Abstract
Background
Eugenol is a natural phenolic compound and possesses anticancer and antibacterial activities. Breast cancer is a major global health problem, and most of the chemotherapeutic agents are highly toxic with long-term side effects. Therefore, this study aimed to explore the possibility of using eugenol as an anti-metastatic and anti-proliferative agent against MDA-MB-231 and SK-BR-3 breast cancer cells.
Methods
Breast cancer cell lines MDA-MB-231 and SK-BR-3 were treated with eugenol and cell proliferation was measured using a real-time cell electronic sensing system. Annexin V analysis with flow cytometry was used to detect the effect of eugenol on cell death. In MDA-MB-231 and SK-BR-3 cells, metastatic potential after eugenol treatment was examined using a wound-healing assay. Real-time PCR was used to study the effect of eugenol on the expression of anti-metastatic genes such as MMP2, MMP9, and TIMP-1, and genes involved in apoptosis including Caspase3, Caspase7, and Caspase9.
Results
Treatment with 4 μM and 8 μM eugenol for 48 h significantly inhibited cell proliferation of MDA-MB-231, with an inhibition rate of 76.4%, whereas 5 μM and 10 μM of eugenol for 48 h significantly inhibited the proliferation of SK-BR-3 cells with an inhibition rate of 68.1%. Eugenol-treated cells showed significantly decreased MMP2 and MMP9 expression and an insignificant increase in TIMP1 expression in HER2 positive and triple negative breast cancer cells. Eugenol significantly increased the proportion of MDA-MB-231 and SK-BR-3 cells in late apoptosis and increased the expression of Caspase3, Caspase7, and Caspase9.
Conclusion
To the best of our knowledge, this is the first study to describe the anti-metastatic effect of eugenol against MDA-MB-231 and SK-BR-3 breast cancer cell lines.
Similar content being viewed by others
Background
Breast cancer is a major health problem with high morbidity and mortality rates among women worldwide [1]. Its high prevalence and differences in response patterns to various treatment modalities and clinical outcomes provides a strong rationale for identifying natural and synthetic cancer preventive agents [2]. Breast cancer is a heterogeneous complex of diseases [3] and can be classified into different subtypes based on biological characteristics (traditional classification systems), or gene expression patterns (molecular classification) [4]. Triple-negative breast cancer (TNBC) has a distinct aggressive molecular subtype, characterized by lack of progesterone receptor (PR), estrogen receptor (ER), and human epidermal growth factor receptor-2 (HER-2) expression [5] resulting in poorer outcomes than other subtypes [6]. Moreover, TNBC can be classified in different subtyping based on gene expression profiling into basal like1, basal-like 2, an immunomodulatory subtype, mesenchymal, mesenchymal stem cell-like and luminal androgen receptor subtype [7]. Because of the absence of commonly targeted receptors present in other breast tumor subtypes, agents that specifically target TNBC are not yet available. Another type of breast cancer that has aggressive biological behavior is HER2- positive breast cancer which categorized by high HER2 expression [3].
In breast cancer patients, metastasis is considered one of the main causes of death [8]. Metastasis starts with degradation of the extracellular matrix, followed by cell invasion and trans-endothelial cell migration and ends with colonization in new site [9]. In metastasis, there was a link between the high levels of a group of matrix metalloproteinases (MMPs), a family of 23 structurally and functionally related endopeptidases [10], and most human tumor cell lines [11]. During tumor progression, the MMPs produce extracellular matrix remodeling and release of cytokines and growth factors that causes modification for the microenvironment [12]. Several MMPs (like MMP-1, − 2, − 3, − 7, − 9, − 11 and − 14) have different roles in different cancer stages [13, 14]. The MMP-2 and -9 are involved in tumor angiogenesis mostly via their matrix-degrading capacity and neovascularization potential [15]. In breast cancer patients, the level MMP-2 and MMP-9 are overexpressed [13] which is associated with a shortened relapse-free survival [16].
Matrix metalloproteinases activities and function were regulated by the tissue inhibitor of metalloproteinase (TIMP) family which includes four subtypes (TIMP-1, 2, 3, and 4). Down-regulation of TIMPS shows some apoptotic properties in different cancer cell lines [17]. TIMP-3 overexpression is associated with apoptosis in lung cancer cell lines. The TIMPs overexpression can reduce the metastasis of cancer [18], for example, TIMP1 overexpression slows the carcinogenesis process in transgenic mice [19], whereas, TIMP-2 is involved in carcinogenesis and metastasis, and is downregulated in prostate cells and tumor samples [20].
A large number of natural products have chemo-preventive potential with no side effects [21]. Eugenol is listed by the Food and Drug Administration as “Generally Regarded as Safe” when consumed orally in the unburned form [22]. Eugenol is a natural phenolic compound available in honey and the essential oils of cloves, cinnamon, and other aromatic spices. It is added as a therapeutic ingredient in various medications to treat digestive disorders [23] and as an antiseptic, analgesic [24], anti-inflammatory, antimicrobial [25] and antioxidant agent [26]. Furthermore, eugenol has several anticancer properties in colon, liver, prostate, and breast cancer [22, 27]. Eugenol prevents cancer progression by modulating the expression of several genes involved in cell growth, angiogenesis, and apoptosis [22]. Moreover, in a rat model of gastric carcinogenesis, eugenol was observed to induce apoptosis and inhibit invasion and angiogenesis [28].
