Abstract
Background
Sesquiterpene lactones (STLs) make a diverse and huge group of bio-active constituents that have been isolated from several plant families. However, the greatest numbers are present in Asteraceae family having more than 3000 different reported structures. Recently several researchers have reported that STLs have significant antioxidant and anticancer potentials.
Methods
To investigate the antioxidant, anticancer and antinociceptive potentials of STLs, gravity column chromatography technique was used for isolation from the biologically rich chloroform fraction of Artemisia macrocephala Jacquem. The antioxidant activity of the isolated STLs was determined by DPPH and ABTS free radical scavenging activity, anticancer activity was determined on 3 T3, HeLa and MCF-7 cells by MTT assay while the antinociceptive activity was determined through acetic acid induced writhings, tail immersion method and formalin induced nociception method.
Results
The results showed that the STLs of Artemisia macrocephala possesses promising antioxidant activity and also it decreased the viability of 3 T3, HeLa and MCF-7 cells and mild to moderate antinociceptive activity.
Conclusion
Sesquiterpenes lactones (STLs) are widely present in numerous genera of the family Asteraceae (compositae). They are described as the active constituents used in traditional medicine for the treatment of various diseases. The present study reveals the significant potentials of STL and may be used as an alternative for the management of cancer. Anyhow, the isolated compound is having no prominent antinociceptive potentials.
Similar content being viewed by others
Background
Sesquiterpenoids are extensively dispersed in plant kingdom. They are natural products with 15 carbons [1–3]. Numerous of them show cyclic chemical structures [4–10]. These compounds are synthesized by plants in several organs such as leaves, fruits or roots [11–14].
Sesquiterpene lactones (STLs) are a sub-class of sesquiterpenoids and are typical secondary compounds of the Asteraceae plant family [15, 16]. In the last several years, the potent bioactivities of STLs, such as anti-malarial activity of artemisinin [17], drew significant interests in the biochemistry of STLs [18]. They show anti-microbial activities, serve as antifeedants [19–21], as an antimigraine [22, 23], anti-inflammatory, for treatment of stomach-ache, skin infection [24], antitumor, antiulcer, neuro-cytotoxic, cardiotonic activities [25] and antineoplastic activity and hence make lead structures for the development of therapeutic agents. According to scientific evidence, the lactone fraction obtained from Artemisia annua L have greater potential for pain relief revealed by chemical-induced nociception assays in mice [26]. Similarly several experiments have shown the antinociceptive potential of terpenes in different painful conditions. They were able to reduce significantly the nociceptive response in various models of nociception with possible involvement of muscarinic, opioid, dopaminergic, adenosinergic and glutamatergic systems and the involvement of ATP-sensitive K+ channels [27]. Citronellal, a monoterpene possess antinociceptive potentials with the involvement of opioid system [28, 29], similarly carvacrol, p-cymene, hydroxydihydrocarvone (monoterpene) possess antinociceptive potentials in several models of animals [30–32]. Through structural analysis and the mechanism proposed by scientific studies, the monoterpenes like myrcene, linalool, citronellol and citronellal usually have antinociceptive potentials via acting on the opioid system [33].
In view of these, we studied the Hirshfeld analysis and fingerprint plots of the crystal of STL isolated from Artemisia macrocephala Jacquem and subjected to antioxidant and cytotoxic study.
Methods
Collection and authentitacation of plant materials
Aerial parts of Artemisia macrocephala (A. macrocephala) were collected from Badwan chowk, Dir Lower, Khyber Pakhtunkhwa, Pakistan in the month of April, 2015. The plant was identified by Professor Jahandar Shah. A voucher specimen, AM-01-2015, has been submitted in Malakand University herbarium.
Extraction fractionation and isolation of sesquiterpene lactone
Shade-dried (10 kg) aerial parts of A. macrocephala were pulverized. This material was then soaked in commercial grade methanol with occasional stirring. Filtered the whole suspension after 22 days and repeated this process three times. Then combined all the filtrates and concentrated through rotary evaporator under reduced pressure at 40 °C. 1 kg of methanolic greenish-black extract was obtained which was subjected to successive fractionation.
The crude extract was added to 5 l distilled water followed by the addition of an equal volume of n-hexane and shaken gently in a separating funnel. Collected the n-hexane layer from the separating funnel and continued this process till there appeared no color in the n-hexane when added further. Then combined the entire n-hexane portion and subjected to rotary evaporator at 45 °C, 120 g n-hexane fraction (12%) was collected. The process was repeated for chloroform (37.8%), ethyl acetate (12.2%) and n-butanol (15.4%) to obtain their respective fractions. The remaining aqueous portion left was approximately 210 g (21%).
Chemical characterization of crude extract/fractions and isolation of sesquiterpene lactone
The crude methanolic extract and fraction were tested for the presence of terpenoids [34]. The fraction showing positive result (chloroform fraction) was subjected to column chromatography for the isolation and purification of terpenoids by elution with 5% ethyl acetate: n-hexane. It gave the compound Ism-1 (1.3 g). TLC plate was used for examining the purity of the compound and visualized under UV lamp. Further spectral and crystal data lead to the confirmation of the compound as sesquiterpene lactone (STL).
DPPH free radical scavenging assay
The antioxidant activity of the isolated STL was carried out through its scavenging ability of DPPH free radical. Various dilutions of 25, 50, 75, 100, 125 and 150 μM of the isolated compound were mixed with 1 ml solution of DPPH (90 μM) and adjusted the final volume 4 ml via methanol. After a time period of 1 h the absorbance of test solutions and the blank was recorded at room temperature. Ascorbic acid helped as positive control. Each reading was taken in triplicate. Percent (%) scavenging of DPPH free radical was calculated as.
