Abstract
Background
Excessive osteoclast activity is a major cause of metabolic bone disorders, such as osteopenia, rheumatoid arthritis, and osteoporosis. Thus, discovery of agents targeting osteoclast differentiation and bone resorption is important for development of novel treatments for bone diseases. It has been demonstrated that ethanolic extract of schizonepeta tenuifolia (EEST) has potent anti-oxidant and anti-inflammatory activities. However, the beneficial effects of EEST on bone metabolism have not been studied. Therefore, we intend to investigate the effects of EEST on osteoclast differentiation.
Methods
We examined the effects and mechanisms of action of the EEST on osteoclastogenesis in vitro in bone marrow macrophages (BMMs) stimulated with receptor activator of nuclear factor kappa-B ligand (RANKL) and in vivo using a mouse model of lipopolysaccharide (LPS)-induced bone destruction.
Results
We found that EEST inhibited phosphorylation of Akt and IkB at early stages of RANKL-induced osteoclastogenesis. Furthermore, EEST negatively controlled the transcription and translation levels of nuclear factor of activated T cells c1 (NFATc1) and the translation level of c-Fos at the final stage of osteoclast differentiation. Reflecting these effects, EEST blocked both filamentous actin (F-actin) ring formation and bone resorbing activity of mature osteoclasts in vitro. The inhibitory effects of EEST on osteoclast formation and activity were observed in an LPS-mediated bone erosion mouse model using micro-CT and histological analysis.
Conclusions
EEST is a potential agent that is able to treat osteoclast-related bone diseases, such as osteoporosis.
Similar content being viewed by others
Background
Skeletal tissue continuously undergoes remodeling. This is defined by three physiological processes. First, in the resorption phase, osteoclasts dissolve the old bone. Next, in the reversal phase, mononuclear cells arrive on the bone surface to complete resorption stage. Finally, in the formation phase, osteoblasts initiate formation of new bone matrix in response to signals of the mononuclear cells [1]. Osteoclast over-activity, caused by such risk factors as an inflammatory response and estrogen hormone deficiency, expedites perturbation of steady-state bone remodeling. This leads to severe bone diseases, including osteoporosis and osteopenia, which are directly associated with excessive bone destruction and impaired bone quality [2, 3].
The initiation of osteoclast differentiation from hematopoietic stem cell of the monocyte/macrophage lineage is dependent on stimulation by two important cytokines: macrophage colony-stimulating factor (M-CSF), which is required for osteoclast proliferation and survival, and receptor activator of nuclear factor kappa-B ligand (RANKL), which triggers various signals for osteoclastogenesis by binding to the RANK receptor, the surface marker of osteoclast precursors [4, 5]. In response to M-CSF and RANKL stimulation, tumor necrosis factor receptor-associated factor6 (TRAF6) is recruited, which leads to subsequent activation of downstream transducers of the RANKL-dependent pathway. The downstream transducers include mitogen-activated protein kinases (MAPKs), such as p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK); Akt; and nuclear factor kappa-B (NFκB). Activation of this signaling pathways leads to the nuclear translocation of c-Fos and nuclear factor of activated T cell c1 (NFATc1), which are recognized as two master osteoclast regulators. This results increase expression of various osteoclast-specific marker genes that are crucial for development and function of mature osteoclasts, such as β3-integrin, dendritic cell-specific transmembrane protein (DC-STAMP), and Cathepsin K [6–10].
Lipopolysaccharide (LPS) leads to the intracellular induction of p38, JNK, and NFκB in macrophages and monocytes, and promotes the differentiation and survival of osteoclasts through the production of other factors such as PGE2, interleukin 1, RANKL, and TNF [11–13]. Therefore, LPS is an important mediator of pathological bone destruction associated with inflammation.