Up to date, we could not find any study in the literature, describing the anti-metastatic activity of eugenol against triple negative (MDA-MB-231) and anti-metastatic, anti-proliferative and apoptotic activity of eugenol against HER2 positive (SK-BR-3) breast cancer cells. Therefore, this study aimed to assess the effect of eugenol on the proliferation, metastasis, and apoptosis of triple-negative MDA-MB-231 and HER2-positive SK-BR-3 breast cancer cell lines.
Methods
Reagents
Eugenol and Trypan blue solution were purchased from Sigma Aldrich (Sigma Aldrich, USA). TaqMan probes, Gene expression PCR Master Mix kit, and High Capacity cDNA Reverse Transcription kit were purchased from Applied Biosystems (Life Technologies, Grand Island, NY, USA). MDA-MB-231 (ATCC HTB-26®) and SK-BR-3 (ATCC HTB-30™) cells were obtained from American Type Culture Collection (Rockville, MD, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), Roswell Park Memorial Institute (RPMI) medium, TRIzol reagent, and Muse™ Annexin V & Dead Cell Kit were purchased from Merck KGaA© (Darmstadt, Germany). MTT reagent was purchased from Roche (Roche Diagnostics, Mannheim, Germany). Western blot detection kits, Luminata® Western HRP Chemiluminescence Substrates were purchased from EMD Millipore (Billerica, MA).
Cell viability assay using MTT
Viability of triple negative- (MDA-MB-231) and HER2 positive- (SK-BR-3) breast cancer cells in response to the different concentrations of eugenol was determined by measuring the capacity of cellular oxidoreductase enzymes present in viable cells to convert the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to its insoluble formazan form. In brief, cells were seeded in 96-well plates (1 × 104 cells/well) and incubated for 24 h. The medium was then replaced with fresh medium containing different concentrations of eugenol; untreated cells were treated with Dimethyl sulfoxide (DMSO). At the end of 24 h, 10 μl of MTT reagent was added to each well, and the plates were incubated for 4 h at 37 °C. Next, 100 μl of isopropanol was added to each well, and after 15 min, the amount of formazan was quantified by measuring absorbance at 450 nm using an ELISA reader. Percentage of cell proliferation was calculated relative to control wells designated as 100% viable cells, where % cell proliferation = (A treated)/(A control) × 100.
Cell proliferation analysis using real-time cell electronic sensing system
MDA-MB-231 and SK-BR-3 were cultured individually in 100 μl complete medium containing 2 × 104 cells in each well of 16-well microtiter Eplates with integrated microelectronic sensor arrays at the bottom of each well. The plates were incubated for 30 min, then inserted into a Real-Time Cell Electronic Sensing System (ACEA Biosciences Inc., San Diego, CA). The eugenol was added to each well after the cell growth reaches the log phase, then the plates were inserted into the Real-Time Cell Electronic Sensing System, finally were incubated for 72 h. (for label-free and dynamic monitoring of cell proliferation). The electronic readout, cell-sensor impedance, is displayed as arbitrary units called the cell index.
RNA extraction and cDNA synthesis
TRIzol reagent (Invitrogen®) was applied to isolate total RNA according to the standard protocol as described previously [29]. The quality and quantity of isolated RNA were assessed by measuring absorbance at 260 nm and maintaining a 260/280 ratio of ~ 2.0. First strand cDNA was synthesized from 1 μg total RNA using the High-Capacity cDNA reverse transcription kit (Applied Biosystems®) according to the manufacturer’s instructions as described previously [30].
Real-time polymerase chain reaction
To determine the effect of eugenol on gene expression levels, quantitative real-time polymerase chain reaction (qRT-PCR) was conducted. MDA-MB-231 cells were treated with 4 μM and 8 μM eugenol whereas SK-BR-3 was treated with 5 μM and 10 μM eugenol for 48 h. These doses and times were chosen based on the cytotoxicity results. Quantitative PCR was performed to detect the expression levels of MMP2 (Hs01548724_m1), MMP9 (Hs00957562_m1), and TIMP1 (Hs01092511_m1) using the gene expression master mix (Applied Biosystems, CA, USA), on the ABI PRISM 7500 Detection System (Applied Biosystems, USA). Glyceraldehyde-3-phosphate dehydrogenase (Hs02786624_g1) was used as an internal control for qRT-PCR. Each sample was analyzed in triplicate, and representative data sets are shown. Results were analyzed using the 2−ΔΔCT method. Data were expressed as mean fold changes ± standard deviation (SD) for three independent amplifications.
