich India Limited, Hi Media, India and E. Merck, India.
Preparation of plant extract
The plant rhizome was dried in shade and then ground into fine powder. Methanolic extract of the rhizome was prepared by Soxhlet extraction method. About 20 g of powdered plant material was uniformly packed into a thimble and extracted with 200 ml of methanol for 24 h or till the solvent in siphon tube of extractor became colorless. The extract obtained was concentrated under reduced pressure in a rotary evaporator to fine powder. The total yield percentage of the plant extract was 39.75% as calculated by the formula, Yield Percentage (%) = (Weight of extract obtained)/ (Total weight of sample loaded) × 100.
Qualitative analysis of phytochemicals
The methanolic rhizome extract was screened for phytochemicals, such as phenols, flavonoids, tannins, saponins, terpenoids, cardiac glycosides and alkaloids. For phenol detection, 100 mg of powdered plant sample was suspended in 5 ml double distilled water, mixed well and filtered through Whatman No.1 filter paper. To 1 ml of filtrate equal volume of 1% FeCl3 was added. Appearance of blue or green color confirmed the presence of phenol [12]. For detection of flavanoid, 1 g sample was mixed with 5 ml of acetone, followed by evaporation of acetone in hot water bath. The precipitate was extracted with 5 ml of warm double distilled water, filtered under hot condition and cooled at room temperature (RT). To 1 ml of the filtrate equal volume of 20% NaOH was added. Appearance of yellow colour indicated the presence of flavonoids [13]. The methanolic filtrate of plant rhizome powder (100 mg ml− 1) was used for detection of alkaloid, terpenoid, cardiac glycoside and saponin. Methanolic filtrate (2 ml) was mixed with 2 ml of 1% HCl and kept over steam for 5 min. To 1 ml of mixture, 6–7 drops of Mayer’s/Wagner’s reagent was added to obtain creamish/ brown/ red/ orange precipitate indicated the presence of alkaloids [14]. 1 ml of methanolic filtrate was mixed with 1 ml of chloroform, 1 ml of acetic anhydride and 0.5 ml of concentrated H2SO4. Appearance of reddish brown coloration at the interface indicated the presence of terpenoid [15]. 1 ml methanolic filtrate was mixed with 0.5 ml glacial acetic acid followed by addition of 3–4 drops of 5% FeCl3 and 0.5 ml of concentrated H2SO4. Appearance of brown ring at the interface indicated presence of cardiac glycosides [14]. For detection of tannins and saponins, 100 mg rhizome powder was suspended in 5 ml of double distilled water and filtered. To 0.5 ml of aqueous filtrate, 5 ml double distilled water was added and shaken vigorously for about 30 s. Formation and persistence of froth indicated the presence of saponins [14]. To 2 ml filtrate, 1 ml of 5% FeCl3 was added. The formation of yellow brown precipitate confirmed the presence of tannins [16].
GC-MS analysis
The chemical constituents of methanolic extract of Astilbe rhizome were identified by GC-MS analysis on GC-MS JEOL, GC Mate II equipped with HP5 silica column (50 m × 0.25 mm i.d.) and secondary electron multiplier. The sample (1 μl) was evaporated in a splitless injector at 300 °C. The analysis conditions were 20 min at 100 °C, 3 min at 235 °C for column temperature and 240 °C for injector temperature. Helium was the carrier gas and split ratio was 5:4. Run was carried out for 22 min. The chemical constituents of the rhizome extract were identified by the comparison of the experimental mass spectra with that of National Institute Standard and Technique (NIST) GC-MS database [17].
DPPH free radical scavenging assay
Antioxidant property of the rhizome extract was determined by its ability to reduce purple-colored methanolic solution of DPPH (2, 2-diphenyl-1-picryllhydrazyl) free-radical to colourless compound [18]. The extract at various concentrations (5–30 μg ml− 1) was mixed with equal volume of methanolic DPPH solution (100 μM), incubated at RT for 30 min in dark and absorbance was recorded at 517 nm. The reaction mixture without extract served as control and L-ascorbic acid was used as antioxidant standard. The percentage inhibition was calculated according to the formula: DPPH inhibition (%) = (Ao – A1)/Ao × 100, where Ao and A1 are absorbance of control and extract/standard, respectively.
