Abstract
Purpose
The weight-adjusted waist index (WWI) is a recently developed index for measuring obesity. The aim of this study was to investigate the association between WWI levels and overactive bladder (OAB) in a nationally representative population.
Methods
This cross-sectional study used data from the National Health and Nutrition Examination Survey (NHANES) database between 2007 and 2016. OAB was defined as the Overactive Bladder Syndrome Symptom Score (OABSS, score ≥ 3). The WWI index was calculated as the square root of waist circumference (WC, cm) divided by body weight (kg). We used weighted logistic regression models to assess the relationship between the WWI index and OAB in adult women. The reliability of the findings was assessed using restricted cubic spline, subgroup analysis.
Results
A total of 10,563 individuals were included in the study, and the prevalence of OAB was 18.6%. Higher WWI was associated with an increased risk of overactive bladder syndrome. In model 1 with unadjusted variables (OR = 1.148; 95% CI = 1.148–1.149, p < 0.001), model 2 (OR = 1.253; 95% CI = 1.253–1.254, p < 0.001) and model 3 with fully adjusted variables (OR = 1.215; 95% CI = 1.214–1.215, p < 0.001) in which the association was significant. The results of the subgroup analyses showed that age stratification and stroke status could modify this association between WWI and OAB. Restricted cubic spline showed a nonlinear relationship between WWI and OAB (p for nonlinear < 0.05).
Conclusion
Weight-adjusted waist circumference index (WWI) values are positively associated with the risk of developing OAB in adult women in the United States, but further studies are needed to elucidate the causal relationship between WWI and OAB.
Similar content being viewed by others
Introduction
Overactive bladder (OAB) is an idiopathic disorder characterized by urinary urgency, usually with nocturia and frequency, with or without urge incontinence [1]. The International Continence Society (ICS) has identified overactivity of the detrusor muscles as the main cause of OAB, but other forms of urethro-bladder dysfunction may also contribute [2]. In recent years, OAB has affected millions of men and women worldwide and the prevalence has increased. The overall prevalence of OAB in the United States has increased from 16.5% in 2000–2001 to 38.5% in 2021–2022, and is increasing with age [3, 4]. In Europe, the prevalence of OAB is 16.6% among people under 40 years of age and the incidence of urge incontinence is higher in women than in men [5]. Some studies have confirmed that moderate-to-severe OAB may lead to mood disorders resulting in a significant reduction in quality-of-life [6, 7]. Urinary incontinence is an underestimated health problem. Patients need to understand their health through detailed memory removal collection and physical examination to determine the type of incontinence and provide the best treatment. Existing available medical techniques and medications have been reported to be effective in improving overactive bladder syndrome [8]. Opemifene has shown high efficacy in improving overactive bladder symptoms in postmenopausal women affected by vulvovaginal atrophy [9]. However, the primary goal of treating OAB is to relieve symptoms because it cannot reverse pathophysiologic abnormalities. OAB also carries a significant economic burden, with the societal costs totaling more than $14 billion [10]. Risk factors for OAB include diet, physical activity, chronic diseases, and neurologic disorders [11]. Few population-based studies have investigated the relationship between OAB and human health. Therefore, there is an urgent need to fill this research gap using reliable and representative data.
The prevalence of obesity has reached epidemic levels globally and is becoming increasingly common among women of childbearing age [12]. Studies have shown that obesity is an important factor in overactive bladder disease [13]. Although body mass index (BMI) is a common assessment of obesity, it cannot be used to differentiate between fat and muscle mass [14]. In addition, BMI is influenced by differences in a variety of demographic factors, such as age, sex, and ethnicity [15]. The obesity paradox challenges trust in the role of BMI. In contrast to BMI, waist circumference (WC) shows a strong correlation with abdominal fat accumulation [16]. However, the correlation between BMI and WC limits the function of WC as an independent obesity marker. The weight-adjusted waist index (WWI) is a novel obesity index that combines the strengths of the WC and shows a stable correlation with abdominal fat accumulation [17]. It is calculated as waist circumference divided by the square root of body weight. WWI corrects for the correlation between WC and BMI primarily by reflecting central obesity independent of body weight. WWI has been shown to correlate with a variety of disorders such as erectile dysfunction, depression, and female infertility [18,19,20,21]. A large number of epidemiological studies have emphasized the important role of obesity, especially abdominal obesity, in OAB [22]. It is valuable that our study introduces the novel obesity indicator WWI to explore this progress. However, to our knowledge, the role of the WWI index concerning OAB in adult women remains unclear. According to previous studies, the transformation of numerical variables into categorical variables by equating them helps to further analyse the sensitivity of the model and assess its robustness. Considering the median after equalisation as a continuous variable, it was assessed whether the non-linear trend was still statistically significant. Using the National Health and Nutrition Examination Survey (NHANES) database, this study sought to explore the association between the WWI index and the prevalence of OAB among adult women in a nationally representative sample.