Where Ablank is absorbance of control, Asample is the absorbance of the test sample.
IC50 values, which represented the concentration of test sample that caused 50% neutralization of DPPH radical, were calculated from the plot of inhibition percentage against concentrations [35].
ABTS free radical scavenging assay
The antioxidant activity of the isolated compound was evaluated against ABTS free radical [36]. This is dependent upon the potential of antioxidants to scavenge ABTS radical cation causing a reduction in absorbance at 734 nm. Shortly, K2S2O4 solution (2.45 mM) was mixed with ABTS (7 mM) solution. Kept this mixture in dark at room temperature for 14 h which gave a dark color solution having cation radical of ABTS. The cation radical solution of ABTS was then diluted with 0.01 M phosphate buffer of pH 7.4 in order to adjust the absorbance 0.70 at 734 nm. ABTS solution (3 ml) was mixed with 300 μl of test sample to determine the radical scavenging potential of the test samples. Reduction in absorbance was determined through spectrophotometer after 1 min of the solutions mixing and continued for 6 min. Ascorbic acid served as positive control. All the readings were taken in triplicate. Percentage scavenging was calculated as:
Antioxidant potential was expressed as percent inhibition and as IC50 (Test sample concentration required for 50% reduction of ABTS radicals).
Anticancer activity
Anticancer activity of the isolated STL was carried out in 96-well flat-bottomed micro plates through standard MTT (3-[4, 5-dimethylthiazole-2-yl]-2, 5-diphenyl-tetrazolium bromide) colorimetric assay [37]. For this purpose, HeLa (Cervical Cancer), MCF-7 (Breast Cancer) and NIH 3 T3 (Mouse fibroblast) were cultured in Minimum Essential Medium Eagle, supplemented with 5% of fetal bovine serum (FBS), 100 IU/ml of penicillin and 100 μg/ml of streptomycin in 75 cm2 flasks, kept at 37 °C in 5% CO2 incubator. Exponentially growing cells were harvested, counted with haemocytometer and diluted with a particular medium. Cell culture with the concentration of 6×104, 5×104 and 5×104 cells/ml for HeLa, MCF-7 and NIH 3 T3 respectively was prepared and introduced (100 μL/well) into 96-well plates. After overnight incubation, medium was removed and 200 μL of fresh medium was added with different concentrations of compound (62.5-1000 μg/mL). After 48 h, 200 μL MTT (0.5 mg/ml) was added to each well and further incubated for 4 h. Subsequently, DMSO (100 μL) was added to each well. The extent of MTT reduction to formazan within cells was calculated by measuring the absorbance at 570 nm, through micro plate reader (Spectra Max plus, Molecular Devices, CA, USA). The cytotoxicity was recorded as concentration causing 50% growth inhibition (IC50) for HeLa. Percent inhibition was determined as:
% inhibition = 100-[(mean of O.D of test compound – mean of O.D of negative control)/(mean of O.D of positive control – mean of O.D of negative control) × 100].
The results (% inhibition) were processed by using Soft- Max Pro software (Molecular Device, USA).
Acute toxicity
Balb/C mice (18–26gm) were purchased from National Institute of Health (NIH) Islamabad and housed in separated cages at University of Malakand animal house with free access to standard diet and water and starved for 12–18 h before experimentation. Ethical Committee of Pharmacy Department, University of Malakand accepted the protocols (No: S-Ter-24-12/2015) of experimental and ensured its compliance with provisions of the “Animal Bye-Laws 2008, Scientific Procedures Issue-I of the University of Malakand”.
The isolated STL was tested for acute toxicity study as per standard protocol [38]. Balb C mice were given 25, 50 and 100 mg/kg of the isolated compound in phase first. While 125, 250 and 500 mg/kg in phase second were given to the experimental animals. One group, given normal saline served as control. They were observed for 6 h continuously for changes in the behavioral or autonomic responses followed by another examination after 24 h. Any mortality for the next 14 days was also noted.
Acetic acid induced writhing test
The peripheral antinociceptive response was determined through acetic acid induced writhing test. Two groups of mice (n = 6) for each sample were prepared. They were given 25 and 50 mg/kg of isolated compound 1 h before the injection of 10 ml/kg of 1% acetic acid intraperitoneally. Negative control group was given 10 ml/kg of 1% solution of Tween 80 while positive control group was given 10 mg/kg of diclofenac sodium intraperitoneally to overnight fasting mice. Writhing and stretching number was noted and percent protection was determined from the data [39].
Thermal nociception (Tail immersion method)
In order to determine the central antinociceptive response of the sample, the animals were given 25 and 50 mg/kg of isolated compound intraperitoneally, 2% vehicle and 10 mg/kg of diclofenac sodium, 30 min prior to the immersion of the tail (3 cm) into hot water at a temperature of 55 ± 0.5 °C. To assist the possible involvement of opioid receptor, morphine (agonist) at a dose of 5 mg and naloxone (antagonist) at a dose of 2 mg were used. The time of reaction taken at 15, 30, 45, 60, 75 and 90 min after administration of sample were noted with a stopwatch [40].
Formalin induced writhings test
This test was carried out with in Balb C mice using formalin. 25 and 50 mg/kg of isolated compound (i.p) were given to the pre labeled groups. 30 min after the treatment of test samples, 2.5% formalin (20 μl) was injected (s.c) in mice hind paw. The time spent in licking the injected paw in early phase (0–5 min) and late phase (15–30 min) was recorded. Naloxone, opioid antagonist (2 mg/kg, s.c) and standard drug indomethacin (10 mg/kg) was also used [41].
Statistical analysis
All the experiments were carried out in triplicate. The data was represented as mean ± SEM. Data was subjected to one way ANOVA followed by Dunnetts test for finding statistical significance. P value less than 0.1 was considered statistically significant.