ABTS scavenging assay
ABTS scavenging assay was performed by standard method with some modifications [19]. Briefly, ABTS stock solution was prepared by mixing potassium per sulfate (2.45 mM) and ABTS (7 mM) in equal ratio and incubating at RT in dark for 12–16 h. The working ABTS+ solution was prepared by dilution of the stock solution with 80% methanol to absorbance A734 = 0.708 ± 0.002. Plant extract (10 μl) at concentrations ranging from 25 to 200 μg ml− 1 in methanol was added to 1 ml of ABTS+ working solution. Thereafter, the reaction mixture was incubated at RT for 7 min in dark and absorbance was recorded at 734 nm. The reaction mixture without extract served as control, whereas L-ascorbic acid and gallic acid served as antioxidant standards. The percentage inhibition was calculated using the formula: ABTS scavenging (%) = (Ao – A1)/Ao × 100, where Ao and A1 absorbance of control and extract/standard, respectively.
Evaluation of rhizome extract for scavenging of induced ROS in WRL-68 cells using fluorescent probe 2′, 7′- dichlorofluorescin diacetate (DCF-DA)
The coverslips seeded with cells (WRL-68) at concentration of 2.5 × 104, were placed over 35 mm petriplates and grown overnight in the Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 5% fetal bovine serum (FBS), 100 IU ml− 1 Penicillin and 100 μg ml− 1 of Streptomycin at 37 °C in 5% CO2 incubator. The culture plates were divided into four sets and three sets were treated with incomplete medium (DMEM without FBS) and one set was treated with complete medium (DMEM with FBS). Out of three sets of cells grown in incomplete media, two sets were treated with 25 μl of methanolic extract at 50 and 100 μg ml− 1 concentrations, whereas third set treated with 25 μl of methanol served as control. The untreated cells grown in complete media served as the positive control. The intracellular oxidative level was examined using the dichlorofluorescein assay [20, 21]. For this both treated and untreated cells were washed with phosphate-buffer saline (PBS) followed by addition of 10 μM carboxy-2′, 7′–dichlorofluorescin diacetate (DCF-DA) and incubated for 30 min at 37 °C in dark in an incubator (5% CO2). Cells washed with PBS were immediately analyzed for generation of ROS under fluorescence microscope (Magnus MLXi, Olympus).
Determination of antibacterial activity
The antibacterial property of the rhizome extract was monitored by agar well diffusion method using Mueller Hinton Agar (MHA) medium [22]. Bacterial culture grown overnight in Muller Hinton broth at 37 °C was swabbed into the surface of MHA plates. Wells were prepared on the plates with the help of sterile 6 mm cork-borer and the (30 μl) plant extract of different concentrations (20–100 mg ml− 1) introduced into the wells followed by incubation of plates at 37 °C for 24 h and thereafter, the zone of inhibition was measured. Antibiotics ampicillin (2 μg ml− 1) and tetracycline (30 μg ml− 1) were used as standards and extraction solvent (methanol) was used as control.
Cytotoxicity analysis
Cytotoxic activity of the plant rhizome extract against normal and cancer cell lines was determined by using standard MTT [(3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay [23]. For this purpose, SH-SY5Y, HEK-293 and WRL-68 were separately cultured in DMEM, supplemented with 5% FBS, 100 IU ml− 1 Penicillin and 100 μg ml− 1 of Streptomycin, in 100 mm petri dishes at 37 °C in 5% CO2 incubator. 100 μl of cells (1 × 105 cells ml− 1) were seeded into each well of 96 well microplate. After 24 h incubation, cells were treated with specified concentrations of plant extract. After 24 h of treatment, 10 μl of MTT (5 mg ml− 1) was added to each well and incubated further for 4 h. The MTT was replaced by 50 μl isopropanol to dissolve the insoluble formazan product. The extent of MTT reduction to formazan within the cells was calculated by measuring the absorbance at 540 nm using a micro plate reader (Spectrostarnano, BMG Labtech). The inhibition of cell growth was calculated by the formula; Percent inhibition (%) = (Y-X)/Y × 100, where Y is the mean optical density of control (DMSO treated cells) and X is the mean optical density of cells treated with plant extract. For cytotoxicity assay, the stock solution of extract was prepared in DMSO, which was diluted to final desired concentrations using the same solvent.