Materials and methods
Data sources
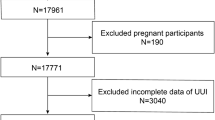
This population-based study used data from NHANES, a national survey conducted by the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention, using a randomized, stratified, multistage approach. Participants underwent a series of assessments, including physical examinations, health and nutrition questionnaires, and laboratory assessments. The NHANES study protocol was approved by the Ethics Review Board of the National Center for Health Statistics. The full detailed design and data are available at https://www.cdc.gov/nchs/nhanes/. Data were obtained and combined from 5 NHANES cycles: 2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016 (50,588 participants). Participants who were male, under 20 years of age, pregnant, and participants without information on WWI index, OAB diagnostic data, and other covariates were excluded. Ultimately, a total of 10,563 eligible patients were enrolled in the study. Figure 1 is a flowchart of participant inclusion and exclusion. The NCHS Research Ethics Review Board approved the NHANES study protocol based on Helsinki declaration, and participants provided written informed consent at enrollment (https://www.cdc.gov/nchs/nhanes/irba98.htm). Ethical approval and consent were not required as the study was based on publicly available de-identified data.
Exposure and outcome definitions
WWI index was used as an exposure variable, defined as the square root of waist circumference divided by body weight. Continuous data were categorized into subtypes according to the trichotomy method. Q1 was defined as WWI ≤ 6.96). Q2 was defined as 6.96 < WWI ≤ 8.07). Q3 was defined as WWI > 8.07. The diagnosis of OAB was defined according to the International Association for Urological Control. OAB is a symptomatic syndrome characterized by concurrent urgency to urinate, usually with urinary frequency and nocturia, with or without urgency urinary incontinence (UUI), and in the absence of urinary tract infections or other obvious pathology. We used the Nephrology Urology Questionnaire from the NHANES database for the assessment of OAB. "Have you ever urinated before going to the bathroom" and "How often does this happen?" were used to assess the severity of UUI. "How many times did you urinate at night? Used to assess the severity of nocturia. Based on previous study [23], we quantified the severity of OAB for each participant by means of the novel Overactive Bladder Symptom Score (OABSS) scale, and participants with an overall OABSS score of ≥ 3 were considered as OAB. Important to emphasize is that participants with an OABSS score of ≥ 3 were considered to have overactive bladder regardless of whether the incontinence score was 0 or not. This study did not provide more detailed typing of overactive bladder syndrome.
Covariate definitions
We collected demographic data and other covariates from the NHANES database, including age, race, Ratio of family income to poverty (RIP), education level, marital status, physical activity, smoking status, alcohol consumption, diabetes, coronary heart disease, stroke, and malignancy. The RIP is the ratio of income to the family poverty line set by the U.S. Census Bureau and is a measure of socioeconomic status. According to "Had at least 12 alcohol drinks/lifetime" and "Had at least 12 alcohol drinks/1 year?", drinkers are categorized as current, former, and never drinkers. Smokers were categorized as current, former, or never smokers based on "Smoked at least 100 cigarettes in life" and "Do you now smoke cigarettes". Self-reported diabetes was also defined based on questionnaire information. The presence of coronary heart disease, stroke, and malignant neoplasm were defined based on self-reported history of specific diseases. The details of the conversion of the Nephrology Urology Questionnaire into OABSS scores in Fig. 2.
