Abstract
Background
There is limited concrete evidence connecting serum uric acid levels to female infertility. Therefore, this study aimed to find out if serum uric acid levels are independently related to female infertility.
Methods
From the National Health and Nutrition Examination Survey (NHANES) 2013–2020, a total sample of 5872 chosen female participants between the ages of 18 and 49 were identified for this cross-sectional study. The serum uric acid levels (mg/dL) of each participant were tested, and the reproductive health questionnaire was used to evaluate each subject's reproductive status. Both in the analyses of the full sample and each subgroup, logistic regression models were used to evaluate the relationship between the two variables. A stratified multivariate logistic regression model was used to perform the subgroup analysis based on serum uric acid levels.
Results
Infertility was found in 649 (11.1%) of the 5,872 female adults in this study, with greater mean serum uric acid levels (4.7 mg/dL vs. 4.5 mg/dL). Serum uric acid levels were associated with infertility in both the initial and adjusted models. According to multivariate logistic regression, the odds of female infertility were found to be significantly higher with rising serum uric acid levels (Q4 [≥ 5.2 mg/dL] vs. Q1 [≤ 3.6 mg/dL]), adjusted odds ratio [aOR] = 1.59, p = 0.002]. The data suggests that there is a dose–response relationship between the two.
Conclusions
The results from this nationally representative sample from the United States confirmed the idea that there is a link between increased serum uric acid levels and female infertility. Future research is necessary to evaluate the relationship between serum uric acid levels and female infertility and explicate the underlying mechanisms of this relationship.
Similar content being viewed by others
Background
Infertility is defined as the inability to conceive after unprotected sexual activity or therapeutic donor insemination in women under the age of 35 or within six months in women over the age of 35. Infertility is estimated to affect 15% of all couples worldwide [1, 2]. The World Health Organization has classified infertility as a social disease, and the Centres for Disease Control and Prevention (CDC) in the United States have named infertility a public health priority [3]. Infertility is more than just a quality-of-life concern and has significant public health repercussions, such as psychological discomfort, social stigmatisation, economic pressure, and marital discord [4, 5].
Serum uric acid (SUA) is a major by-product of purine metabolism catalysed by xanthine oxidoreductase (XOR). XOR is a source of reactive oxygen species, which can lead to oxidative stress and endothelial dysfunction. When SUA becomes an oxidant, it contributes to the development of various pathological processes in the body that are ruled by oxidative stress [6]. SUA might behave as an antioxidant, which might have some protective effects, or as a pro-oxidant, which might accelerate a chain reaction of free radicals and cause oxidative damage to cells [7]. In addition to inducing oxidative stress, SUA has a role in the metabolism of lipids, glucose, and inflammation [8, 9]. Hyperuricemia (HUA) is a chronic metabolic condition, defined by unusually elevated SUA levels, which has been identified to have effects on multiple body organs through its numerous effects and contribute to the emergence of several disease states [10]. In the female reproductive system, hyperuricemia with SUA deposition may cause female sexual dysfunction [11]. According to research on buffalo ovaries, disruption of the plasma-follicular barrier structure is linked to higher levels of SUA [12]. Oocyte meiosis can be inhibited by hypoxanthine, which is a precursor to SUA [13]. SUA has potential mechanisms such as oxidative stress, promotion of inflammation, endothelial damage and thrombosis, and therefore, high levels of SUA may be correlated with incread clinical severity of polycystic ovarian syndrome (PCOS), endometriosis, or adverse pregnancy outcomes [14,15,16,17].
Our hominoid ancestors had gene alterations that led to the lack of uricase. As a consequence, humans must adapt to SUA levels that are comparatively greater [18]. In addition to genes, the risk of hyperuricemia is associated with ethnicity, age, lifestyle, and dietary factors [19]. Unhealthy living and eating habits in modern society lead to an increased incidence of hyperuricemia. High SUA levels are involved in the development of several diseases, including obesity, diabetes, metabolic syndrome, kidney disease, cardiovascular disease, and female reproductive disorders [10, 17, 20,21,22]. At the same time, unhealthy lifestyle and dietary factors will increase the prevalence of female infertility [23, 24]. Hence, we hypothesise that increased SUA levels may lead to decreased female fertility. Ultimately, this leads to an increased incidence of female infertility.