Western blot analysis
Total protein was extracted from cells after treatment with eugenol, and protein concentrations were determined using NanoDrop (NanoDrop 8000, Thermo Scientific, USA). Western blot was performed according to our previous study [31] using appropriate primary antibodies (Cell Signaling, Danvers, MA, USA): Total and cleaved caspase-3 (9662), Total and cleaved caspase-9 (9508), Caspase-7 (D2Q3L), MMP-9 (D6O3H) XP, MMP-2 (D4M2N), TIMP1 (D10E6), GAPDH (D16H11) XP, followed by HRP-linked secondary Antibody (7074) or (7076). Then the Bands were visualized using the Documentation System; images were captured and analyzed using Quantity One software, (Bio-Rad, USA). The relative quantification was calculated by dividing the density of the target protein with the respective loading control. GAPDH was used as the loading control.
Flow cytometric analysis of apoptosis
The percentage of cells undergoing apoptosis was determined by flow cytometry using annexin V staining. According to the manufacturer’s instructions, MDA-MB-231 and SK-BR-3 cells were treated with different doses of eugenol for 48 h, the medium was removed, and cells were washed with cold PBS. Cells were collected after trypsinization by centrifugation at 300×g for 5 min and then resuspended in 0.5 ml PBS. Further, 100 μl of cell suspension was incubated with 100 μl Muse™ Annexin V & Dead Cell reagent in the dark for 20 min at 25 °C and immediately analyzed on the Muse™ Cell Analyzer (Merck KGaA Co., Darmstadt, Germany) to determine the viable, apoptotic, and necrotic cells populations.
Wound healing assay
Migration potential of MDA-MB-231 and SK-BR-3 cells after eugenol treatment was determined using a wound healing assay. MDA-MB-231 and SK-BR-3 cells (5 × 105) were cultured in 6-well plates and grown to 70–80% confluence. Subsequently, the cells were scratched using a 200-μl pipette tip when they grew to 90% confluence. The cells were washed twice using PBS, and the specific medium for each cell was completely replaced. Different concentrations of eugenol were added to the plate and incubated for 48 h. Wound closure was recorded using an Olympus inverted microscope (Olympus, Tokyo, Japan). To evaluate cell migration ability, at least 5 random fields were captured for each time point at 0, 24, and 48 h. The distance migrated by the cell monolayer to close the wounded area during this period was measured and analyzed using Image J software. Experiments were performed in triplicate and repeated at least three times.
Statistical analyses
Comparative analysis of results from various experimental groups with their corresponding controls was performed using Graph Pad 5.0 Prism software (GraphPad Software, Inc., La Jolla, CA, USA). Data were stated as mean ± standard deviation (SD) (from triplicate experiments). One-way analysis of variance (ANOVA) followed by Tukey–Kramer post-ANOVA test was used to measure the treated groups. Differences were considered significant when p < 0.05.
Results
Effect of eugenol on cell viability
To determine the anti-proliferative effect of different eugenol concentrations against MDA-MB-231 and SK-BR-3 cell growth, the cell proliferation assay was performed using the MTT method and real-time cell analysis. Figure 1 shows that eugenol at concentrations up to 2.5 μM did not significantly affect cell viability. However, concentrations of 5, 10, and 20 μM significantly decreased the viability of MDA-MB-231 cells by approximately 20, 35, and 58%, respectively, at 24-h incubation period, whereas, at 48-h incubation with eugenol 5, 10, and 20 μM significantly reduced the cell viability by approximately 40, 65, and 80%, respectively. On the other hand, high eugenol concentrations (40 and 60 μM) markedly inhibit cell proliferation by > 90%. Eugenol at concentrations 5, 10, and 20 μM significantly decreased SK-BR-3 cell viability by approximately 15, 30, and 70% respectively upon 24 h incubation, whereas 48 h incubation with eugenol at 5, 10, and 20 μM reduced the cell viability by approximately 32, 72, and 80%, respectively. The data obtained data from MTT, and real-time cell analysis showed almost the same results of cell viability.
Effect of eugenol on cell growth and proliferation
The effect of eugenol concentrations on MDA-MB-231 and SK-BR-3 cell proliferation was also investigated using real-time cell analysis. Results indicated that eugenol could regulate the cell proliferation of MDA-MB-231 and SK-BR-3 cells (Fig. 2). Figure 2a shows that incubation of MDA-MB-231 for 24 and 48 h with 4 μM eugenol significantly inhibits MDA-MB-231 cell proliferation by 29.7 and 24.5% respectively (p < 0.05) compared to untreated cells, and incubation with 8 μM eugenol for the same period results in significantly reduced cell proliferation by 70 and 76.4% respectively compared to that of untreated cells (p < 0.05). Eugenol 10 μM could thus be a toxic dose wherein incubation with 10 μM eugenol for 24 and 48 h significantly decreased MDA-MB-231 cell proliferation by 95.1 and 97.1%, respectively compared to untreated cells (p < 0.001). Similarly, incubation with 5 μM eugenol for 24 and 48 h significantly reduced SK-BR-3 proliferation by 20 and 5% respectively (p < 0.05). Eugenol at 10 μM significantly decreased SK-BR-3 proliferation by 63.3 and 68.1% at 24 and 48 h, respectively, compared to untreated cells (p < 0.001). Similarly, incubation with 12 μM eugenol for 24 and 48 h caused a significant decrease in SK-BR-3 cell proliferation by 66.7 and 67.3%, respectively, compared to untreated cells (Fig. 2b). Based on these findings, eugenol at 4 and 8 μM for MDA-MB-231 and 5 and 10 μM for SK-BR-3 at the 48 h time interval were used in all subsequent experiments.