Statistical analysis
Participants' baseline characteristics were described as continuous and categorical variables based on OAB prevalence. Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as numbers and percentages. Weighted t-tests and chi-square tests were used to compare the baseline characteristics of these participants. Logistic regression models were used to assess the association between OAB and WWI. Three models with different levels of covariate adjustment were used (model 1, no adjustment for covariates; model 2, adjustment for age, race, RIP, education level, marital status; and model 3, adjustment for all covariates). Odds ratios (ORs) and 95% confidence intervals (CIs) for OAB patients with different WWIs. In the model 3, we used WWI as a continuous variable and restricted cubic spline (RCS) to reveal the dose–response relationship between WWI and OAB risk. The multiple logistic regression models were categorized into categorical and continuous models. Based on previous studies of the correlation between obesity indicator measures and disease, this study used tertiles for grouping variables [24,25,26]. In the categorical model, WWI was categorized into thirds and nonlinear trends were assessed by treating the median of each trichotomy as a continuous variable. Finally, we used subgroup analyses stratified by age, ethnicity, RIP, education level, marital status, physical activity, smoking status, alcohol consumption, hypertension, diabetes, coronary heart disease, stroke, and malignancy, and performed interaction analyses to check for differential associations between subgroups. A bilateral p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 26.0.
Results
Baseline characteristics of participants
A total of 10,563 eligible participants were ultimately enrolled in the study (from 2007–2016 NHANES). Statistically, a total of 2,412 participants (18.6%) with OAB and 8,142 healthy participants(81.4%). Table 1 shows the characteristics of the study participants. The population-weighted mean age was 57.24 ± 15.91 years. We observed statistically significant differences in all variables in the OAB prevalent population (p value < 0.05). Participants in the prevalent group were older, less educated, had low RIP, comorbid diabetes, coronary heart disease, stroke, and malignant tumors compared to the non-OAB group. In addition, participants in the disease group were more likely to drink alcohol, smoke, and engage in excessive physical activity.
The relationship between OAB and WWI
Three weighted univariate and multivariate regression models were used to assess whether the relationship between WWI and the prevalence of OAB in adult women in the United States was statistically significant. Based on these three models, we observed a positive and statistically significant association between WWI and OAB prevalence. Higher WWI was associated with an increased risk of overactive bladder syndrome. In our model 1 (OR = 1.148; 95% CI = 1.148—1.149, p < 0.001), model 2 (OR = 1.253; 95% CI = 1.253—1.254, p < 0.001) and model 3 (OR = 1.215; 95% CI = 1.214—1.215, p < 0.001) in which the association was significant. In the fully adjusted model, each unit increase in WWI was associated with a 21.5% increased risk of developing overactive bladder syndrome (Table 2).
Using restricted cubic spline, a nonlinear relationship between WWI and OAB risk was found in all three models adjusted for multiple covariates (Fig. 3) (p < 0.05).
Analysis of the Measured Response Relationship Between WWI and OAB. A Model 1 was adjusted for none. B Model 2 was adjusted for age, sex, race, education level, and marital status. C Model 3 was adjusted for age, race, RIP, education level, marital status, physical activity, smoking status, alcohol use, diabetes, coronary heart disease, stroke, and malignancy. The solid blue line indicates the OR and the blue shaded area indicates the 95% CI
Subgroup analysis
Subgroup analyses were performed to assess the association between WWI and the prevalence of OAB in different populations. As shown in Table 3, WWI was positively associated with the risk of OAB prevalence in specific populations (P < 0.05). These characteristics included age, race, education level, marital status, RIP, history of alcohol consumption, history of smoking, diabetes mellitus, recreational activities, history of coronary heart disease, history of stroke, and malignancy. Significant interactions were found within subgroups based on age (≤ 40, 41–60, > 60) (P for Interaction = 0.032). In individuals under 40 years of age, each 1-unit increase in WWI was associated with a 25% increase in the odds of having OAB. For individuals aged 41 to 60 years, each 1-unit increase in WWI was associated with a 26.7% increase in the odds of having OAB. For those older than 60 years, each 1-unit increase in WWI was associated with a 17.4% increase in the odds of having OAB. We also found some degree of interaction in the stroke (yes/no) subgroup (P for Interaction = 0.030). In the non-stroke population, each 1-unit increase in WWI was associated with a 15.9% increase in the incidence of OAB.
Discussion
In this study, based on a representative sample of US adult women, we observed a significant association between WWI and OAB. Both univariate and multivariate logistic regression analyses showed that WWI levels were associated with increased prevalence of OAB. Conversion of WWI from a continuous to a categorical variable showed a significant increase in the prevalence of OAB in Q2 (6.96 < WWI ≤ 8.07) and Q3 (> 8.07) compared to Q1 (WWI ≤ 6.96)). Further analysis of the dose–response relationship showed a nonlinear positive correlation between WWI and OAB prevalence. Subgroup analyses showed that the positive correlation between WWI and OAB prevalence varied across age and stroke populations. The stability of the correlation between WWI and OAB prevalence in other subgroups remained consistent. Stroke and age can modify the relationship between WWI and OAB. In particular, increased WWI was associated with a significantly higher risk of OAB in the non-stroke group, but no significant correlation was found between WWI and OAB in the stroke group. In addition, the correlation between WWI and OAB was stronger in the 41–60-year-old group.