Results
Structures of isolated compound
Colorless needle. 1H-NMR (CD3OD, 400 MHz): δ: 4.81 (dd, 1H, J= 1.0 Hz, J= 11.0 Hz, H-6), 2.64 (m, 1H, H-11), 2.61 (m, 1H, H-2β), 2.47 (m, 1H, H-2α), 2.39 (m, 1H, H-3β), 2.34 (m, 1H, H-3α), 2.17 (m, 1H, H-7), 1.94 (s, 3H, H-15), 1.84 (m, 1H, H-9β), 1.81 (m, 1H, H-8α), 1.66 (m, 1H, H-8β), 1.51 (m, 1H, H-9α), 1.30 (s, 3H, H-14), 1.19 (d, 3H, J 7.5 Hz, H-13). EI-MS (m/z) 248 [M] + [42].
The title compound (C15H20O3), comprises on one hexahydroxynaphthol ring (C3/C4/C6-C9/C11-C14), having a ketonic functionality at C-11 and dimetyl substitutions at C-8 and C-15 along with five membered cyclic ester [lactone, C1-C4/O1/O2 (Fig. 1a)].
Ring A adopts a half-chair conformation while ring B adopts complete chair conformation and ring C possess enveloped conformations (Fig. 1b). The title compound has three methyl substitutions, one methyl group is located on fused C-8, the second methyl group is located on C-14 and both has axial orientations. The third methyl group has pseudo-axial orientation on C-2 make the dihedral angle to the lactone ring (C-C2-C5) is 112.7 (3) °. The torsion angle of the plane (C8-C9-C14-C13) of ring A is 1.4 (6)°.
In the crystal, asymmetric molecule is directly interlinked with other four neighboring molecules through intermolecular interactions. Molecules are arranged in the crystal packing in such a way that making two dimer motifs via C-H•••O (C6-H6A•••O2 = 153.8° and H6A•••O2 = 2.709 Ǻ) and C-H•••C (C5-H5A•••C1 = 164.9° and H5A•••C1 = 2.892 Ǻ) intermolecular hydrogen bonding (Fig. 2c). Additionally, the O•••H (O2•••H2 = 2.507Ǻ) intermolecular interactions are involved in the formation of zig-zag anti-parallel chain in a two-dimensional network (Fig. 2d).
Hirshfeld surface and fingerprint plots analysis
Hirshfeld surface analysis is a powerful tool in the estimation and understanding the nature of intermolecular interactions involving in the packing of molecular crystal by using their fingerprint plots. In the title molecule, the Hirshfeld surface analysis shows a higher proportion of H•••H contacts, ranging from 60 to 70% as compared to O•••H, C•••H contacts (Fig. 3). In this case, the H•••H (Fig. 4) interactions are represented by the mid area of the fingerprint plots, which represents an asterisk oxygen interacting with the neighboring α-hydrogen of location, forming the zig-zag network of hydrogen bonds (Fig. 5).
The sharp “wings” seen in the plot belong to C-H•••O interactions, with the “wings” in the lower area of the fingerprint plot representing C-H•••O acceptor (H•••O) interactions. The proportion of O•••H interactions has a larger contribution than its H•••O counterparts. The proportion of O•••H interactions vary from 10.0 to 13.4%, is mainly due to neighboring ester group pointing toward the oxygen of the lactone moiety whereas proportion of O•••H interactions ranging from 12.0 to 15.7%.
Antioxidant activity of the STL towards DPPH and ABTS free radical scavenging radicals
This method of stable free radical DPPH is normally used to investigate the anti-oxidant potentials. Here the test was conducted to determine the IC50 value of STL to scavenge DPPH radicals. The isolated compound shows a dose dependent activity starting from 26.65 ± 1.04 at the lowest concentration to 68.76 ± 0.82 at the highest concentration used. The value is reaching to that of ascorbic acid at the same concentration used as standard. Other standards, tocopherol and rutin are anyhow having higher values at the same concentration of 150 \( \mu \)g/mL Table 1.
In the ABTS assay, the STL again showed an activity of 71.76 ± 0.82 at 150 \( \mu \)g/mL, having an excellent activity reaching to that of ascorbic acid again where as the other standards tocoperol and rutin are having the reading of 94.33 ± 0.49 and 94.67 ± 0.51 at the same concentration Table 2.
Inhibitory effects of the STL towards the proliferation of 3 T3, HeLa and MCF-7 cells
The sesquiterpenoids have prominent antitumor [25] and antineoplastic activity [43]. To evaluate such potentials of the isolated STL, we investigated its effects on 3 T3, HeLa and MCF-7 cells. The anti-proliferative potentials of the tested STL are expressed in terms of % cells growth inhibition and are depicted in Table 3. The isolated compound was found to be very active against HeLa MCF-7 cells. It showed 64.39 ± 2.40 and 81.25 ± 3.07% cell growth inhibitions against HeLa cell line at 500 and 1000 μg/mL concentrations respectively. Similarly, it revealed 65.34 ± 2.00 and 73.41 ± 2.11% cell growth inhibition against MCF-7 cells at 500 and 1000 μg/mL concentrations respectively. The compound mostly showed no anti-proliferative activity against 3 T3 cells even at the highest concentration of 1000 μg/mL (43.11 ± 1.34%).
Acute toxicity
According to International regulations concerning human health oblige that all novel pharmaceutical drugs are tested for safety before use in human beings. An important phase in ensuring drug safety is toxicity tests conduction in proper animal models. The studies of acute toxicity are among the series of toxicity tests used. The aim of this test is the identification of a dose that cause serious adverse effects and evaluation of lowest dose that cause lethality. The International Conference on Harmonization in its 3rd meeting (ICH M3) suggests the acute toxicity studies or appropriate alternatives are essential before administering new medicine in humans for the first time [44].