Statistical analysis
All experimental results are mean ± SD of three parallel measurements. The data were analyzed by analysis of variance (P < 0.05). Results were processed in Excel and SPSS programmes.
Results
The preliminary in vitro phytochemical analysis of methanolic extract of AR rhizome revealed the presence of various classes of phytochemicals, such as tannins, phenols, cardiac-glycosides, saponins, terpenoids and alkaloids with terpenoids being the most abundant one (Table 1). Further the qualitative and quantitative analyses of phytochemicals were performed by GC-MS. The chromatogram showed nine prominent peaks based on their retention time indicating the presence of nine major phytochemical constituents (Fig. 1). The structure, molecular formula, molecular weight and percent peak area of these compounds are mentioned in Table 2.
The methanolic extract was analyzed for antioxidant activities based on different working principles. AR rhizome showed the ability to scavenge DPPH free radical in a concentration dependent manner; however, its scavenging capacity was lower than that of the standard antioxidant ascorbic acid (Fig. 2). Consequently, the IC50 values as calculated from linear regression analysis curve were 15 and 5 μg ml− 1 for rhizome extract and ascorbic acid, respectively. ABTS free radical scavenging potential of AR and of the standard antioxidants such as ascorbic acid and gallic acid, were observed at different concentrations i.e. 25, 50, 100, 150 and 200 μg ml− 1 and their IC50 values were 138, 128 and 40 μg ml− 1, respectively (Fig. 3). 6-carboxy-2′, 7 dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) is a non-fluorescent reagent, which in presence of free radicals undergoes oxidation to produce green fluorescence. Although it mainly detects peroxides, it also gets oxidized by other ROS generated in the cells due to stress [24]. In our study, the normal liver cell line (WRL-68) grown in incomplete media led to production of ROS, that reacted with carboxy-H2DCFDA to give intense fluorescent signal (Fig. 4b). However, the level of ROS generation on growing in incomplete medium significantly reduced in the presence of various doses of plant extract. The results in Fig. 4c and d demonstrate the dose dependent lowering of fluorescent signal in the cells on exposure to the crude extract at 50 and 100 μg ml− 1 as compared to untreated control (Fig. 4a).
Effects of AR rhizome extract on oxidative stress in the WRL-68 cells. a) Unstressed cells grown in CM showed basal level ROS b) Control cells grown in IM showing increased ROS generation c) Cells in IM treated with 50 μgml− 1 of extract d) Cells in IM treated with 100 μgml− 1 of extract. **CM- Complete Media (DMEM with FBS), IM- Incomplete Media (DMEM without FBS)
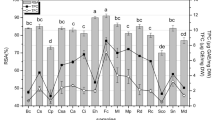
Antibacterial effect of AR extract was evaluated against five different bacteria, including Bacillus subtilis, Bacillus amyloliquefaciens, Aeromonas liquefaciens, Flexibactor sp. and Psedomonas sp., by agar well diffusion method and zone of inhibition (ZOI) formed on the plates was measured. From the results in Table 3 it is evident that the rhizome extract showed antibacterial effect against both gram +ve and gram –ve bacteria. The extract at 20–100 mg ml− 1 concentration produced ZOI in the range of 13–24 mm (P < 0.001) with the highest ZOI of 23 and 24 mm against A. liquefaciens and B.amyloliquefaciens at 100 and 80 mg ml− 1, respectively.
The plant extract also showed cytotoxic effect against neuroblastema cell line (SHSY5Y) and two normal cell lines namely, HEK-296 and WRL-68, with IC50 value of 83.7, 193.8 and 389.3 μg ml− 1, respectively (Fig. 5). The results thus indicate a relatively greater inhibition of cancer cells proliferation by the active components of the plant rhizome extract.