In this study, we screened several plant-derived extracts by tartrate-resistant acid phosphate (TRAP) staining and confirmed that ethanolic extract of Schizonepeta tenuifolia (EEST) can suppress osteoclast activity. Although previous reports demonstrated that EEST exerts various pharmacological effects, including anti-inflammatory, anti-oxidant, and hemostatic activity, the effects of EEST on bone metabolism have not been studied [14–16]. Therefore, we investigated the effects of EEST on RANKL-induced osteoclast differentiation and its underlying intracellular mechanisms in vitro. Furthermore, we performed in vivo experiments using a LPS-mediated bone erosion mouse model in order to verify the therapeutic value of EEST for treatment of osteoporosis.
Methods
Plant materials and EEST preparation
The 95 % EEST (sale number: CA03-094) of the Korean Plant Extract Bank (KPEB) at the Korea Research Institute of Bioscience and Biotechnology (KRIBB) (Daejeon, Korea) was acquired from the plant samples purchased from an Oriental medicine market in Korea and then authenticated by three taxonomic experts at Chungbuk, Chungnam, and Pusan National University. Also, all forms of extraction from the KPEB were produced through standardization procedure. The KPEB extraction protocol consists of 5 stages: extraction, filtration and yield testing, concentration, drying, and storage. First, extraction of ST was performed using 95 % ethanol with a sonicator (SDN-900H, SD Ultrasonic Cleaner, Seoul, Korea) at 45 °C for 3 days (15 min sonication followed by 2 h standing; repeated 10 times per day). Next, The EEST was filtered through Whatman filter paper No.2 (Advantec, Tokyo, Japan). The filtrates were combined, evaporated under vacuum, and then lyophilized with a CleanVac 12 vacuum freeze dryer (Biotron; Gangneung, Korea) at -70 °C for 24 h under reduced pressure (<20 Pa). A 50 mg/mL stock solution of EEST was prepared in dimethyl sulfoxide (DMSO) and stored at -20 °C.
Reagents
A TRAP staining solution was obtained from Sigma Aldrich (St. Louis, MO, USA) and a sodium 3ʹ-[1-(phenyl-aminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) (XTT) assay kit was purchased from Roche (Indianapolis, IN, USA). The α-minimum essential medium (α-MEM), fetal bovine serum (FBS), and penicillin-streptomycin were purchased from Gibco-BRL (Grand Island, NY, USA), and soluble human recombinant M-CSF and RANKL were purchased from Peprotech (London, UK). Specific antibodies against c-Fos and NFATc1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Specific primary antibodies against phospho-p38, p38, phospho-Akt, Akt, phospho-ERK, ERK, phospho-JNK, JNK, phospho-IκB, and IκB were purchased from Cell Signaling Technology (Beverly, MA, USA), and that against the house-keeping gene GAPDH was purchased from Santa Cruz Biotechnology.
Osteoclast differentiation from mouse bone marrow macrophages (BMMs)
To obtain osteoclast precursors, we prepared mouse BMMs as described previously [17] and BMMs were incubated with M-CSF (30 ng/mL) and RANKL (50 ng/mL) in the absence and presence of EEST (1–50 μg/mL). In this experiment, the control group was treated with 0.1 % DMSO, and the other 5 groups were treated with EEST at concentrations of 1, 5, 10, 25, and 50 μg/mL. After 3 days, the culture medium was replaced with fresh medium with the same composition. After an additional day, cells were stained with a TRAP solution and TRAP-positive multinucleated cells (TRAP+ MNCs) containing more than 5 nuclei were observed and counted as described previously [17].
Evaluation of cytotoxicity, analysis of western blotting and quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR), retroviral gene transfection, and assay of bone resorption in co-culture system of BMCs and primary osteoblasts
The experiments were performed as described previously [17]. The primer sets used for the real-time PCR were listed in Table 1. The retroviral vectors used for gene transfection were pMX-IRES-EGFP, pMX-Akt-IRES-EGFP, and pMX-constitutively active (CA)-IKKβ-IRES-EGFP packaging.