The isolated STL at a highest dose of 500 mg/kg body weight showed no adverse effect on the behavioral responses in the tested mice for 14 days observation. No mortality or weight change observed. The mice were sacrificed for checking the gross anatomy of liver and kidney, anyhow, no significant changes were observed when compared to normal. Therefore, the dose considered to be safe is 50 mg/kg.
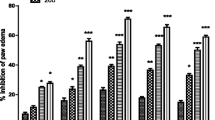
Acetic acid induced writhing
The acetic acid test is implicated for the determination of peripheral activity. In performing the acetic acid induced writhing test for the determination of antinociceptive affect, the sample showed a moderate antinociceptive effect. The sample mild to moderately inhibited the acetic acid induced writings with a maximum value of 53.62% (P < 0.01, n = 6) at 50 mg/kg as shown in Table 4. All the results were compared to the standard (diclofenac sodium, 10 mg/kg) with 83.29% response.
Formalin test
Formalin test is used for continuous moderate pain. It is a valid model for pain determination in animal model. After the administration of the sample to the animals treated with formalin, it again mildly inhibited the first phase with 45.16% (** P < 0.01, n = 6) and the second phase with 51.47% (** P < 0.01, n = 6) at 50 mg/kg.
Animals treated with morphine (5 mg) inhibited significantly both the phases with 86.48% (*** P < 0.001, n = 6) and 95.82% (*** P < 0.001, n = 6) for first phase and second phase respectively as shown in Table 5. Indomethacin (10 mg/kg) mildly inhibited the first phase with 27.69% (*** P < 0.001, n = 6) and significantly inhibited the second phase with 73.47% (*** P < 0.001, n = 6).
Thermal nociception (tail immersion) test
This test is performed for finding out the central analgesic response. The samples when tested for its effectiveness in tail flick model, significantly increased the latency time at both the doses to 60.00% (** P < 0.01, n = 6) and 57.89% (** P < 0.01, n = 6) respectively at 25 mg/kg and 62.10% and 61.05% at 60 min at which morphine (opioid analgesic, centrally acting), showed 85.07% (*** P < 0.001, n = 6) activity. Naloxone treated animals significantly reduced the analgesic potentials of morphine and the isolated compounds (Table 6).
Discussion
STLs are pharmacologically active molecules and have been reported for various potentials like antitumor, anti-inflammatory, antibacterial, antifungal, antiviral, antiprotozoal, antihelminthic, antiulcer, molluscicidal, hepatoprotective, hepatocurative, and antidepressant effects [45–65]. The first-line therapy against malaria caused by Plasmodium falciparum is, as recommended by the World Health Organization (WHO), a combination therapy of a STL (artemisinin) and its derivatives with other antimalarials, such as mefloquine and amodiaquine [66, 67]. Semisynthetic derivatives with improved pharmacokinetic profiles include the active principle dihydroartemisinin, artemether, artelinic acid and artesunate [66]. Free radicals production in the living systems leads to a series of chemical reactions and thus gives rise to serious tissues injuries and ultimately cancer. STLs have been reported to be effective in inhibiting free radicals [68]. Data from the literature show that some species of the genera Artemisia possess analgesic activity and these pharmacological effects have been attributed mainly to flavonoids, alkaloids, sesquiterpene lactones and essential oils [69]. Currently, STLs have been the subject of greater scientific interest due to their ever-increasing evidence concerning their antitumor properties.
The cytotoxic and apoptotic effects of STLs have been investigated in vitro against several cancer cell lines [70–75]. In result of all the chemical and pharmacological research on STLs, substances such as parthenolide and its synthetic analog, dimethylaminoparthenolide (DMAPT), thapsigargin, the artemisinin derivatives artemether and artesunate are presently in cancer clinical trials [56]. Similarly thapsigargin pro-drug (G-202, thapsigargin coupled with a masking peptide which is cleaved at the tumor site, releasing the cytotoxic drug) is in phase I clinical trials for advanced solid tumors. These substances exert diverse mechanisms of antitumoral action, such as ROS formation, epigenetic modulation of gene expression, targeting the sarco/endoplasmic reticulum calcium ATPase (SERCA) pump, the NF-kB signaling pathway, the p53 pathway, and inhibiting angiogenesis and metastasis [76, 77]. Most recently, Vernolide from Vernonia cinerea, has been reported for immunostimulatory effects, inducing enhanced cellular and humoral responses against tumors [78]. Our STL showed good inhibition of gowning cancer cell (HeLa and MCF-7 in case of this study). Although it did not show good results against 3 T3 cell line, may be due the cell line nature that is not enough sensitive like the other two. The possible mechanism for the anti-proliferative activity of the tested STL may be its ability to simultaneously target two molecular pathways (NF-kB and p53) as reported for other STLs elsewhere in literature [79]. Thus, the results of the present study reveal the possible potentials of the isolated STL for its anti-proliferative activity.
The scavenging of free radicals has been important as it helps in preventing the tissues and other vital organs injuries. The isolated STL was screened for free radicals scavenging potentials using DPPH and ABTS free radicals. It caused the scavenging of both the free radicals in a concentration dependent manner. It was found quite effective against the tested free radicals. The isolated STL will require to be extensively studied for its antioxidant activity possible mechanism but mostly STLs have been reported to exert their antioxidant activity through the activation of antioxidant response element (ARE) gene [68]. The results of the present study suggest the possible uses of the isolated STL in free radicals caused tissues injuries.
antinociceptiveantinociceptive. Three different models, visceral nociception, inflammatory nociception and thermal and neurogenic nociception, were used to investigate the antinociceptive effects of the STL. Abdominal constriction assay induced with acetic acid [80] is considered to be sensitive using minimum quantity of noxious stimulus and the results can detect even a weak analgesic. This method is used for investigation of peripheral analgesic response. The acetic acid increases prostaglandin level (especially PGE2) in the mice peritoneal fluid [81]. Prostaglandins activate abdominal constriction via sensitizing and activating peripheral chemo-sensitive nociceptors [82] which are mainly involved in inflammatory pain [83].