Discussion
The rhizome of A. rivularis is ethanopharmacologically known to treat a number of ailments including stomach ache, diarrhoea, dysentery, headache, cough, rheumatism, back pain, wound healing, weakness, avian plague, peptic ulcer and malaria [25, 26]. Moreover, powdered root is taken with curd to cure jaundice and with honey to control excessive bleeding after child birth [27]. However, the plant has not been explored much for identification of phytoconstituents and their biological activities. Hence, in the present work methanolic extract of the rhizome was analyzed for phytoconstituents, antimicrobial and antioxidant activities, and in vitro cytotoxicity against normal and cancer cell lines. Primarily, plant water extracts are being used for their medicinal use, however, plants extracted in organic solvents have been found to give more consistent in vitro biological activities [22, 28]. Therefore, in our study we used methanol as polar organic solvent for extraction of A. rivularis rhizome. Phytochemical assay of the rhizome extract showed the presence of terpenoids, alkaloids, tannins, flavanoids and phenols that are known for several bioactive functions, such as antioxidant, antimicrobial and anticancer activities. The qualitative analysis by GC-MS identified different types of compounds that include 2-coumaranone; 2-buten-1-one, 1-phenyl; Undecanoic acid, 2-methyl; 2-piperidinone, 3,6-bis (1-methyllethenyl)-1-phenyl,trans; Crinan 1,2-didehydro; 9-Octadecenoic acid (z)-,methyl ester; [1,1-bicyclopropyl]-2-octanoic acid, 2-hexyl-, methyl ester; 17a-ethyl-3a-methoxy-17a-aza-D-homoandrost-5-ene-17- one and Butanedioic acid, 2,3-bis (8-nonen-1-yl)-, dimethyl ester. In a previous study, coumaranone terpenoid isolated from Astilbe species has been found to inhibit acetyl choline esterase [29]. The compound also showed antidiabetic effect by enhancing glucose uptake via activation of Akt and Erk1/2 in C2C12 myotubes. Undecanoic methyl esters are reported to have cytotoxic effect against breast, ovarian, prostrate and liver cancer cell lines with IC50 values in the range of 10–140 μM [30].
The incidence of drug resistance against microorganisms is a leading cause of ineffectiveness of antimicrobial agents. Medicinal plants could act as potential source of new antibacterial agents even against some resistant strains of microorganisms. In earlier research works, several plant extracts were found to be more effective against gram positive bacteria than gram negative ones because of the presence of impermeable lipopolysacharide layer [31]. In the present study, methanolic AR rhizome extract was found to be effective against both gram +ve and gram -ve bacteria and with greater ZOI at higher extract concentrations. In an earlier study, the compounds arbutin and bergenin isolated from methanolic extract of rhizome of A. rivularis inhibited the growth of E.coli [25, 32].
Phytochemicals are considered to have multiple beneficial effects through neutralization of free radicals associated with several diseases [33]. They neutralize the free radical by either donating hydrogen or quenching singlet oxygen. Astilbe rhizome extract showed antioxidant activity, with a more effective scavenging of DPPH as compared to that of ABTS. In an earlier research on A. rivularis, the methanolic leaf extract exhibited 96% DPPH scavenging activity at a dose of 100 μg ml− 1 [34], whereas we found almost similar level of DPPH scavenging at 25 μg ml− 1 of methanolic rhizome extract. The results thus indicate markedly greater antioxidant potential of rhizome compared with leaf. Even though the scavenging activities of the extract were significantly lower in comparison to standards (gallic acid and ascorbic acid), the reduced activities could be related to the lesser amounts of antioxidant compounds in the extract [22]. GC-MS analysis of Astilbe rhizome extract indicated the presence of fatty acid ester i.e. methyl ester of 9-Octadecenoic acid as one of the most abundant molecule with 29.8% peak area, which has also been earlier reported of having reducing potential [3].
Serum deprivation in the growth medium has been reported to trigger cellular ROS generation [35]. Hence in our study, ROS production was induced by growing the normal liver cell line in serum deprived medium, which was effectively lowered down in presence of the plant extract.
The in vitro cytotoxic activity of plant extracts with IC50 < 100 μg ml− 1 is generally considered to be therapeutically important [36]. The rhizome extract exhibited in vitro cytotoxic activity for cancer cell line i.e. SHSY5Y, with IC50 < 100 μg ml− 1, whereas IC50 > 100 μg ml− 1 was obtained for normal cell lines. The results thus suggest A. rivularis rhizome extract as a potential candidate for cancer research.