a Proliferation curve of MDA-MB-231 cells obtained by real-time analysis. Cells were seeded in E-16 Plates at 2 × 104 cells/well and were continuously observed by measuring the cell index (CI) values for 72 h. Cell proliferation was observed at intervals of 15 min. The black line marks the normalization of the CI time point at 20 h. MDA-MB-231 cells were then treated with different doses of eugenol. Colored curves represent the various concentrations of eugenol. Pink line: Control (DMSO); Blue line: 4 μM Eugenol; Green line: 8 μM Eugenol; Red line: 10 μM Eugenol. Data are presented as mean ± SD (n = 3). *, # and $ indicate a significant change from untreated cells, 4, and 8 μM eugenol, respectively, at p < 0.05 using ANOVA followed by Tukey–Kramer post-ANOVA test. b Proliferation curve of SK-BR-3 cells obtained by real-time analysis. Cells were seeded in E-16 Plates at 2 × 104 cells/well and were continuously observed by measuring CI values for 72 h. Cell proliferation was observed at intervals of 15 min. The black line marks the normalization of the CI time point at 20 h. SK-BR-3 cells were then treated with different doses of eugenol. Colored curves represent various concentrations of eugenol. Pink line: Control (DMSO); Blue line: 5 μM Eugenol; Green line: 10 μM Eugenol; Red line: 12 μM Eugenol. Data are presented as mean ± SD (n = 3). * and # indicate significant changes from untreated and 5 μM eugenol, respectively, at p < 0.05 using ANOVA followed by Tukey–Kramer post-ANOVA test
Effect of different concentrations of eugenol on cell migration
Wound healing assay was performed using eugenol treated MDA-MB-231 (4 and 8 μM) and SK-BR-3 (5 and 10 μM) cells to investigate the effect of eugenol on cell migration. Figure 3 shows that incubation with eugenol significantly reduced the migration ability of MDA-MB-231 and SK-BR-3 cells compared to untreated cells (p < 0.05). As shown in Fig. 3a, eugenol concentrations of 4 and 8 μM after 24 h delay MDA-MB-231 cell migration by 43 and 62%, respectively from 24 h control and, after 48 h delay MDA-MB-231 cell migration by 95 and 96%, respectively from 48 h control. Whereas eugenol concentrations of 5 and 8 μM after 48 h delay SK-BR-3 cell migration by 97% (Fig. 3b).
a, b Effect of eugenol on cell migration of MDA-MB-231 (a) and SK-BR-3 (b). Eugenol delayed the wound-healing time in human MDA-MB-231 (a) and SK-BR-3 (b) cells. The inhibitory effects of eugenol on the cells were determined using a wound healing assay. The distance of the scratch was measured in the control and eugenol groups using Image J software. Data are presented as mean ± SD (n = 3). * and # indicate a significant change from 0 and 24 h of incubation with eugenol respectively, at p < 0.05 using ANOVA followed by Tukey–Kramer post-ANOVA test
Effect of eugenol on MMP2 expression levels
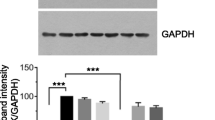
To determine the ability of eugenol to regulate the expression levels of genes involved in tumor metastasis, MDA-MB-231, and SK-BR-3 cells were incubated with two concentrations of eugenol, 4 and 8 μM, and 5 and 10 μM, respectively for 48 h. Subsequently, mRNA and protein levels were determined by real-time PCR and western blot. Figure 4a shows that incubation of MDA-MB-231 with 4 μM eugenol decreases the expression MMP-2 by 1.25-fold whereas 8 μM significantly decreases it by 2-fold compared to both untreated and 4 μM treated cells. Similarly, SK-BR-3 treated with 5 and 10 μM eugenol shows a significant decrease in MMP-2 expression levels by 1.6- and 1.72-fold, respectively, compared to untreated cells. To investigate whether the changes in MMP2 mRNA levels are associated with alteration of protein levels, MDA-MB-231 and SK-BR-3 cells were incubated for 48 h with eugenol at 4 and 8 μM, and 5 and 10 μM, respectively. MMP-2 protein levels were then determined by western blot analysis. Similar to mRNA results, Fig. 4b shows that eugenol significantly decreases the protein levels of MMP2 in MDA-MB-231 by approximately 1.4- and 1.8-fold, and in SK-BR-3 by 1.75- and 2.1-fold, respectively compared to untreated cells, after normalization to GAPDH levels.