Immunofluorescence staining and confocal microscopy
BMMs were incubated with M-CSF (30 ng/mL) and RANKL (50 ng/mL) in the presence and absence of EEST (50 μg/mL). After 3 days, the culture medium was replaced with fresh medium containing the same constituents. After an additional day, cells were stained with phalloidin and a DAPI solution for visualization of filamentous actin (F-actin) and nuclei as described previously [17]. The fluorescence signal was observed using a laser scanning confocal microscope (Olympus FV1200; Olympus, Shinjuku, Japan), and images representative of 5 experiments were analyzed using Image-Pro Plus software (Media Cybernetics Inc., Rockville, MD, USA).
LPS-mediated bone erosion mouse model and micro-CT and histological analysis
Five-week-old male ICR mice were purchased from Samtako Inc. (Osan, Korea). The mice were kept under controlled temperature (22–24 °C) and humidity (55–60 %) with a 12 h light/dark cycle. The use of experimental animals was reviewed by the institutional animal care and use committee (IACUC) and approved under WKU15-91. To examine the effect of EEST on LPS-induced bone destruction, 5-week-old male ICR mice were randomly divided into 3 groups (5 mice per group): Control (treated with PBS), LPS (treated only with LPS), and LPS + ST (treated with both LPS and EEST). EEST (200 mg/kg) or PBS was administered orally 1 day before LPS injection (5 mg/kg), and then every other 8 days. LPS was injected intraperitoneally on day 2 and 6. All mice were sacrificed after 8 days and femur of each mouse were examined by high-resolution micro-CT analysis and histological analysis including hematoxylin and eosin (H&E) and TRAP staining as described previously [17]. Briefly, micro-CT analysis was performed using bone-related parameters, including bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) which are minimal set of variables that should be investigated for trabecular bone regions [18]. Nomenclature, symbols, and units used in this study were recommended by the American Society for Bone Mineral Research (ASBMR) Nomenclature Committee.
Statistical analysis
Each experiment was conducted at least 3 times, and data were expressed as mean ± standard deviation (SD). All statistical analyses were performed using Statistical Package for the Social Sciences Software (SPSS; Korean version 14.0). Student’s t-test was used to compare parameters between 2 groups, while analysis of variance followed by Tukey post-hoc test was used to compare parameters among 3 groups. P < 0.05 was considered statistically significant.
Results and discussion
EEST exerts inhibitory effects on TRAP-positive osteoclast formation induced by RANKL treatment without cytotoxicity
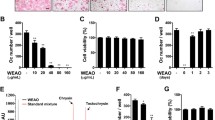
To screen the effects of EEST on osteoclast formation, we treated mouse BMMs with the indicated concentrations of EEST in culture medium (α-MEM containing 30 ng/mL M-CSF and 50 ng/mL RANKL). We observed that EEST suppressed the number of TRAP+ MNCs with more than 5 nuclei in a dose-dependent manner compared to DMSO-treated control group (Fig. 1a and b). In addition, EEST did not exert any cytotoxic effects during the differentiation of BMMs into osteoclasts (Fig. 1c). Our results indicated that EEST effectively suppressed RANKL-dependent osteoclast formation without cytotoxicity at various concentrations (1–50 μg/mL).