Conclusion
The methanolic extract of A. rivularis rhizome showed the presence of various phytochemical constituents, such as terpenoids, alkaloids, tanins and phenols. Further the GC-MS analysis of the extract confirmed the presence of some antibacterial and anticancer compounds. The extract was the source of effective antioxidants as revealed by DPPH, ABTS and DCF-DA based ROS scavenging assays and of effective antimicrobial compounds against both gram positive and gram negative bacteria. Astilbe rhizome extract also exhibited moderate cytotoxicity against the cancer cell line with limited effect on the normal cell lines. The results suggest that A. rivularis has the potential as therapeutic agent for disease conditions. Further, the study also validates its traditional use by ethnic people. Hence, our further research work has been directed towards the isolation of pure compound(s) from the methanolic rhizome extract of A. rivularis and evaluation of their biological activity and mechanism of action.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article. The.
Datasets/materials used and/or analyzed during the current study are available from the.
corresponding author on reasonable request.
References
Stephanie D, Xavier V, Philippe C, Marion W, Jean M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–74.
Hajar II, Kim WC, Abdalbasit AM, Maznah I. Phenolic content and antioxidant activity of cantaloupe (cucumismelo) methanolic extracts. Food Chem. 2009;119:643–7.
Kabir O, Damilola BO, Abdulfatai A, Jimoh AA. Chemical composition, antioxidant and antimicrobial potentials of Icacina trichantha Oliv. Leaf extracts. Nat Prod Chem. 2015;3:188–95.
Shabina IA, Muhammad QH, Muhammad T, Qaisar M, Muhammad I, Kristen K, Bates RB. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. Compl. Alt. Med. 2016;16:460–8.
George S, Bhalerao SV, Lidstone EA, Ahmad IS, Abbasi A, Cunningham BT, Watkin KL. Cytotoxicity screening of Bangladeshi medicinal plant extracts on pancreatic cancer cells. Compl. Alt. Med. 2010;10:52–62.
Bhanot A, Sharma R, Noolvi MN. Natural sources as potential anti-cancer agents: a review. Int J Phytomed. 2011;3:9–26.
Bhandari J, Muhammad BT, Thapa P, Shrestha BG. Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. Compl Alt Med. 2017;17:102–11.
Neill AR, Badola HK, Dhyani PP, Rana SK. Integrating ethnobiological knowledge into biodiversity conservation in the eastern Himalayas. J Ethn Ethnomedicine. 2017;13:21–34.
Hafidh RR, Abas F, Abdulamir AS, Jahanashiri F, Bakar FA, Sekawi Z. A review: cancer research of natural products in Asia. Int J Cancer Res. 2009;5:69–82.
Joo HH, Wei Z, Wei L, Pham QT, Nguyen MK, Thuong PT, Min KN, Seon MC. Pentacyclic triterpenoids from Astilbe rivularis that enhance glucose uptake via the activation of Akt and Erk1/2 in C2C12 Myotubes. JNat Prod. 2015;78:1005–14.
Mitra PK. Isolation of antiulcerogenic compound (AR-I) from Astilbe rivularis leaves and effect of season on yield of the compound. World J Pharm Sci. 2014;2:1489–94.
Sonam M, Singh RP, Pooja S. Phytochemical screening and TLC profiling of various extracts of Reinwardtia indica. Int J Pharmacognosy Phytochem Res. 2017;9:523–7.
Evans WC. Trease and Evans Pharmacognosy.(15th Ed.), 15th edition: Sanders Co. Ltd. Singapore; 2002.
Trease GE, Evans WC. Isolation, purification and partial characterization of prunellin an anti – HIV compound from aqueous extract of Prunella vulgaris, ant. Res. 1989;11:263–73.
Harborne JB. Phytochemical methods. London: Chapman and Hall; 1973.
Jigna P, Sumitra VC. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plant. Turk J Bio. 2007;31:53–8.
Radhakrishnan K, James F, Mohan A, Mohan SC. Gas chromatography and mass spectrometry analysis of Canthium parviflorum leaves. Inn J Sci. 2017;5:1.
Singh RP, Murthy KN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extract using in vitro models. J Agr Food Chem. 2002;50:81–6.
Igwaran A, Iweriebor BC, Okoh SO, Nwodo UU, Obi LC, Okoh AI. Chemical constituents, antibacterial and antioxidant properties of the essential oil flower of Tagetes minuta grown in Cala community eastern cape, South Africa. Comp Alter Med. 2017;17:352–60.
Myoung J, Lee JS, Lee KR, Ha SJ, Hong EK. Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence. Nutrients. 2014;6:3711–26.