Effect of eugenol treatment on the mRNA expression of MMP2 in MDA-MB-231 and SK-BR-3 (a), and on MMP2 protein levels (b), mRNA expression of MMP9 in MDA-MB-231 and SK-BR-3 (c) and MMP 9 protein levels (d), mRNA expression of TIMP-1 in MDA-MB-231 and SK-BR-3 (e), and TIMP-1 protein levels (f). Cells were treated for 48 h with eugenol (4 and 8 μM and 5 and 10 μM for MDA-MB-231 and SK-BR-3, respectively). Data are presented as mean ± SD (n = 3). * and # indicate a significant change from untreated, 4, and 5 μM eugenol respectively, at p < 0.05 using ANOVA followed by Tukey–Kramer post-ANOVA test
Effect of eugenol on MMP-9 expression levels
Figure 4c shows that eugenol concentrations of 4 and 8 μM significantly decrease the expression levels of MMP-9 in MDA-MB-231 by 1.5- and 2.5-fold, respectively, compared to untreated cells. Incubation of SK-BR-3 with 5 μM eugenol causes an insignificant decrease in MMP-9 expression whereas a high concentration (10 μM) significantly decreases its expression compared to untreated cells (Fig. 4c). Similar to the mRNA results, Fig. 4d shows that eugenol significantly decreases the protein levels of MMP-9 in MDA-MB-231 by approximately 1.5- and 1.7-fold, and in SK-BR-3 by 1.65- and 1.8-fold, respectively compared to untreated cells, after normalization to GAPDH levels.
Effect of eugenol on TIMP-1 expression levels
Figure 4e shows the effect of different concentrations of eugenol (4 and 8 μM) on the expression levels of TIMP-1 in MDA-MB-231 and (5 and 10 μM) in SK-BR-3 cells. Incubation of MDA-MB-231 with different concentrations of eugenol did not significantly alter the levels of TIMP-1. Likewise, incubation of SK-BR-3 with 5 and 10 μM eugenol increased the expression levels of TIMP-1 compared to untreated cells, albeit insignificantly (Fig. 4e). Similarly, eugenol treatment to MDA-MB-231 and SK-BR-3 resulted in insignificant changes in TIMP-1 protein levels in both cells (Fig. 4f).
Effect of eugenol treatment on the expression levels of apoptotic genes
To determine the ability of eugenol to modulate the expression levels of caspase-3,-7, and − 9, MDA-MB-231 and SK-BR-3 cells were incubated for 48 h with increasing concentrations of eugenol (4 and 8 μM, and 5 and 10 μM, respectively). Subsequently, mRNA expression levels of apoptotic genes were quantified by real-time PCR. Figure 5 (a, c, e) shows that cells incubation with eugenol for 48 h significantly increased the mRNA expression levels of caspase-3, − 7, and − 9 in MDA-MB-231 and SK-BR-3 in a concentration-dependent manner. In MDA-MB-231, the maximum induction levels of caspase-3 (6.3-fold), caspase-7 (5.5-fold), and caspase-9 (4-fold) were observed at the highest concentration of eugenol tested, i.e., 8 μM. Similarly, in SK-BR-3, the maximum induction levels of caspase-3 (5.8-fold), caspase-7 (8-fold), and caspase-9 (6.7-fold) were observed at the highest concentration tested, i.e., 10 μM. To confirm the effect of eugenol on the expression of apoptotic genes, breast cancer cell lines (MDA-MB-231 and SK-BR-3) were treated with different concentrations of eugenol and harvested after 48 h. Whole cell extracts were used to evaluate the levels of total and cleaved caspase-3, cleaved caspase-7, and total and cleaved caspase-9 proteins using immunoblotting and specific antibodies. Figure 5b, f show that eugenol triggered the cleavage of caspase-3 and -9, respectively, leading to a significant elevation in their active forms. Eugenol significantly overexpressed cleaved caspase-7 protein expression level (Fig. 5d) confirming the induction of apoptosis by eugenol in both triple negative and HER2 positive breast cancer cells.
Effect of eugenol treatment on the expression levels of caspases in MDA-MB-231 and SK-BR-3 cell lines. MDA-MB-231 and SK-BR-3 cells were treated for 48 h with eugenol (4 and 8 μM, and 5 and 10 μM, respectively). The mRNA levels of caspase-3 (a), caspase-7 (c), and caspase-9 (e) genes were quantified by RT-PCR and normalized to GAPDH. Protein expression levels of total and cleaved caspase-3 (b), cleaved caspase-7 (d) and total and cleaved caspase-9 (f) were determined by western blotting. Data are presented as mean ± SD (n = 3). * and # indicate a significant change from untreated, 4, and 5 μM eugenol respectively, at p < 0.05 using ANOVA followed by Tukey–Kramer post-ANOVA test
To investigate the effect of eugenol concentrations on apoptotic genes and cell death, the percentage of apoptotic MDA-MB-231 and SK-BR-3 cells was determined by flow cytometry assay after 48 h of incubation with different doses of eugenol. Figure 6 shows that 97.82–97.38% of the untreated MDA-MB-231 and SK-BR-3 cells were healthy. However, MDA-MB-231 treated cells significantly increased the percentage of cells in late apoptosis from 0.59% (in untreated cells) to 58.10 and 61.55% and increased the percentage of dead cells from 0.55 to 19.8% and 26% in response to 4 and 8 μM of eugenol, respectively. Likewise, in SK-BR-3 cells, eugenol significantly increased the percentage of cells that experienced late apoptosis from 0.98% (untreated cells) to 98.40–97.65% in response to 5 and 10 μM eugenol respectively.