EEST attenuates RANKL-induced osteoclast differentiation in a dose-dependent manner with no cytotoxicity. a BMMs were cultured for 4 days in the presence of M-CSF (30 ng/mL) and RANKL (50 ng/mL) with the indicated concentrations of EEST. Cells were fixed in 3.7 % formalin, permeabilized with 0.1 % Triton X-100, and stained with TRAP solution. TRAP+ MNCs were photographed under a light microscope. b TRAP+ MNCs with more than 5 nuclei were counted. ***P < 0.001, **P < 0.01 vs. control. c BMMs were seeded into a 96-well plate and cultured for 3 days in the presence of M-CSF (30 ng/mL) and the indicated concentrations of EEST. After 3 days, the absorbance at 450 nm was determined using an ELISA reader
EEST affects early signaling events of osteoclastogenesis via dephosphorylation of Akt and IkB
Next, we performed western blotting to confirm whether EEST was related with RANKL-dependent signal transducers, such as MAPKs, including p38, ERK, and JNK; Akt; and IκB. As shown in Fig. 2a, EEST strongly reduced the phosphorylation of Akt and weakly suppressed the phosphorylation of IkB. In addition, we reaffirmed the role of EEST on the activation of Akt and IκB using retroviral vectors. Overexpression of Akt and CA form of IKKβ, a catalytic subunit of IκB kinase complex, was sufficient to reverse the inhibitory effect of EEST on osteoclast formation. Previously, it has been shown that Akt plays a critical role in osteoclast survival by regulating its downstream target, GSK3β, and the signaling cascade of NFATc1, a master transcription factor for osteoclastogenesis. An Akt inhibitor, LY294002, significantly suppresses osteoclast formation and NFATc1 expression in vitro, and systemic injection of LY294002 attenuates multiple myeloma-induced abnormal osteoclast formation and osteolysis in vivo [19, 20]. The other early signaling molecule, IκB, is also essential for the regulation of osteoclast activity. The inhibitor of IκB kinase (IKK), which induces phosphorylation of IkB, suppresses RANKL-induced osteoclast formation and activity in vitro, and ovariectomy-mediated bone erosion in vivo by targeting osteoclastic bone resorption [21]. Therefore, our results suggested that EEST suppresses osteoclast formation by targeting two signal transducers, Akt and IκB at the early stage of osteoclast differentiation.
EEST down-regulates RANKL-mediated phosphorylation of Akt and IkB. a BMMs were cultured for 1 day in the presence of M-CSF (10 ng/mL). Next, BMMs were starved for 3 h, pretreated with EEST (50 μg/mL) for 1 h and then stimulated with RANKL (50 ng/mL) for the indicated times. Cell lysates were analyzed by western blotting with antibodies against p-p38, p38, p-ERK, ERK, p-JNK, JNK, p-Akt, Akt, p-IκB, IκB, and GAPDH. b BMMs were infected with retroviruses expressing pMX-IRES-EGFP (pMX), pMX- CA-IKKβ-EGFP, and pMX-Akt-EGFP. Infected BMMs were cultured with or without EEST (50 μg/mL) in the presence of M-CSF (30 ng/mL) and RANKL (50 ng/mL) for 4 days. After culturing, the cells were fixed and stained for TRAP (left). The TRAP+ MNCs with more than 5 nuclei were counted (right). *P < 0.05, **P < 0.01 vs. the indicated group
EEST suppresses mRNA and protein expressions of NFATc1 and protein expression of c-Fos
Through the verification of the role of EEST on the early stage of osteoclastogenesis, we assumed that EEST was also associated with the final stage of RANKL-mediated osteoclast differentiation. At this stage, the transcription factors c-Fos and NFATc1 are activated in response to the activation of early signal transducers [22, 23]. It has previously been shown that embryonic stem cells with NFATc1 deficiency are not capable of differentiating into functional osteoclasts, and the retrovirus-mediated overexpression of NFATc1 induces normal osteoclast differentiation even in the absence of RANKL [22]. Ectopic expression of c-Fos reverses the osteoclast dysfunction-induced symptom of osteopetrosis, which is observed in c-Fos knockout mice [23]. In this study, we found that EEST blocked the expression of NFATc1 gene at both the transcription and translation levels and c-Fos gene at the translation level (Fig. 3). Collectively, EEST showed suppression effects on the expression of two major transcription factors in response to downregulation of Akt and IκB during osteoclast differentiation.