Cui Y, Li H, Wu S, Zhao R, Du D, Ding Y, Nie H, Ji HL. Formaldehyde impairs transepithelial sodium transport Sci Rep. 2016;6:1–11.
Wintola FA. The antibacterial, phytochemicals and antioxidants evaluation of the root extracts of Hydnora africana Thunb. Used as antidysenteric in eastern Cape Province, South Africa. Comp Alter Med. 2015;15:1–12.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Imm Meth. 1983;65:55–6.
Danli W, Patricia Y. Production and detection of reactive oxygen species (ROS) in cancers. J Vis Exp. 2011;57:3357–61.
Adhikary P, Roshan KC, Kayastha D, Thapa D, Shrestha R, Shrestha TM, Gyawali R. In vitro evaluation of antimicrobial and cytotoxic potential of dry rhizome extract of Astilbe rivularis. Int J Pharm and Phyt Res. 2012;4:122–6.
Kirtikar KR, Basu BD. Indian Medicinal Plants. vol. 9, 2nded. Uttaranchal, India, 2001. 2832–2836.
Hussain S, Hore DK. Collection and conservation of major meditional plants of Darjeeling and Sikkim Himalayas. Indian J Trad Know. 2007;6:352–7.
Parekh J, Jadeja D, Chanda S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk J Biol. 2005;29:203–10.
Asadipour A, Alipour M, Jafari M, Khoobi M, Emami S, H d N, Sakhteman A, Moradi A, Sheibani V, Moghadam FH, Shafiee A, Foroumadi A. Novel coumarin-3-carboxamides bearing N-benzylpiperidine moiety as potent acetylcholinesterase inhibitors. Europ J MedChem. 2013;70:623–30.
Narra N, Kaki SS, Prasad RBN, Misra S, Dhevendar K, Kontham V, Korlipara PV. Synthesis and evaluation of anti-oxidant and cytotoxic activities of novel 10-undecenoic acid methyl ester based lipoconjugates of phenolic acids. Beilstein J Org Chem. 2017;13:26–32.
Fennell CW, Lindsey KL, McGawb LJ, Sparg SG, Stafford GI, Elgorashi EE, Grace OM, Staden J. Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J Ethnopharmacol. 2004;94:205–17.
Timalsena S, Lamichhane PP. Astible rivularis: bioactive compounds and pharmacological functions. Chin J Integr Med. 2016:1–6.
Gülçin I, Topal F, Sarıkaya SB, Bursal E, Bilsel G, Goren AC. Polyphenol contents and antioxidant properties of Medlar (Mespilus germanica L.). Rec Nat Prod. 2011;5(3):158–75.
Mitra PK, Ghosh T, Mitra P. Effect of season on in vitro anti oxidant activity of Astilbe rivularis Buch – ham. Ex D. Don leaves. Biomed J Sci & Tech Res. 2017;1:1–4.
Yun SB, Hyunjin O, Sue GR, Young. Regulation of reactive oxygen species generation in cell signaling. Mol Cells 2011, 32:491–509.
Hendra R, Ahmad S, Oskoueian E, Sukari A, Shukor MY. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff fruit. Compl. Alt. Med. 2011;11:110–9.
Acknowledgements
The first author, Vijeta Rai is much thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of Junior Research Fellowship (CSIR Award letter no.09/285(0067)/2015-EMR-1). The authors acknowledge IITM SAIF, Tamil Nadu for the GC MS analysis.
Funding
The study did not receive any research funding from any agency.
Author information
Authors and Affiliations
Contributions
SG conceived and designed the work. SG and VR wrote and edited the manuscript. VR performed all the experiments including antioxidant, antibacterial and cytotoxic activities. VD collected the plant sample and performed in vitro phytochemical analysis, and AK performed the experiments related to cytotoxity and scavenging of induced ROS. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rai, V., Kumar, A., Das, V. et al. Evaluation of chemical constituents and in vitro antimicrobial, antioxidant and cytotoxicity potential of rhizome of Astilbe rivularis (Bodho-okhati), an indigenous medicinal plant from Eastern Himalayan region of India. BMC Complement Altern Med 19, 200 (2019). https://doi.org/10.1186/s12906-019-2621-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-019-2621-6