Nociception induced with formalin is used to measure the potentials of a substance to relieve continuous moderate pain generated as a result of tissue injury [84]. Formalin induced acute and chronic phases of nociception are regarded to indicate neurogenic and inflammatory pain behaviors respectively. The acute phase is due to direct chemical stimulation of nociceptive afferent fibers (mostly C fibers) which can be suppressed by opiate like morphine [85]. The 2nd phase is due to release of inflammatory mediators such as histamine, prostaglandins, bradykinin, serotonin in the peripheral tissues [85], and from functional changes in the spinal dorsal horn [86]. The tail immersion test is used to determine the spinal pathways in regulation of pain response [87]. This method utilizes elevated thermal nociception and test samples showing good antinociceptive results in this way are regarded potent analgesics [86].
In tail immersion test, a little increase in reaction time after treatment with the sample indicates that the isolated compound is having moderate efficacy in a model of thermal nociception. antinociceptiveThe nociceptive patterns after formalin injection were particularly recorded in two phases. In first phase, paw licking/biting response starts immediately after injection and this may be considered due to direct stimulation of nociceptors [88]. Second phase appears little later and is regarded to be due to combination of inflammatory reactions in the peripheral tissue and changes in central processing’s [89]. A moderate increase in the antinociceptive effect was observed against both neurogenic (early phase) and inflammatory (late phase) pain behavior caused by formalin. The magnitude of inhibition in both the phases was moderate for the isolated compound. It may be suggested from the results that the isolated compound have no prominent antinociceptive potentials.
Conclusion
Sesquiterpenes lactones (STLs) have been isolated from numerous genera of the family Asteraceae (compositae) and can also be found in other angiosperm families. They are described as the active constituents of a variety of medicinal plants used in traditional medicine for the treatment of various diseases. They are known to possess wide variety of biological and pharmacological activities such as antimicrobial, cytotoxic, anti-inflammatory, antiviral, antibacterial, antifungal activities, effects on the central nervous and cardiovascular systems as well as allergenic potency. Their wide structural diversity and potential biological activities have made further interest in the field of Pharmacy and Pharmacology. The present study was designed to isolate the STLs from A. macrocephala and to investigate their possible antioxidant, anticancer and antinociceptive potentials. The study reveals the significant potentials of STL and may be used as an alternative for the management of cancer. Anyhow, the isolated compound is having no prominent antinociceptive potentials.
References
Cane DE. Enzymic formation of sesquiterpenes. Chem Rev. 1990;90(7):1089–103.
Cane DE. Sesquiterpene biosynthesis: cyclization mechanisms. Compr Nat Prod Chem. 1999;2:155–200.
Davis EM, Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. In: Biosynthesis. edn.: Springer; 2000: 53–95.
Chen F, Tholl D, D’Auria JC, Farooq A, Pichersky E, Gershenzon J. Biosynthesis and emission of terpenoid volatiles from arabidopsis flowers. Plant Cell. 2003;15(2):481–94.
Degenhardt J, Gershenzon J, Baldwin IT, Kessler A. Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol. 2003;14(2):169–76.
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A. 2004;101(6):1781–5.
Kappers IF, Aharoni A, Van Herpen TW, Luckerhoff LL, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts bodyguards to arabidopsis. Science. 2005;309(5743):2070–2.
Keeling CI, Bohlmann J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006;170(4):657–75.
Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291(5511):2141–4.
Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry. 2003;64(1):3–19.
Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel W-J, Verstappen FW, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. Terpenoid metabolism in wild-type and transgenic arabidopsis plants. Plant Cell. 2003;15(12):2866–84.
Martin DM, Bohlmann J. Identification of Vitis vinifera (−)-α-terpineol synthase by in silico screening of full-length cDNA ESTs and functional characterization of recombinant terpene synthase. Phytochemistry. 2004;65(9):1223–9.
Pichersky E, Noel JP, Dudareva N. Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science. 2006;311(5762):808–11.
Rodríguez‐Concepción M, Ahumada I, Diez‐Juez E, Sauret‐Güeto S, Lois LM, Gallego F, Carretero‐Paulet L, Campos N, Boronat A. 1-Deoxy-d-xylulose 5-phosphate reductoisomerase and plastid isoprenoid biosynthesis during tomato fruit ripening. Plant J. 2001;27(3):213–22.
Picman AK. Biological activities of sesquiterpene lactones. Biochem Syst Ecol. 1986;14(3):255–81.
Seaman FC. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot Rev. 1982;48(2):121–594.
Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228(4703):1049–55.
Göpfert JC, MacNevin G, Ro D-K, Spring O. Identification, functional characterization and developmental regulation of sesquiterpene synthases from sunflower capitate glandular trichomes. BMC Plant Biol. 2009;9(1):86.
Knight DW. Feverfew: chemistry and biological activity. Nat Prod Rep. 1995;12(3):271–6.
Mori K, Matsushima Y. Synthesis of mono-and sesquiterpenoids; XXIV:(−)-homogynolide A, an insect antifeedant isolated from Homogyne alpina. Synthesis Stuttgart. 1993;1(7):845.
Mullin CA, Alfatafta AA, Harman JL, Everett SL, Serino AA. Feeding and toxic effects of floral sesquiterpene lactones, diterpenes, and phenolics from sunflower (Helianthus annuus L.) on western corn rootworm. J Agric Food Chem. 1991;39(12):2293–9.
