Abstract
Background
Quercetin (QR), is a polyphenolic flavonoid compound which is found in large amounts in certain foods, and protects against oxidative stress. The current study was conducted to determine whether Quercetin can possibly exert hepatoprotective and antioxidant activity against acrylamide (ACR) induced toxicity in rats.
Methods
Four groups of Wistar rats consisting of six rats each: (i) control group; (ii) ACR treated group (50 mg/kg bw); (iii) QR group: rats were treated with QR (10 mg/kg bw); (iv) QR (10 mg/kg bw) was given i.p. for 5 days followed by ACR (50 mg/kg bw) on 5th day (single dose).
Results
ACR caused an elevation in 8-OH guanosine level and a reduction in Glutahione S-transferase (GST) activity. Administration of QR significantly protected liver tissue against hepatotoxic effect of acrylamide from amelioration of the marker enzyme (p < 0.05) and DNA damage (p < 0.01) as evident by comet assay and, besides some indices of histopathological alterations.
Conclusion
It is concluded that QR could protect the liver against DNA damage induced by ACR probably is thus capable of ameliorating ACR-induced changes in the rat livers.
Similar content being viewed by others
Background
Several studies have reported that acrylamide (ACR) is formed in heat-treated food mainly containing car bohydrates [1–7]. ACR has been reported to form acrylamide-protein adducts in laboratory animals [3]. Earlier carcinogenic action of ACR has been reported in detail [8]. Recently, not only major metabolic pathways and enzymes of ACR have been summarized, but also the inter individual and the interspecies differences of ACR metabolism among humans, rats and mice have been reported [9].
Due to ACR exposure damage of biological macromolecules and disruption of normal metabolism leads to oxidative stress and imbalance in antioxidant activity [10]. Oxidative stress causes enhanced generation of reactive oxygen species (ROS) and depletion of antioxidant defense system in the tissues. ROS can stimulate free radical chain reactions, leading to the enhancement of lipid peroxidation [11].
Plants contain numerous polyphenols, which have been shown to reduce inflammation and thereby increase resistance to disease [12]. Flavonoids are present in high concentration, as polyphenols in vegetables, fruits, and beverages [13–15]. Flavonoids are known to have anti inflammatory [16], anti-allergic [17], cardio protective [18], and anti-cancer activities [19]. Also, flavonoids protect against DNA damage in certain oxidative stress conditions [20].
Quercetin is one of the most common flavonoids in the diet and exhibits therapeutic potential, including hepatoprotection and the inhibition of liver fibrosis, against many diseases [12, 21, 22]. It contains a number of phenolic hydroxyl groups that have strong antioxidant activity [15, 23–26]. Moreover, QR has been shown to protect against carbon tetrachloride, ethanol, and paracetamol-induced hepatotoxicity [27]. Our recent studies have shown that quercetin can restore against oxidative damage against acrylamide induced neurotoxicity [28]. Additionally, recent published reports have shown protective effect of QR against various toxic insults [29–32]. In this study, the effect of QR on acrylamide caused hepatotoxicity in rats has been investigated.
Methods
Chemicals
ACR, QR, and other reagents were bought from Sigma–Aldrich Chemicals Co., St. Louis, USA. Quercetin (Sigma) was resuspended immediately before administration in a 2 % tween aqueous solution. ACR was dissolved in saline and/or distilled water.
Animals and experimental procedures
Male wistar rats weighing approximately 200–220 g were procured. Animal utilization protocols were performed in accordance with the guidelines provided by the Experimental Animal Laboratory and approved by the Animal Care and Use Committee. All the animals used in this study were placed in cages in an air conditioned room maintained at 12 h light/dark cycle.
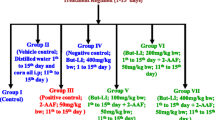
Study design: Twenty-four rats were randomly divided into 4 groups (6 rats in each group). Group I received saline (0.85 % NaCl i.p) at 10 ml/kg bodyweight. Group II received ACR at a dose of 50 mg/kg b.w. Group III received pretreatment with QR -10 mg/kg body weight, and groups IV received the pretreatment with QR -10 mg/kg body weight. After the last treatment with QR, the rats of groups IV received a single i.p. injection of ACR at 50 mg/kg body weight. Forty-eight hours later, the rats were sacrificed. The dose of QR and ACR used in the present study was in accordance with previous study, respectively [33, 34]. The livers were excised, weighed, and divided for histological and biochemical analyses.