EEST inhibits protein expression of c-Fos and both mRNA and protein expression of NFATc1. a BMMs were pretreated with or without EEST (50 μg/mL) for 1 h and then stimulated with M-CSF (30 ng/mL) and RANKL (50 ng/mL) for the indicated times. The cell lysates were analyzed by western blotting with antibodies against c-Fos, NFATc1, and GAPDH. b BMMs were stimulated with RANKL (50 ng/mL) and M-CSF (30 ng/mL) in the presence or absence of EEST (50 μg/mL) for the indicated times. Total RNA was isolated from cells using the QIAzol reagent, and mRNA expression of c-Fos and NFATc1 was determined using quantitative real-time RT-PCR. ***P < 0.001 vs. control in the indicated time
EEST inhibits formation of F-actin structure and bone resorption activity of mature osteoclasts
During the development of functional osteoclasts, the organization of F-actin structure is required for bone resorption activity. Once the multinucleated osteoclast attaches to the bone surface, its membrane becomes polarized and secretes both hydrogen ions and lytic enzymes into the resorption lacuna in order to dissolve the bone matrix. This region is surrounded by a tight sealing zone that is recognized as a physiological feature of mature osteoclasts and composed of F-actin ring-like structures [24, 25]. In this study, we confirmed that EEST significantly inhibited the formation F-actin ring-positive osteoclasts and subsequently reduced the pit area formed as a result of the bone resorption activity of mature osteoclasts (Fig. 4a and b). Also, this phenomenon was induced by the decreased expression of various osteoclast-marker genes, including β3-integrin, DC-STAMP, Atp6v0d2, and Cathepsin K, which are required for the cell-to-cell fusion needed to organize F-actin structure and bone resorption [26–29]. Our results demonstrated that EEST negatively regulated the development of functional osteoclasts by attenuating the transcription of several osteoclast-specific marker genes.
EEST negatively controls F-actin structure, bone-resorbing activity, and expression of osteoclast-specific genes. a BMMs were cultured for 4 days in the presence of M-CSF (30 ng/mL) and RANKL (50 ng/mL) with or without EEST (50 μg/mL). Cells were fixed with 3.7 % formalin, permeabilized with 0.1 % Triton X-100, and stained with phalloidin and DAPI. Mature osteoclasts were seeded on hydroxyapatite-coated plates for 24 h with EEST (50 μg/mL) treatment. Adherent cells were removed and photographed under a light microscope. b The relative ratio of pit areas was quantified using Image J. ***P < 0.001 vs. control. c BMMs were stimulated with RANKL (50 ng/mL) and M-CSF (30 ng/mL) in the presence or absence of EEST (50 μg/mL) for the indicated times. Total RNA was isolated from cells using QIAzol reagent, and mRNA expression of β3-integrin, DC-STAMP, Atp6v0d2, and Cathepsin K was determined using quantitative real-time RT-PCR. ***P < 0.001 vs. control in the indicated time
EEST exerts a protective effects in LPS-induced bone erosion mice model
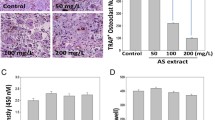
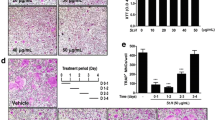
Finally, to determine if the in vitro effect of EEST on osteoclast activity could be confirmed in vivo, commonly used mouse model of osteoporosis was applied. Among the osteoporotic models, in this study, we focused on the therapeutic value of EEST on inflammation-induced bone loss because EEST has been proved to exert a significant anti-inflammatory effect [14]. LPS is an efficient tool for the induction of inflammatory osteoporosis in mice [30]. Thus, we selected bone loss model induced by LPS injection to suggest the therapeutic value of EEST on inflammatory osteoporosis. Mice were injected with LPS intraperitoneally on day 1 and 4 to induce systemic inflammatory response and subsequent bone loss and orally administered with EEST or PBS every 8 days. After, the left femora of sacrificed mice were analyzed by micro-CT and the right femora were stained with H&E and TRAP solution. While the bone mass in the femora of LPS-treated mice was lower than that of controls, there was a partial recovery of bone density in mice treated with both LPS and EEST (Fig. 5a). Morphometric analysis of the femora of LPS-treated mice showed decreased BV/TV and Tb.N and increased Tb.Sp, while restoration of these parameters was observed in the LPS + EEST group (Fig. 5b). Histological analysis confirmed that LPS + EEST treatment inhibited LPS-induced erosion of bone matrix and formation of TRAP-positive osteoclasts within growth plates (Fig. 5c and d). The present findings suggested that EEST exhibited protective effects on osteoclast differentiation and subsequent bone resorption in vivo. Also, it was thought that our further study could be in need of demonstrating the effect of EEST on OVX-mediated bone loss to more clarify the protective effect of EEST on osteoporosis.