Pfaffenrath V, Diener H, Fischer M, Friede M, Henneicke‐von Zepelin H. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis—a double-blind, multicentre, randomized placebo-controlled dose–response study. Cephalalgia. 2002;22(7):523–32.
Tassorelli C, Greco R, Morazzoni P, Riva A, Sandrini G, Nappi G. Parthenolide is the component of Tanacetum parthenium that inhibits nitroglycerin‐induced Fos activation: studies in an animal model of migraine. Cephalalgia. 2005;25(8):612–21.
Heinrich M, Robles M, West JE, de Montellano BR O, Rodriguez E. Ethnopharmacology of Mexican asteraceae (compositae). Annu Rev Pharmacol Toxicol. 1998;38(1):539–65.
Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398(3):399–407.
Faveri FF, Grando R, Nonato FR, Sousa IM, Queiroz NC, Longato GB, Zafred RR, Carvalho JE, Spindola HM, Foglio MA. Artemisia annua L.: evidence of sesquiterpene lactones’ fraction antinociceptive activity. BMC Complement Altern Med. 2014;14(1):1.
Batista PA, de Paula Werner MF, Oliveira EC, Burgos L, Pereira P, da Silva Brum LF, dos Santos ARS. Evidence for the involvement of ionotropic glutamatergic receptors on the antinociceptive effect of (−)-linalool in mice. Neurosci Lett. 2008;440(3):299–303.
Quintans-Júnior LJ, Melo MS, De Sousa DP, Araújo AAS, Onofre A, Gelain DP, Goncalves J, Araujo D, Almeida J, Bonjardim LR. Antinociceptive effects of citronellal in formalin, capsaicin and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. J Orofac Pain. 2010;24(3):305–12.
Quintans-Júnior LJ, Guimarães AG, De Santana MT, Araújo BE, Moreira FV, Bonjardim LR, Araújo AA, Siqueira JS, Antoniolli ÂR, Botelho MA. Citral reduces nociceptive and inflammatory response in rodents. Revista Brasileira de Farmacognosia. 2011;21(3):497–502.
Guimarães AG, Xavier MA, de Santana MT, Camargo EA, Santos CA, Brito FA, Barreto EO, Cavalcanti SC, Antoniolli ÂR, Oliveira RC. Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(3):253–63.
Brito RG, Santos PL, Prado DS, Santana MT, Araújo AA, Bonjardim LR, Santos MR, Lucca Júnior W, Oliveira AP, Quintans‐Júnior LJ. Citronellol reduces orofacial nociceptive behaviour in mice–evidence of involvement of retrosplenial cortex and periaqueductal grey areas. Basic Clin Pharmacol Toxicol. 2013;112(4):215–21.
Gonçalves JCR, Oliveira Fd S, Benedito RB, de Sousa DP, de Almeida RN, de Araújo DAM. Antinociceptive activity of (−)-carvone: evidence of association with decreased peripheral nerve excitability. Biol Pharm Bull. 2008;31(5):1017–20.
Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65(19):2979–99.
Kumar BR. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. J Pharmacognosy Phytochemistry. 2015;4(1):7-9.
Shekhar TC, Anju G. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. leaves. Am J Ethnomed. 2014;1(4):244–9.
Floegel A, Kim D-O, Chung S-J, Koo SI, Chun OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal. 2011;24(7):1043–8.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
Ali N, Shah I, Shah SWA, Ahmed G, Shoaib M, Junaid M, Ali W, Ahmed Z. Antioxidant and relaxant activity of fractions of crude methanol extract and essential oil of Artemisia macrocephala Jacquem. BMC Complement Altern Med. 2013;13(1):1.
Khan H, Saeed M, Khan MA, Khan I, Ashraf N. Antinociceptive activity of aerial parts of Polygonatum verticillatum: attenuation of both peripheral and central pain mediators. Phytother Res. 2011;25(7):1024–30.
Vidyalakshmi K, Kamalakannan P, Viswanathan S, Ramaswamy S. Antinociceptive effect of certain dihydroxy flavones in mice. Pharmacol Biochem Behav. 2010;96(1):1–6.
Bhutia YD, Vijayaraghavan R, Pathak U. Analgesic and anti-inflammatory activity of amifostine, DRDE-07, and their analogs, in mice. Indian J Pharmacol. 2010;42(1):17.
Ishikawa H, Nakashima T, Inaba K, Mitsuyoshi H, Nakajima Y, Sakamoto Y, Okanoue T, Kashima K, Seo Y. Proton magnetic resonance assay of total and taurine-conjugated bile acids in bile. J Lipid Res. 1999;40(10):1920–4.
Schmidt TJ, Lyß G, Pahl HL, Merfort I. Helenanolide type sesquiterpene lactones. Part 5: the role of glutathione addition under physiological conditions. Bioorg Med Chem. 1999;7(12):2849–55.
Ali N, Aleem U, Shah SWA, Shah I, Junaid M, Ahmed G, Ali W, Ghias M. Acute toxicity, brine shrimp cytotoxicity, anthelmintic and relaxant potentials of fruits of Rubus fruticosus Agg. BMC Complement Altern Med. 2013;13(1):138.
Akkol EK, Arif R, Ergun F, Yesilada E. Sesquiterpene lactones with antinociceptive and antipyretic activity from two Centaurea species. J Ethnopharmacol. 2009;122(2):210–5.
Reyhan A, KÜPELİ E, ERGUN F. The biological activity of Centaurea L. species. Gazi Univ J Sci. 2004;17(4):149–64.
Barrera PA, Jimenez-Ortiz V, Tonn C, Giordano O, Galanti N, Sosa MA. Natural sesquiterpene lactones are active against Leishmania mexicana. J Parasitol. 2008;94(5):1143–9.