Biochemical analysis
The liver homogenates were centrifuged at 3000 rpm for 10 min at 4 °C. The supernatants were used for the various biochemical determinations.
Liver homogenates were analyzed for Glutathione S-transferase (GST) measured by Biovision assay kit, DNA damage by comet assay and 8-OH deoxyguanosine (8-OHdG) by ELISA kit (Abnova, cat: 1221).
Histological examinations
Small pieces of liver tissue were used for histopathological studies. Fixed tissues were dehydrated in serial ethanol series, trimmed, embedded in paraffin, sectioned into 2-μm sections and stained with hematoxylin and eosin (H&E). Morphological examination was conducted under a light microscope (Nikon Eclipse E600).
DNA damage assay
The DNA damage evaluation was performed by single cell gel electrophoresis (comet) assay as described by [35]. For visualization of DNA damage, observations were made of Ethedium Bromide -stained DNA using a 40x objective on a fluorescent microscope. Generally, 50–100 randomly selected cells were analyzed per sample.
Statistical analysis
Results were analyzed using SPSS software and values are given as arithmetic means standard error of the mean (S.E.M.). Data was statistically analyzed by using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests.
Results
Assessment of Glutathione S-Transferase (GST) activity
ACR exposure significantly decreased GST activity in the liver cells (~30 %), and this was prevented in the QR + ACR treated group (Table 1). QR pretreatment elevated the activity of GST significantly (p < 0.05) compared to the ACR group-2. GST activity was no different between QR alone and control groups.
Assessment of 8-OH dG levels
The levels of 8-OHdG was significantly elevated in liver tissues of the rats treated with ACR (p < 0.05). QR significantly decreased 8-OHdG levels as compared with ACR-treated rats (p < 0.05) (Table 1). There was no significant difference between the control group1 and QR group 3.
Assessment of DNA damage
The DNA damage was expressed as tail length, tail DNA, and tail moment in the liver Fig. 1, Table 2. DNA damage was increased significantly in the ACR group compared to the control (p < 0.05). QR reduced DNA damage significantly as observed in the ACR + QR group as compared to the ACR group (p < 0.01). There was no significant difference in DNA damage between the control group and the QR + ACR group.
Histopathological findings
Figure 2 shows liver sections: Control I-A, showing normal orientation of hepatic parenchyma. Liver sections show disarrangement and mild degeneration of cells in cytoplasm from group II-B. Liver sections from group III-C and group IV-D show normal hepatic cells, and sinusoid spaces as compared to group II-B.
Discussion
Food during baking and frying at high temperatures leads to formation of acrylamide at higher levels [36]. Recent studies have reported that a fried potato dish as large contributor to acrylamide exposure [37]. Furthermore, acrylamide has been reported as a carcinogen showing hazardous effects [38]. Acrylamide’s mechanism of action is greatly enhanced as it can be readily absorbed through the intestinal tract. Present study demonstrates that QR protects against DNA damage indicating that QR possesses DNA-protective properties. These findings are in accordance with other studies that used quercetin or other antioxidant substances, such as rutin, nacetylcysteine, and vitamins E and C, all of which decreased the severity of hepatic fibrosis [39–43].
The GST enzyme catalyzes the conjugation of the reduced form of glutathione (GSH) to xenobiotic and protect cells and tissues against oxidative stress. A reduction of GST activity was observed in homogenized liver in ACR treated group, and this reduction was blocked after quercetin administration in this study. These findings support the hypothesis that QR exerted a protective effect in vivo [29]. The histopathological profile of liver damage demonstrates that pretreatment of QR in ACR-treated group exhibits less damage to the hepatic cells as compared to the rats treated with the toxic group. It could be suggested that QR scavenges free-radical generation and inhibits ACR–induced injury in hepatic tissues.