EEST recovers LPS-induced inflammatory bone loss in mice. a Mice were sacrificed 8 days after the first LPS injection and 2D or 3D radiographs of the coronal and transverse planes of the proximal femora were obtained by micro-CT. b The BV/TV, Tb.Sp, Tb.Th, and Tb.N of the femora were determined using the micro-CT data and analyzed by INFINITT-Xelis software. ***P < 0.001 vs. control; ##P < 0.01, ###P < 0.001 vs. LPS group. c Dissected femora were fixed, decalcified, embedded, and sectioned. Sections were stained with TRAP and H&E. d The number of osteoclasts per field of tissue was counted by histomorphometric analysis
Conclusions
In the present study, we demonstrated that EEST attenuated RANKL-induced osteoclast differentiation by downregulating Akt and IkB phosphorylation in the early signaling event and subsequently targeted NFATc1 at the transcriptional and translational levels and c-Fos at the translational level. Moreover, EEST exerted suppression effects on F-actin ring formation and bone resorption in vitro and LPS-mediated bone erosion in vivo. Taken together, our findings supported the potential value of EEST as a plant-derived therapeutic agent to treat bone-related disorders, particularly osteoporosis.
Abbreviations
- BMCs:
-
Bone marrow cells
- BMMs:
-
Bone marrow macrophages
- BV/TV:
-
Bone volume per tissue volume
- DC-STAMP:
-
Dendritic cell-specific transmembrane protein
- EEST:
-
Ethanolic extract of Schizonepeta tenuifolia
- ELISA:
-
Enzyme-linked immunosorbent assay
- ERK:
-
Extracellular signal-regulated kinase
- F-actin:
-
Filamentous actin
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- H&E:
-
Hematoxylin and eosin
- IKK:
-
Inhibitor of IkB kinase
- JNK:
-
c-Jun N-terminal kinase
- LPS:
-
lipopolysaccharide
- MAPKs:
-
Mitogen-activated protein kinases
- M-CSF:
-
Macrophage colony-stimulating factor
- NFATc1:
-
Nuclear factor of activated T cells c1
- NFκB:
-
Nuclear factor kappa-B
- PGE2:
-
Prostaglandin E2
- RANKL:
-
Receptor activator of nuclear factor kappa-B ligand
- Tb.N:
-
Trabecular number
- Tb.Sp:
-
Trabecular separation
- Tb.Th:
-
Trabecular thickness
- TRAF6:
-
Tumor necrosis factor receptor-associated factor6
- TRAP+ MNCs:
-
TRAP-positive multinucleated cells
- Vitamin D3:
-
Dihydroxyvitamin D3
- XTT:
-
Sodium 3ʹ-[1-(phenyl-aminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)
- α-MEM:
-
α-minimum essential medium
References
Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–96.
Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):325–8.
Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2(10):1132–6.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42.
Suda T, Kobayashi K, Jimi E, Udagawa N, Takahashi N. The molecular basis of osteoclast differentiation and activation. Novartis Found Symp. 2001;232:235–47.
Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25.
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901.
Rodan SB, Rodan GA. Integrin function in osteoclasts. J Endocrinol. 1997;154(Suppl):S47–56.
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202(3):345–51.
Goto T, Yamaza T, Tanaka T. Cathepsins in the osteoclast. J Electron Microsc (Tokyo). 2003;52(6):551–8.