Borkosky S, de León SP, Juárez G, Sierra MG, Bardón A. Molluscicidal sesquiterpene lactones from species of the tribe Vernonieae (Compositae). Chem Biodivers. 2009;6(4):513–9.
Chaturvedi D. Sesquiterpene lactones: structural diversity and their biological activities, In-opportunity, challanges and scope of natural products in medicinal chemistry. ISBN: 978-81-308-0448-4, Research Signpost, Trivandrum 2011:313–334.
Cheng G, Xie L. Parthenolide induces apoptosis and cell cycle arrest of human 5637 bladder cancer cells in vitro. Molecules. 2011;16(8):6758–68.
Dupuy OA, Bonilla JA. Lactonas sesquiterpénicas de las plantas Viguiera sylvatica y Decachaeta thieleana (Asteraceae) modulan la producción de óxido nítrico y la fagocitosis de macrófagos RAW. Rev Biol Trop. 2008;56(3):1063–73.
Emami SA, Taghizadeh Rabe SZ, Iranshahi M, Ahi A, Mahmoudi M. Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression through the inactivation of NF-κB. Immunopharmacol Immunotoxicol. 2010;32(4):688–95.
Fonseca LC, Dadarkar SS, Lobo AS, Mishra PB, Thakkar AD, Chandrababu S, Padigaru M. NF-κB-mediated anti-inflammatory activity of the sesquiterpene lactone 7-hydroxyfrullanolide. Eur J Pharmacol. 2011;657(1):41–50.
Fortuna AM, Juárez ZN, Bach H, Nematallah A, Av-Gay Y, Sánchez-Arreola E, Catalan C, Turbay S, Hernández LR. Antimicrobial activities of sesquiterpene lactones and inositol derivatives from Hymenoxys robusta. Phytochemistry. 2011;72(18):2413–8.
Foster JG, Cassida KA, Turner KE. In vitro analysis of the anthelmintic activity of forage chicory (Cichorium intybus L.) sesquiterpene lactones against a predominantly Haemonchus contortus egg population. Vet Parasitol. 2011;180(3):298–306.
Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today. 2010;15(15):668–78.
Gonçalves AE, Bürger C, Amoah SK, Tolardo R, Biavatti MW, de Souza MM. The antidepressant-like effect of Hedyosmum brasiliense and its sesquiterpene lactone, podoandin in mice: Evidence for the involvement of adrenergic, dopaminergic and serotonergic systems. Eur J Pharmacol. 2012;674(2):307–14.
Hwang D-R, Wu Y-S, Chang C-W, Lien T-W, Chen W-C, Tan U-K, Hsu JT, Hsieh H-P. Synthesis and anti-viral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorg Med Chem. 2006;14(1):83–91.
Julianti T, Hata Y, Zimmermann S, Kaiser M, Hamburger M, Adams M. Antitrypanosomal sesquiterpene lactones from Saussurea costus. Fitoterapia. 2011;82(7):955–9.
Kang K, Lee HJ, Kim CY, Lee SB, Tunsag J, Batsuren D, Nho CW. The chemopreventive effects of Saussurea salicifolia through induction of apoptosis and phase II detoxification enzyme. Biol Pharm Bull. 2007;30(12):2352–9.
Kreuger MRO, Farias BG, Moreira J, Blind LZ, Amoah SKS, Leite AS, Biavatti MW, van Hoof T, D’Herde K, Cruz AB. Effects of the topical application of an ethyl acetate fraction from Vernonia scorpioides on excisional wounds infected with Staphylococcus aureus in rats. Revista Brasileira de Farmacognosia. 2012;22(1):123–30.
Li X-W, Weng L, Gao X, Zhao Y, Pang F, Liu J-H, Zhang H-F, Hu J-F. Antiproliferative and apoptotic sesquiterpene lactones from Carpesium faberi. Bioorg Med Chem Lett. 2011;21(1):366–72.
Maas M, Hensel A, da Costa FB, Brun R, Kaiser M, Schmidt TJ. An unusual dimeric guaianolide with antiprotozoal activity and further sesquiterpene lactones from Eupatorium perfoliatum. Phytochemistry. 2011;72(7):635–44.
Mahesh A, Jeyachandran R, Cindrella L, Thangadurai D, Veerapur V, Muralidhara Rao D. Hepatocurative potential of sesquiterpene lactones of Taraxacum officinale on carbon tetrachloride induced liver toxicity in mice. Acta Biol Hung. 2010;61(2):175–90.
Merfort I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr Drug Targets. 2011;12(11):1560–73.
Aquino I, Perazzo FF, Maistro EL. Genotoxicity assessment of the antimalarial compound artesunate in somatic cells of mice. Food Chem Toxicol. 2011;49(6):1335–9.
Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IR, Johnston GL, Tatem AJ, Hay SI. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10(1):1–16.
Fischedick JT, Standiford M, Johnson DA, De Vos RC, Todorović S, Banjanac T, Verpoorte R, Johnson JA. Activation of antioxidant response element in mouse primary cortical cultures with sesquiterpene lactones isolated from Tanacetum parthenium. Planta Med. 2012;78(16):1725–30.
Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc Sci Med. 1998;47(11):1859–71.
Bach SM, Fortuna MA, Attarian R, de Trimarco JT, Catalán C, Av-Gay Y, Bach H. Antibacterial and cytotoxic activities of the sesquiterpene lactones cnicin and onopordopicrin. Nat Prod Commun. 2011;6(2):163–6.
Berger TG, Dieckmann D, Efferth T, Schultz ES, Funk J-O, Baur A, Schuler G. Artesunate in the treatment of metastatic uveal melanoma-first experiences. Oncol Rep. 2005;14(6):1599–603.