Effect of eugenol treatment on the percentage of apoptotic MDA-MB-231 and SK-BR-3 cells. Cells were treated for 48 h with eugenol (4 and 8 μM, and 5 and 10 μM, respectively). The percentage of cells undergoing apoptosis was determined using annexin-V staining. Cells were immediately analyzed on the Muse™ Cell Analyzer (Merck KGaA Co., Darmstadt, Germany)
Discussion
Eugenol is a member of the phenyl-propanoid category of compounds. It is used in medicine as a local antiseptic and anesthetic and in food products as a flavoring agent; however, it has some toxicity when used at high concentrations. The present study intended to investigate the possible anti-proliferative and apoptotic effects of eugenol against triple negative and HER2 positive breast cancer cell lines. Results of the proliferation assay indicated that eugenol had a concentration- and time-dependent effect on MDA-MB-231 and SK-BR-3 cell proliferation, suggesting the potential anti-proliferative and apoptotic effects of eugenol in MDA-MB-231 and SK-BR-3 cells. Eugenol at 40 and 60 μM markedly inhibited cell proliferation by more than 90% in both MDA-MB-231 and SK-BR-3 as determined by MTT assay. Another study suggested that 4 μM eugenol could inhibit cell proliferation in normal, ER-negative, and ER-positive breast cancer cells [22]. The cytotoxic effect of eugenol on G361 cells in the range of 0.5 to 2 mM was also reported. Moreover, the cell proliferation and migration of MCF7 ER + breast cancer cell line show dependence in time and eugenol dose [32]. The observed variation in the cytotoxic effect of eugenol across the literature could be due to the variation in used concentrations, purity of eugenol, and the source or types of cell lines.
In cancer, invasion, and metastasis, the primary cause of death, is mediated by a group of genes (e.g., MMPs) via degradation of the extracellular matrix with complicated steps [29, 33, 34]. Therefore, suppression of MMPs (expression or secretion) may be an effective strategy in preventing cell migration and invasion. Earlier trials with MMPs inhibitors in breast cancer revealed serious dose-limiting toxicity or failure to reach therapeutic plasma levels, which may be due in part, to their inability to target specific MMPs.
Multiple signaling pathways regulate the expression of MMPs [35, 36]. Thus, targeting genes in these signaling pathways may suppress or decrease metastasis and consequently reduce cancer mortality. The MMP-2 and -9 are collagenases involved in tumor invasion and metastasis through the degradation of collagen IV [37]. A correlation has been reported between a higher rate of distant metastases and high expression levels of MMP-2 and -9 [38]. Tissue inhibitor of metalloproteinases-1 has been suggested as a marker of prognosis and response to treatment in breast cancer. In the present study, MMP-2 and -9 mRNA and protein expression levels were significantly suppressed in cells after eugenol treatment, even though TIMP-1 was not changed. Further, eugenol treatment reduced the invasion and migration of breast cancer cells as shown in the wound healing assay. To the best of our knowledge, this is the first study to describe the anti-metastatic effect of eugenol against MDA-MB-231 and SK-BR-3 breast cancer cell lines. The observed efficient reduction of MMP gene expression levels during eugenol treatment suggested that eugenol can suppress triple negative as well as HER2-positive breast cancer metastasis. A study found that 100 μM eugenol has an antioxidant effect on human fibrosarcoma cells, and can inhibit MMP-9 activity and expression [39]. Another study found a correlation between high expression levels of MMP-2 and MMP-9 and lymph node metastasis and tumor staging in breast cancer patients [40]. Low expression levels of MMP-2 and MMP-9 in breast cancer patients may indicate a relatively good patient prognosis.
Eugenol is a cytotoxic compound and can trigger caspase-mediated cell death in breast cancer cells. Caspases have affected the mitochondria and upstream events of intrinsic apoptosis. Caspase-3, − 7, and − 9 have distinct roles during apoptosis with a possible feedback loop in the mitochondria. In the present study, eugenol significantly induced late apoptosis in MDA-MB-231 and SK-BR-3 in a dose-depended manner. It upregulated caspase-3, − 7, and − 9 expression levels in both MDA-MB-231 and SK-BR-3 along with a proportional increase in apoptosis. A previous study showed that eugenol suppressed E2F1/survivin and triggered apoptosis in breast cancer cells [22]. Eugenol may cause DNA degradation or cracking in MDA-MB-231 and SK-BR-3 cells and provoke a robust cytotoxic response [41]. Another study found that eugenol induces apoptosis in human osteosarcoma cells via activating caspase-3 and may play an important role in antitumor activity [42]. Cells deficient in caspase-3 or − 7 showed a delay in mitochondrial events of intrinsic apoptosis while Caspase-9 uncouples the mitochondria and increases ROS production [43]. A recent study found that eugenol enhances Bax, which may increase the release of cytochrome C from mitochondria and activate the caspase pathway needed for apoptosis [44].