Our results in subgroup analyses found a significant positive correlation between WWI and OAB in the non-stroke population (OR = 1.159 95% CI 1.123—1.196, P < 0.001). Post-stroke develop neurogenic detrusor overactivity is the most common urodynamic manifestation in stroke patients. Approximately 10% of stroke patients may develop sphincter pseudo-synergia after stroke [27]. After the acute phase, it is the post-stroke patient whose lower urinary tract symptoms are primarily characterized by bladder overactivity. After the recovery period, bladder dysfunction and urge incontinence may still be present in post-stroke patients [28]. A growing number of studies have shown that overactive bladder disorder is a common disorder in middle-aged women [27]. The OAB prevalence increases with age, especially in the middle-aged population (age ≥ 40 years) [29].
Numerous studies have confirmed that high BMI is a risk factor for urinary incontinence in women [30, 31]. A longitudinal study based on a Chinese population suggests that incontinence remission rates are lower in women with higher BMIs [32]. Women with larger abdominal diameters have a greater chance of experiencing lower urinary tract symptoms (urinary incontinence, nocturia, and urgency) [33]. Since obesity is a modifiable risk factor, weight loss programs may help reduce symptoms or incidence of OAB [34]. BMI is widely used worldwide as an indicator of overweight and obesity. However, it does not reflect obesity types such as apple-shaped obesity (upper body obesity) and pear-shaped obesity (lower body obesity) due to poor estimates of overall obesity. But the obesity paradox remains. The reason for the controversy may partly stem from the inability of traditional metrics to differentiate between fat distribution and muscle mass [14]. The WWI takes into account the ratio of waist circumference to height, which provides more precise information about fat distribution [17]. There is a lack of studies exploring the correlation between OAB and the WWI, which may be one of the directions for future research in assessing obesity and OAB.
Several potential mechanisms could explain this positive correlation in the relationship between WWI and OAB. First, increased WWI may reflect visceral fat accumulation, particularly in the abdomen. This fat accumulation can exert physical pressure on the bladder and pelvis, leading to compression of the bladder neck and urethra, which can increase the risk of urinary incontinence. Visceral fat may also affect the function of the pelvic floor muscles, which in turn affects the ability of the urethra to close and control urination [35]. Second, obesity is closely associated with a state of chronic inflammation [36]. The accumulation of large amounts of visceral fat can lead to the release of pro-inflammatory factors, which can damage the pelvic floor structures and weaken the tone and elasticity of the urinary sphincter. Chronic inflammation may directly or indirectly affect the mechanisms of urinary incontinence [37]. Inflammation may affect nerve conduction, the pelvic floor nerves, and the central nervous system that controls urethral and bladder function. This may include changes in nerve conduction velocity, neuronal damage or degeneration, which in turn affects urethral perception and control and increases the risk of urinary incontinence [38]. In addition, obesity can lead to changes in hormone levels, such as insulin resistance. These hormonal changes may affect the strength and function of the pelvic floor muscles, which may affect urethral control. Interaction between reduced oestrogen production and prevalence of obesity in menopausal women. Oestrogen and the oestrogen receptor regulate glucose and lipid metabolism, and disturbances in this metabolic signaling contribute to the risk of metabolic syndrome. It is characterized by changes in the lipid profile and the accumulation of predominantly abdominal fat [39]. Decreased estrogen levels may lead to atrophy and decreased function of the pelvic floor muscles as well as damage to the urethral mucosal barrier which can increase the risk of urinary incontinence [40]. Finally, people with obesity usually have unhealthy diet and exercise habits. They may consume large amounts of stimulating foods or beverages, such as coffee and carbonated beverages [41], which may irritate the bladder and lead to frequent or urgent urination. They may often be physically inactive, and prolonged physical inactivity may lead to weakening and decreased function of the pelvic floor muscles [42]. Obesity may lead to psychological problems such as body image dissatisfaction, social rejection, or anxiety. Anxiety, depression, or stress may lead to tension in the pelvic floor muscles or overactive bladder [6].