Buskuhl H, de Oliveira FL, Blind LZ, de Freitas RA, Barison A, Campos FR, Corilo YE, Eberlin MN, Caramori GF, Biavatti MW. Sesquiterpene lactones from Vernonia scorpioides and their in vitro cytotoxicity. Phytochemistry. 2010;71(13):1539–44.
Chew AL, Bashir SJ, Hawk JL, Palmer R, White IR, McFadden JP. Contact and photocontact sensitization in chronic actinic dermatitis: a changing picture. Contact Dermatitis. 2010;62(1):42–6.
Choi J-H, Lee K-T. Costunolide-induced apoptosis in human leukemia cells: involvement of c-jun N-terminal kinase activation. Biol Pharm Bull. 2009;32(10):1803–8.
Cotugno R, Fortunato R, Santoro A, Gallotta D, Braca A, De Tommasi N, Belisario M. Effect of sesquiterpene lactone coronopilin on leukaemia cell population growth, cell type‐specific induction of apoptosis and mitotic catastrophe. Cell Prolif. 2012;45(1):53–65.
Büchele B, Zugmaier W, Lunov O, Syrovets T, Merfort I, Simmet T. Surface plasmon resonance analysis of nuclear factor-κB protein interactions with the sesquiterpene lactone helenalin. Anal Biochem. 2010;401(1):30–7.
Khan M, Yi F, Rasul A, Li T, Wang N, Gao H, Gao R, Ma T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life. 2012;64(9):783–94.
Pratheeshkumar P, Kuttan G. Modulation of cytotoxic T lymphocyte, natural killer cell, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by Vernonia cinerea L. and vernolide-A in BALB/c mice via enhanced production of cytokines IL-2 and IFN-γ. Immunopharmacol Immunotoxicol. 2012;34(1):46–55.
Dey A, Tergaonkar V, Lane DP. Double-edged swords as cancer therapeutics: simultaneously targeting p53 and NF-κB pathways. Nat Rev Drug Discov. 2008;7(12):1031–40.
Neto A, Costa J, Belati C, Vinholis A, Possebom L, Da Silva FA, Cunha W, Carvalho J, Bastos J, e Silva M. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J Ethnopharmacol. 2005;96(1):87–91.
Deraedt R, Jouquey S, Delevallée F, Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61(1):17–24.
Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharm Exp Ther. 1998;285(3):1031–8.
Bley KR, Hunter JC, Eglen RM, Smith JA. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol Sci. 1998;19(4):141–7.
Oliveira RR, Góis RM, Siqueira RS, Almeida JR, Lima JT, Nunes XP, Oliveira VR, Siqueira JS, Quintans-Júnior LJ. Antinociceptive effect of the ethanolic extract of Amburana cearensis (Allemão) AC Sm., Fabaceae, in rodents. Revista Brasileira de Farmacognosia. 2009;19(3):672–6.
do Amaral JF, Silva MIG, de Aquino Neto MRA, Neto PFT, Moura BA, de Melo CTV, de Araújo FLO, de Sousa DP, de Vasconcelos PF, de Vasconcelos SMM. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol Pharm Bull. 2007;30(7):1217–20.
Dalal A, Tata M, Allegre G, Gekiere F, Bons N, Albe-Fessard D. Spontaneous activity of rat dorsal horn cells in spinal segments of sciatic projection following transection of sciatic nerve or of corresponding dorsal roots. Neuroscience. 1999;94(1):217–28.
Khatun A, Imam MZ, Rana MS. Antinociceptive effect of methanol extract of leaves of Persicaria hydropiper in mice. BMC Complement Altern Med. 2015;15(1):1.
Oyadeyi A, Ajao F, Afolabi A, Udoh U, Azeez O. The formalin test in African toad (Bufo regularis)-a novel pain model in amphibians. Am-Eurasian J Sci Res. 2007;2(1):24–8.
Al Amin M, Chowdhury IA, Mahbub K, Sattar M, Shahriar M, Kuddus MR, Rashid MA. Anti-inflammatory and analgesic activities of Asteracantha longifolia Nees. Bangladesh Pharm J. 2012;15(2):171–6.
Acknowledgements
The authors are thankful to University of Malakand, Pakistan, International Center for Chemical and Biological Sciences HEJ Research Institute of Chemistry, University of Karachi, Pakistan, and Department of Physics University of Sargodha, Sarghodah Punjab, Pakistan for providing research facilities.
Funding
No fund was provided by any funding agency for this study.
Availability of data and materials
The data sets supporting the conclusions of this article are presented in this paper. The isolated compound has been characterized by Dr. Achyut Adikhari and Dr. Muhammad Nawaz Tahir. The spectroscopic data is submitted as supporting/Additional file 1.
Authors’ contributions
MS: Supervision of whole project. IS: Performed pharmacological tests and collaborated in manuscript preparation. NA: Supervision of the pharmacological tests. AA: Carried out the spectroscopic data. MNT: Carried out the crystal data. SWAS: Helped in pharmacological activity and manuscript preparation. SI: Carried out the Hirshfeld analysis. JK: Helped in pharmacological tests. SK: Helped in manuscript preparation and English correction. MNU: Interpret the data. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have competing interests.
Consent for publication
All the authors have agreed and given approval for publication of the manuscript.
Ethics approval and consent to participate
Standard experimental protocols were followed as per the guidelines of ethical committee of Department of Pharmacy (No: S-Ter-24-12/2015) University of Malakand as per Bye Laws 2008 of the University of Malakand (Scientific Procedures Issue-I).
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
NMR spectra of compound. (DOCX 696 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shoaib, M., Shah, I., Ali, N. et al. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement Altern Med 17, 27 (2017). https://doi.org/10.1186/s12906-016-1517-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-016-1517-y