Conclusion
The present study demonstrates that eugenol exhibits an anti-breast cancer effect via targeting the caspase pathway and may be considered as a potential therapeutic agent for both triple negative and HER2-positive breast cancer.
Abbreviations
- cDNA:
-
Complementary DNA
- DMSO:
-
Dimethyl sulfoxide
- ECM:
-
Extracellular Matrix
- ELISA:
-
enzyme-linked immunosorbent assay
- ER:
-
Estrogen Receptor
- FDA:
-
Food and Drug Administration
- HER2:
-
Human epidermal growth factor receptor 2
- MMP2:
-
Matrix metalloproteinases 2
- MMP9:
-
Matrix metalloproteinases 9
- MMPs:
-
Matrix metalloproteinases
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PR:
-
Progesterone Receptor
- qRT-PCR:
-
Quantitative Real-time polymerase chain reaction
- RNA:
-
Ribonucleic acid
- TIMP-1:
-
Tissue inhibitor metalloproteinases
- TIMP-2:
-
Tissue inhibitor metalloproteinases
- TIMP-3:
-
Tissue inhibitor metalloproteinases
- TNBC:
-
Triple-negative breast cancer
References
Becker S. A historic and scientific review of breast cancer: the next global healthcare challenge. Int J Gynaecol Obstet. 2015;131 Suppl 1:S36–9.
Maayah ZH, Ghebeh H, Alhaider AA, El-Kadi AO, Soshilov AA, Denison MS, Ansari MA, Korashy HM. Metformin inhibits 7,12-dimethylbenz[a]anthracene-induced breast carcinogenesis and adduct formation in human breast cells by inhibiting the cytochrome P4501A1/aryl hydrocarbon receptor signaling pathway. Toxicol Appl Pharmacol. 2015;284(2):217–26.
Yersal O, Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol. 2014;5(3):412–24.
Rychly B, Sidlova H, Danis D. The 2007 World Health Organisation classification of tumours of the central nervous system comparison with 2000 classification. Cesk Patol. 2008;44(2):35–6.
Korlimarla A, Prabhu JS, Remacle J, Rajarajan S, Raja U, Srinath BS, Manjunath S, Correa M, Sridhar TS. Identification of BRCA1 deficiency using multi-Analyte estimation of BRCA1 and its repressors in FFPE tumor samples from patients with triple negative breast Cancer. PLoS One. 2016;11(4):e0153113.
Simon N, Antignani A, Sarnovsky R, Hewitt SM, FitzGerald D. Targeting a Cancer-specific epitope of the epidermal growth factor receptor in triple-negative breast Cancer. J Natl Cancer Inst. 2016;108(8):djw028.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67.
Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112(1):3–25.
McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16(8):717–27.
Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73.
Baker EA, Bergin FG, Leaper DJ. Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging. Br J Surg. 2000;87(9):1215–21.
Gonzalez-Arriaga P, Pascual T, Garcia-Alvarez A, Fernandez-Somoano A, Lopez-Cima MF, Tardon A. Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer risk and survival. BMC Cancer. 2012;12:121.
Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44-46:200–6.
Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2(9):657–72.
Fink K, Boratynski J. The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth metastasis and angiogenesis. Postepy Hig Med Dosw (Online). 2012;66:609–28.
Johanson M, Zhao XR, Huynh-Ba G, Villar CC. Matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and inflammation in cyclosporine A-induced gingival enlargement: a pilot in vitro study using a three-dimensional model of the human oral mucosa. J Periodontol. 2013;84(5):634–40.
Hassan ZK, Daghestani MH. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac J Cancer Prev. 2012;13(7):3259–64.
Khokha R. Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melanoma cells in vivo by the overexpression of the tissue inhibitor of the metalloproteinases-1. J Natl Cancer Inst. 1994;86(4):299–304.
Buck TB, Yoshiji H, Harris SR, Bunce OR, Thorgeirsson UP. The effects of sustained elevated levels of circulating tissue inhibitor of metalloproteinases-1 on the development of breast cancer in mice. Ann N Y Acad Sci. 1999;878:732–5.
Hannocks MJ, Zhang X, Gerwien H, Chashchina A, Burmeister M, Korpos E, Song J, Sorokin L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2017.
Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of Cancer chemotherapeutic and Chemopreventive agents. Med Princ Pract. 2016;25(Suppl 2):41–59.
Al-Sharif I, Remmal A, Aboussekhra A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer. 2013;13:600.