Results in this study show that ACR induced DNA damage was significantly decreased after the treatment of QR. The antioxidant effects of QR may be due to flavonoid’s high diffusion into the membranes allowed it to scavenge oxy radicals at several sites throughout the lipid bilayer or its pentahydroxyl flavones structure allowed it to chelate metal ions via the orthodihydroxy phenolic structure, thereby scavenging lipid alkoxyl and peroxyl radicals [19, 44] or flavanoids might be also involved in the indirect induction of detoxifying genes [25, 27, 45, 46], which might promote detoxification of ACR and decrease their toxicity. Enhanced chemiluminescence studies haves shown antioxidant role of flavonoids and other polyphenols found in tea [47]. Some recent studies also support the finding that QR has also been shown to suppress toxicity and oxidative stress in vivo and in vitro [48–52].
Once ingested ACR can interact with other proteins at the cellular level and bind to DNA to form adducts [38]. It has been shown earlier that QR as flavanoides may bind DNA at sites that would normally react with the active metabolites of carcinogen [18, 20, 53]. Recently, in vivo genotoxicity assessment of acrylamide has been shown and it is reported that genotoxicity of ACR is tissue specific [54]. Acrylamide can cause gene interactions and chromosomal aberrations and has been classified as being genotoxic.
Conclusions
In conclusion, the study revealed that ACR induces toxicity by increasing DNA damage and decreasing the glutathione S transferase activity. QR protects ACR toxicity indicating that QR possesses a spectrum of antioxidant and DNA-protective properties. However, further studies are required to elucidate the precise mechanisms of protection of QR against ACR toxicity.
References
Alpozen E, Uren A. Determination of acrylamide levels of “Izmir gevregi” and effects of cooking parameters on acrylamide formation. J Agric Food Chem. 2013;61(30):7212–8.
Rosen J, Hellenas KE. Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry. Analyst. 2002;127(7):880–2.
Barber DS, LoPachin RM. Proteomic analysis of acrylamide-protein adduct formation in rat brain synaptosomes. Toxicol Appl Pharmacol. 2004;201(2):120–36.
Becalski A, Lau BP, Lewis D, Seaman SW, Hayward S, Sahagian M, Ramesh M, Leclerc Y. Acrylamide in French fries: influence of free amino acids and sugars. J Agric Food Chem. 2004;52(12):3801–6.
Jin X, Coughlan M, Roberts J, Mehta R, Raju J. Dietary acrylamide exposure in male F344 rats: dataset of systemic oxidative stress and inflammation markers. Data Brief. 2016;7:460–7.
Nguyen HT, Van der Fels-Klerx HJ, Peters RJ, Van Boekel MA. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: part I: effects of sugar type. Food Chem. 2016;192:575–85.
Obon-Santacana M, Lujan-Barroso L, Freisling H, Cadeau C, Fagherazzi G, Boutron-Ruault MC, Kaaks R, Fortner RT, Boeing H, Ramon Quiros J, et al. Dietary and lifestyle determinants of acrylamide and glycidamide hemoglobin adducts in non-smoking postmenopausal women from the EPIC cohort. Eur J Nutr. 2016. doi:10.1007/s00394-016-1165-5.
Besaratinia A, Pfeifer GP. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis. 2007;28(3):519–28.
Li D, Wang P, Liu Y, Hu X, Chen F. Metabolism of acrylamide: interindividual and interspecies differences as well as the application as biomarkers. Curr Drug Metab. 2016;17(4):317–26.
Trevisan M, Browne R, Ram M, Muti P, Freudenheim J, Carosella AM, Armstrong D. Correlates of markers of oxidative status in the general population. Am J Epidemiol. 2001;154(4):348–56.
Zhu YJ, Zeng T, Zhu YB, Yu SF, Wang QS, Zhang LP, Guo X, Xie KQ. Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem Res. 2008;33(11):2310–7.
Bengmark S, Mesa MD, Gil A. Plant-derived health: the effects of turmeric and curcuminoids. Nutr Hosp. 2009;24(3):273–81.
Middleton Jr E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673–751.
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–84.
Martinez-Florez S, Gonzalez-Gallego J, Culebras JM, Tunon MJ. Flavonoids: properties and anti-oxidizing action. Nutr Hosp. 2002;17(6):271–8.
Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96(3):229–45.