According to recent research, obesity is strongly associated with a wide range of deleterious health outcomes in the context of the current global obesity rate that continues to rise, and in particular is strongly linked to the problem of OAB. This study provides new evidence of a significant association between WWI and the development of OAB, which is one of the key therapeutic targets for future OAB interventions. This study also has several limitations. First, NHANES utilized a multistage sampling design, but selection bias may still exist. Participants may not be representative of the entire population, which may affect the extrapolation of findings. Second, some information was obtained through respondent self-report, such as disease information. This self-reporting bias may have resulted in inaccurate information, which could have affected the results of the study. In addition, this study was a cross-sectional study and only correlations could be inferred and causality over time could not be obtained.
Availability of data and materials
NHANES data used in this work are publicly available. All raw data are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/). Further inquiries can be directed to the corresponding author.
References
Nambiar AK, Arlandis S, Bo K, et al. European association of urology guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: Diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence. Eur Urol. 2022;82(1):49–59.
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49.
Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–36.
Sebesta EM, Gleicher S, Kaufman MR, et al. Associations between unmet social needs and overactive bladder. J Urol. 2022;208(5):1106–15.
Milsom I, Abrams P, Cardozo L, et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87(9):760–6.
Zuluaga L, Caicedo JI, Mogollon MP, et al. Anxiety and depression in association with lower urinary tract symptoms: results from the COBaLT study. World J Urol. 2023;41(5):1381–8.
Hughes FJ, Odom MR, Cervantes A, et al. Why are some people with Lower Urinary Tract Symptoms (LUTS) depressed? New evidence that peripheral inflammation in the bladder causes central inflammation and mood disorders. Int J Mol Sci. 2023;24(3):2821.
Monti M, Fischetti M, Santangelo G, et al. Urinary incontinence in women: state of the art and medical treatment. Minerva Obstet Gynecol. 2021;73(2):135–9.
Schiavi MC, Zullo MA, Faiano P, et al. Retrospective analysis in 46 women with vulvovaginal atrophy treated with ospemifene for 12 weeks: improvement in overactive bladder symptoms. Gynecol Endocrinol. 2017;33(12):942–5.
Durden E, Walker D, Gray S, et al. The economic burden of overactive bladder (OAB) and its effects on the costs associated with other chronic, age-related comorbidities in the United States. Neurourol Urodyn. 2018;37(5):1641–9.
Mckellar K, Bellin E, Schoenbaum E, et al. Prevalence, risk factors, and treatment for overactive bladder in a racially diverse population. Urology. 2019;126:70–5.
Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Gregg EW, Shaw JE. Global health effects of overweight and obesity. N Engl J Med. 2017;377(1):80–1.
Heymsfield SB, Scherzer R, Pietrobelli A, et al. Body mass index as a phenotypic expression of adiposity: quantitative contribution of muscularity in a population-based sample. Int J Obes (Lond). 2009;33(12):1363–73.
Jackson AS, Stanforth PR, Gagnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26(6):789–96.
Lam BC, Koh GC, Chen C, et al. Comparison of Body Mass Index (BMI), Body Adiposity Index (BAI), Waist Circumference (WC), Waist-To-Hip Ratio (WHR) and Waist-To-Height Ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS ONE. 2015;10(4):e122985.
Yu S, Wang B, Guo X, et al. Weight-adjusted-waist index predicts newly diagnosed diabetes in Chinese rural adults. J Clin Med. 2023;12(4):1620.
Cao S, Hu X, Shao Y, et al. Relationship between weight-adjusted-waist index and erectile dysfunction in the United State: results from NHANES 2001–2004. Front Endocrinol (Lausanne). 2023;14:1128076.
Li M, Yu X, Zhang W, et al. The association between weight-adjusted-waist index and depression: Results from NHANES 2005–2018. J Affect Disord. 2024;347:299–305.
Ye J, Hu Y, Chen X, et al. Association between the weight-adjusted waist index and stroke: a cross-sectional study. BMC Public Health. 2023;23(1):1689.
Wen Z, Li X. Association between weight-adjusted-waist index and female infertility: a population-based study. Front Endocrinol (Lausanne). 2023;14:1175394.
Lai HH, Helmuth ME, Smith AR, et al. Relationship between central obesity, general obesity, overactive bladder syndrome and urinary incontinence among male and female patients seeking care for their lower urinary tract symptoms. Urology. 2019;123:34–43.