Yoo CB, Han KT, Cho KS, Ha J, Park HJ, Nam JH, Kil UH, Lee KT. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005;225(1):41–52.
Pramod K, Ansari SH, Ali J. Eugenol: a natural compound with versatile pharmacological actions. Nat Prod Commun. 2010;5(12):1999–2006.
Benencia F, Courreges MC. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother Res. 2000;14(7):495–500.
Rossi GR, Mautino MR, Awwad DZ, Husske K, Lejukole H, Koenigsfeld M, Ramsey WJ, Vahanian N, Link CJ. Allogeneic melanoma vaccine expressing alphaGal epitopes induces antitumor immunity to autologous antigens in mice without signs of toxicity. J Immunother. 2008;31(6):545–54.
Ghosh R, Ganapathy M, Alworth WL, Chan DC, Kumar AP. Combination of 2-methoxyestradiol (2-ME2) and eugenol for apoptosis induction synergistically in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol. 2009;113(1–2):25–35.
Manikandan P, Murugan RS, Priyadarsini RV, Vinothini G, Nagini S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. 2010;86(25–26):936–41.
Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819–29.
Hafez MMHS, El-Khadragy MF, Hassan ZK, Al Rejaie SS, Sayed-Ahmed MM, Al-Harbi NO, Al-Hosaini KA, Al-Harbi MM, Alhoshani AR, Al-Shabanah OA, Alsharari SD. Effect of ginseng extract on the TGF-β1 signaling pathway in CCl4-induced liver fibrosis in rats. BMC Complement Altern Med. 2017;13(17):1507–10.
Sayed-Ahmed MM, Aldelemy ML, Hafez MM, Al-Shabanah OA. Inhibition of gene expression of organic cation/carnitine transporter and antioxidant enzymes in oxazaphosphorines-induced acute cardiomyopathic rat models. Oxidative Med Cell Longev. 2012;2012:452902.
Baharara TR J, Mousavi M, Kouhestanian K. Eugenol suppressed metastasis of breast carcinoma cells and migration by regulation of MMP-9 & Paxilin gene expression. Scholars Journal of Agriculture and Veterinary Sciences. 2015;2B(2):125–30.
Hong BH, Wu CH, Yeh CT, Yen GC. Invadopodia-associated proteins blockade as a novel mechanism for 6-shogaol and pterostilbene to reduce breast cancer cell motility and invasion. Mol Nutr Food Res. 2013;57(5):886–95.
Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel). 2014;6(3):1298–327.
Reddy KB, Krueger JS, Kondapaka SB, Diglio CA. Mitogen-activated protein kinase (MAPK) regulates the expression of progelatinase B (MMP-9) in breast epithelial cells. Int J Cancer. 1999;82(2):268–73.
Pujada A, Walter L, Patel A, Bui TA, Zhang Z, Zhang Y, Denning TL, Garg P. Matrix metalloproteinase MMP9 maintains epithelial barrier function and preserves mucosal lining in colitis associated cancer. Oncotarget. 2017;8(55):94650–65.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67.
Vizoso FJ, Gonzalez LO, Corte MD, Rodriguez JC, Vazquez J, Lamelas ML, Junquera S, Merino AM, Garcia-Muniz JL. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer. 2007;96(6):903–11.
Nam H, Kim MM. Eugenol with antioxidant activity inhibits MMP-9 related to metastasis in human fibrosarcoma cells. Food Chem Toxicol. 2013;55:106–12.
Hai Li ZQ, Li F, Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. oncology letter. 2017;14(5):5865–70.
Wan X, Zheng X, Pang X, Zhang Z, Zhang Q. Incorporation of lapatinib into human serum albumin nanoparticles with enhanced anti-tumor effects in HER2-positive breast cancer. Colloids Surf B Biointerfaces. 2015;136:817–27.
Jaganathan SK, Supriyanto E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules. 2012;17(6):6290–304.
Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32.
Habtemariam S, Belai A. Natural therapies of the inflammatory bowel disease: the case of Rutin and its Aglycone, quercetin. Mini Rev Med Chem. 2017.
Acknowledgments
The authors thank the Deanship of Scientific Research and RSSU at King Saud University for providing technical support. And Dr. Abdelilah Aboussekhra Department of Molecular Oncology, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia for technical support.
Funding
This study was supported by King Abdul-Aziz City for Science and Technology (Grant No.1–17–03-001-0008).
The funder had no implication in the design of the study, collection, analysis and interpretation of data; and in writing the manuscript; and the decision to submit the article for publication.
Availability of data and materials
All the data obtained and materials analyzed in this research are available with the corresponding author.
Authors' contributions
MLA, MMH, AA, and OA: participated in the study design and treatment, participated in practical work, collated, analyzed and interpreted the data, and also drafted the manuscript. All authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Abdullah, M.L., Hafez, M.M., Al-Hoshani, A. et al. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement Altern Med 18, 321 (2018). https://doi.org/10.1186/s12906-018-2392-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-018-2392-5