Kawai M, Hirano T, Higa S, Arimitsu J, Maruta M, Kuwahara Y, Ohkawara T, Hagihara K, Yamadori T, Shima Y, et al. Flavonoids and related compounds as anti-allergic substances. Allergol Int. 2007;56(2):113–23.
Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342(8878):1007–11.
Kuo SM. Dietary flavonoid and cancer prevention: evidence and potential mechanism. Crit Rev Oncog. 1997;8(1):47–69.
Webster RP, Gawde MD, Bhattacharya RK. Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett. 1996;109(1-2):185–91.
Gonzalez-Gallego J, Sanchez-Campos S, Tunon MJ. Anti-inflammatory properties of dietary flavonoids. Nutr Hosp. 2007;22(3):287–93.
Amalia PM, Possa MN, Augusto MC, Francisca LS. Quercetin prevents oxidative stress in cirrhotic rats. Dig Dis Sci. 2007;52(10):2616–21.
Tieppo J, Cuevas MJ, Vercelino R, Tunon MJ, Marroni NP, Gonzalez-Gallego J. Quercetin administration ameliorates pulmonary complications of cirrhosis in rats. J Nutr. 2009;139(7):1339–46.
Ruiz PA, Braune A, Holzlwimmer G, Quintanilla-Fend L, Haller D. Quercetin inhibits TNF-induced NF-kappaB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J Nutr. 2007;137(5):1208–15.
Polat C, Tokyol C, Kahraman A, Sabuncuoglu B, Yilmaz S. The effects of desferrioxamine and quercetin on hepatic ischemia-reperfusion induced renal disturbance. Prostaglandins Leukot Essent Fatty Acids. 2006;74(6):379–83.
Singh D, Chander V, Chopra K. The effect of quercetin, a bioflavonoid on ischemia/reperfusion induced renal injury in rats. Arch Med Res. 2004;35(6):484–94.
Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26(10):1398–402.
Zargar S, Siddiqi NJ, Ansar S, Alsulaimani MS, El Ansary AK. Therapeutic role of quercetin on oxidative damage induced by acrylamide in rat brain. Pharm Biol. 2016:1-5. doi:10.3109/13880209.2015.1127977.
Taslidere E, Dogan Z, Elbe H, Vardi N, Cetin A, Turkoz Y. Quercetin protection against ciprofloxacin induced liver damage in rats. Biotech Histochem. 2016;91(2):116–21.
Carrasco-Pozo C, Castillo RL, Beltran C, Miranda A, Fuentes J, Gotteland M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: role of NF-kappaB and Nrf2. J Nutr Biochem. 2016;27:289–98.
Chen L, Sun L, Liu Z, Wang H, Xu C. Protection afforded by quercetin against H2O2-induced apoptosis on PC12 cells via activating PI3K/Akt signal pathway. J Recept Signal Transduct Res. 2016;36(1):98–102.
Rezaei-Sadabady R, Eidi A, Zarghami N, Barzegar A. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif Cells Nanomed Biotechnol. 2016;44(1):128–34.
Duarte J, Galisteo M, Ocete MA, Pérez-Vizcaino F, Zarzuelo A, Tamargo J. Effects of chronic quercetin treatment on hepatic oxidative status of spontaneously hypertensive rats. Mol Cell Biochem. 2011;221:155–60.
Paulsson B, Grawe J, Tornqvist M. Hemoglobin adducts and micronucleus frequencies in mouse and rat after acrylamide or N-methylolacrylamide treatment. Mutat Res. 2002;516(1-2):101–11.
Collins AR, Dobson VL, Dusinska M, Kennedy G, Stetina R. The comet assay: what can it really tell us? Mutat Res. 1997;375(2):183–93.
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S. Acrylamide from Maillard reaction products. Nature. 2002;419(6906):449–50.
McCombie G, Biedermann M, Biedermann-Brem S, Suter G, Eicher A, Pfefferle A. Acrylamide in a fried potato dish (rosti) from restaurants in Zurich, Switzerland. Food Addit Contam Part B Surveill. 2016;9(1):21–6.
Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C. Acrylamide: review of toxicity data and dose–response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36(6-7):481–608.