Suda K, Woo JT, Takami M, Sexton PM, Nagai K. Lipopolysaccharide supports survival and fusion of preosteoclasts independent of TNF-alpha, IL-1, and RANKL. J Cell Physiol. 2002;190(1):101–8.
Sakuma Y, Tanaka K, Suda M, Komatsu Y, Yasoda A, et al. Impaired bone resorption by lipopolysaccharide in vivo in mice deficient in the prostaglandin E receptor EP4 subtype. Infect Immun. 2000;68(12):6819–25.
Orcel P, Feuga M, Bielakoff J, De Vernejoul MC. Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am J Physiol. 1993;264(3 Pt 1):E391–7.
Byun MW. Schizonepeta tenuifolia ethanol extract exerts anti-inflammatory activity through the inhibition of TLR4 signaling in lipopolysaccharide-stimulated macrophage cells. J Med Food. 2014;17(3):350–6.
Wang BS, Huang GJ, Tai HM, Huang MH. Antioxidant and anti-inflammatory activities of aqueous extracts of Schizonepeta tenuifolia Briq. Food Chem Toxicol. 2012;50(3-4):526–31.
Ding AW. Hemostatic effect of Schizonepeta tenuifolia before and after carbonation. Zhong Yao Tong Bao. 1986;11(3):23–5.
Kim JY, Cheon YH, Kwak SC, Baek JM, Yoon KH, Lee MS, et al. Emodin regulates bone remodeling by inhibiting osteoclastogenesis and stimulating osteoblast formation. J Bone Miner Res. 2014;29(7):1541–53.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86.
Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee SY, et al. Akt induces osteoclast differentiation through regulating the GSK3β/NFATc1 signaling cascade. J Immunol. 2012;188(1):163–9.
Cao H, Zhu K, Qiu L, Li S, Niu H, Hao M, et al. Critical role of AKT protein in myeloma-induced osteoclast formation and osteolysis. J Biol Chem. 2013;288(42):30399–0410.
Idris AI, Krishnan M, Simic P, Landao-Bassonga E, Mollat P, Vukicevic S, et al. Small molecule inhibitors of IkappaB kinase signaling inhibit osteoclast formation in vitro and prevent ovariectomy-induced bone loss in vivo. FASEB J. 2010;24(11):4545–55.
Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–37.
Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–8.
Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85(3-4):195–202.
Kanehisa J, Yamanaka T, Doi S, Turksen K, Heersche JN, Aubin JE, et al. A band of F-actin containing podosomes is involved in bone resorption by osteoclasts. Bone. 1990;11(4):287–93.
McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105(4):433–40.
Yagi M, Miyamoto T, Toyama Y, Suda T. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J Bone Miner Metab. 2006;24(5):355–8.
Wilson SR, Peters C, Saftig P, Brömme D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J Biol Chem. 2009;284(4):2584–92.
Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrionol. 2008;22(1):176–85.
Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, et al. Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J Immunol. 2003;170(7):3688–95.
ᅟ
ᅟ
Funding
This study was supported by a grant from the Wonkwang University in 2015.
Availability of data and materials
All data and materials are contained and described within the manuscript.
Authors’ contributions
JYK and JMB: conception and design, equally performed most of the experiments in this study, drafted the manuscript, and will provide final approval of the version to be published; SJA and SHP: performed the in vivo study; YHC, MY and MKC: drafted the manuscript and revised it critically for important intellectual content; JO: conception and design, and will provide final approval of the version to be published. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The use of experimental animals was reviewed by the IACUC and was approved under WKU15-91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kim, JY., Baek, J.M., Ahn, SJ. et al. Ethanolic extract of Schizonepeta tenuifolia attenuates osteoclast formation and activation in vitro and protects against lipopolysaccharide-induced bone loss in vivo. BMC Complement Altern Med 16, 301 (2016). https://doi.org/10.1186/s12906-016-1300-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-016-1300-0