Lin W, Wang H, Wu Z, et al. Associations between exposure to organophosphate esters and overactive bladder in U.S. adults: a cross-sectional study. Front Public Health. 2023;11:1186848.
Yin YH, Zhou SY, Lu DF, et al. Higher waist circumference is associated with increased likelihood of female infertility: NHANES 2017–2020 results. Front Endocrinol (Lausanne). 2023;14:1216413.
Zhou Y, Yang G, Peng W, et al. Relationship between depression symptoms and different types of measures of obesity (BMI, SAD) in US women. Behav Neurol. 2020;2020:9624106.
Zhang Y, Song J, Li B, et al. Association between body roundness index and overactive bladder: results from the NHANES 2005–2018. Lipids Health Dis. 2024;23(1):184.
Yang CF, Huang CY, Wang SY et al. Prevalence of and associated factors for overactive bladder subtypes in middle-aged women: a cross-sectional study. Medicina (Kaunas). 2022;58(3):383.
Chiang CH, Chen SF, Kuo HC. Video-urodynamic characteristics of lower urinary tract dysfunctions in patients with chronic brain disorders. Neurourol Urodyn. 2022;41(1):255–63.
Przydacz M, Golabek T, Dudek P, et al. Prevalence and bother of lower urinary tract symptoms and overactive bladder in Poland, an Eastern European Study. Sci Rep. 2020;10(1):19819.
Pang H, Lv J, Xu T, et al. Incidence and risk factors of female urinary incontinence: a 4-year longitudinal study among 24 985 adult women in China. BJOG. 2022;129(4):580–9.
Townsend MK, Danforth KN, Rosner B, et al. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet Gynecol. 2007;110(2 Pt 1):346–53.
Pang H, Xu T, Li Z, et al. Remission and transition of female urinary incontinence and its subtypes and the impact of body mass index on this progression: a nationwide population-based 4-year longitudinal study in China. J Urol. 2022;208(2):360–8.
de Oliveira MC, Varella LR, Angelo PH, et al. The relationship between the presence of lower urinary tract symptoms and waist circumference. Diabetes Metab Syndr Obes. 2016;9:207–11.
Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(5):481–90.
Pang H, Yin Y, Xue J, et al. Associations between visceral adipose index and stress urinary incontinence among US adult women: a cross-sectional study. World J Urol. 2023;41(12):3671–8.
Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–15.
Marcelissen T, Anding R, Averbeck M, et al. Exploring the relation between obesity and urinary incontinence: Pathophysiology, clinical implications, and the effect of weight reduction, ICI-RS 2018. Neurourol Urodyn. 2019;38(Suppl 5):S18–24.
Wyndaele M, Charrua A, Herve F et al. Beyond the urothelium: Interplay between autonomic nervous system and bladder inflammation in urinary tract infection, bladder pain syndrome with interstitial cystitis and neurogenic lower urinary tract dysfunction in spinal cord injury-ICI-RS 2023. Neurourol Urodyn. 2023;43(6):1283–92.
Lizcano F, Guzman G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int. 2014;2014:757461.
de Vries AM, Venema PL, Heesakkers J. Midurethral support is also necessary for reflex closure of the urethra. Neurourol Urodyn. 2018;37(8):2965–72.
Tang F, Zhang J, Huang R, et al. The association between wet overactive bladder and consumption of tea, coffee, and caffeine: Results from 2005–2018 National Health and Nutrition Examination Survey. Clin Nutr. 2024;43(6):1261–9.
Hagovska M, Svihra J, Bukova A, et al. The impact of different intensities of exercise on body weight reduction and overactive bladder symptoms- randomised trial. Eur J Obstet Gynecol Reprod Biol. 2019;242:144–9.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
SY: Project development, Data collection, Data analysis, Manuscript writing. CJ: Project development, Data collection, Data analysis, Manuscript writing. TY: Manuscript editing, Result inspection.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NCHS Research Ethics Review Board approved the NHANES study protocol, and participants provided written informed consent at enrollment (https://www.cdc.gov/nchs/nhanes/irba98.htm). Ethical approval and consent were not required as the study was based on publicly available de-identified data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shang, Y., Chen, J. & Tai, Y. Association between weight-adjusted waist index and overactive bladder syndrome among adult women in the United States: a cross-sectional study. BMC Women's Health 24, 488 (2024). https://doi.org/10.1186/s12905-024-03339-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-03339-x