Tieppo J, Vercelino R, Dias AS, Silva Vaz MF, Silveira TR, Marroni CA, Marroni NP, Henriques JA, Picada JN. Evaluation of the protective effects of quercetin in the hepatopulmonary syndrome. Food Chem Toxicol. 2007;45(7):1140–6.
Boots AW, Li H, Schins RP, Duffin R, Heemskerk JW, Bast A, Haenen GR. The quercetin paradox. Toxicol Appl Pharmacol. 2007;222(1):89–96.
Ara C, Kirimlioglu H, Karabulut AB, Coban S, Ay S, Harputluoglu M, Kirimlioglu V, Yilmaz S. Protective effect of resveratrol against oxidative stress in cholestasis. J Surg Res. 2005;127(2):112–7.
Montilla P, Cruz A, Padillo FJ, Tunez I, Gascon F, Munoz MC, Gomez M, Pera C. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J Pineal Res. 2001;31(2):138–44.
Pastor A, Collado PS, Almar M, Gonzalez-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27(2):363–70.
Lien EJ, Ren S, Bui HH, Wang R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic Biol Med. 1999;26(3-4):285–94.
Youdim KA, Spencer JP, Schroeter H, Rice-Evans C. Dietary flavonoids as potential neuroprotectants. Biol Chem. 2002;383(3-4):503–19.
Ha HJ, Kwon YS, Park SM, Shin T, Park JH, Kim HC, Kwon MS, Wie MB. Quercetin attenuates oxygen-glucose deprivation- and excitotoxin-induced neurotoxicity in primary cortical cell cultures. Biol Pharm Bull. 2003;26(4):544–6.
Robinson EE, Maxwell SR, Thorpe GH. An investigation of the antioxidant activity of black tea using enhanced chemiluminescence. Free Radic Res. 1997;26(3):291–302.
Dobrikova AG, Apostolova EL. Damage and protection of the photosynthetic apparatus from UV-B radiation. II. Effect of quercetin at different pH. J Plant Physiol. 2015;184:98–105.
Fatokun AA, Tome M, Smith RA, Darlington LG, Stone TW. Protection by the flavonoids quercetin and luteolin against peroxide- or menadione-induced oxidative stress in MC3T3-E1 osteoblast cells. Nat Prod Res. 2015;29(12):1127–32.
Hu J, Yu Q, Zhao F, Ji J, Jiang Z, Chen X, Gao P, Ren Y, Shao S, Zhang L, et al. Protection of Quercetin against Triptolide-induced apoptosis by suppressing oxidative stress in rat Leydig cells. Chem Biol Interact. 2015;240:38–46.
Ji LL, Sheng YC, Zheng ZY, Shi L, Wang ZT. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12–23.
Storniolo A, Raciti M, Cucina A, Bizzarri M, Di Renzo L. Quercetin affects Hsp70/IRE1alpha mediated protection from death induced by endoplasmic reticulum stress. Oxid Med Cell Longev. 2015;2015:645157.
Frank AA, Collier JM, Forsyth CS, Zeng W, Stoner GD. Ellagic acid embryoprotection in vitro: distribution and effects on DNA adduct formation. Teratology. 1993;47(4):275–80.
Dobrovolsky VN, Pacheco-Martinez MM, McDaniel LP, Pearce MG, Ding W. In vivo genotoxicity assessment of acrylamide and glycidyl methacrylate. Food Chem Toxicol. 2016;87:120–7.
Acknowledgement
This work was supported by “Research Center of the Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Funding
The authors declare that they have received no specific funding for the research reported.
Availability of data and materials
The datasets supporting the conclusion of this article are presented in this manuscript.
Authors’ contributions
SA wrote the manuscript. MA and MAG helped with analysis and interpretation of data. NJS and SZ conducted the biochemical experiments. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable in this section.
Ethic approval and consent to participate
Animal utilization protocols were performed in accordance with the guidelines provided by the Experimental Animal Laboratory, approved by the Animal Care Committee of University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ansar, S., Siddiqi, N.J., Zargar, S. et al. Hepatoprotective effect of Quercetin supplementation against Acrylamide-induced DNA damage in wistar rats. BMC Complement Altern Med 16, 327 (2016). https://doi.org/10.1186/s12906-016-1322-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-016-1322-7