There are no studies that we are aware of that used a nationally representative sample to study the link between SUA levels and female infertility. In this cross-sectional study, we identified and examined correlations between SUA levels and female infertility using the latest nationally representative data from the National Health and Nutrition Examination Survey (NHANES) 2013–2020.
Methods
Data sources and study population
The National Centre for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC) collects data on nutritional status and health information for the NHANES which is a national population-based survey. All data for this study were provided in NHANES cycles 2013–2020. We used this data to describe the demographic characteristics of our population, obtain female self-reported infertility rates, and assess SUA levels in women 18 to 49 years old. This study was based on NHANES public data, and all information was collected from the official website [25]. The NHANES protocols are approved by the NCHS Research Ethics Review Board, and every respondent provided their signed informed consent [26].
The study enrolled women aged 18–49 years old who completed an interview using the reproductive health questionnaire and had a physical examination at the mobile examination centre (MEC). A multistage, stratified probability strategy was used to choose survey respondents [27]. Demographic and health history information was obtained through an extensive household interview. Physical assessments included the collection of blood samples at the MEC. Samples of serum were examined by the CDC Division of Laboratory Sciences. Analyses of the samples were performed in the United States.
Fertility assessment
Responses from the reproductive health questionnaire were used to calculate the dependent variable of infertility (variable name in the questionnaire: RHQ074). Those who answered affirmatively to the survey question, "Have you ever attempted to become pregnant for at least a year without becoming pregnant?" were presumed to have infertility [28].
Measurement and classification of SUA
The main independent variable was SUA measured in mg/dL. SUA levels were collected during subject enrolment in the NHANES using a colorimetric method in which uricase oxidises UA to allantoin and hydrogen peroxide (the Beckman Coulter UniCel DxC 800 Synchron chemistry analyzer between 2013 to 2014, the Beckman Coulter UniCel DxC 800 Synchron and the Beckman Coulter UniCel DxC 660i Synchron since 2015). Quality-control procedures' specifics have been disclosed elsewhere [29]. Values are reported in mg/dL and can be converted to μmol/L by multiplying by 59.48.
Covariates
In the NHANES database, factors were classified as demographics or possible confounders that could influence SUA or fertility status [30, 31]. We considered demographic characteristics (age, sex, race or ethnicity, education, marital status, and ratio of family income to poverty); lifestyles (drinking and smoking); health insurance coverage; physical examinations; and laboratory tests (serum lipids, creatinine (Cr), blood urea nitrogen(BUN), and estimated glomerular filtration rate (eGFR)). In addition, we considered body mass index (BMI) and waist circumference (WC). Disease history was also taken into account and included the following diagnoses: hypertension [32] (characterised as being on anti-hypertensive medication and having a systolic blood pressure ≤ 140 mmHg or a diastolic blood pressure ≤ 90 mmHg); diabetes mellitus [32] (obtained through self-report and using diabetes medications); and metabolic syndrome (MetS). BMI was coded into three categories [33]: (underweight or normal weight (< 25 kg/m2), overweight (25–29.9 kg/m2), and obese (> 30 kg/m2). MetS was diagnosed in respondents when at least three of the following five symptoms were present [34]: WC ≥ 88 cm, triglycerides (TG) ≥ 150 mg/dL, high-density lipoprotein (HDL) < 50 mg/dL, systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg (averaged over three readings), or fasting plasma glucose (FPG) ≥ 100 mg/dL. CKD-EPI Creatinine Eq. (2021): [35].
Statistical analysis
Means and standard errors (SE) were used for continuous variables, as well as percentages and standard errors for categorical variables. The t-test (normal distribution) and Kruskal–Wallis test (skewed distribution) were used to assess continuous variables. The stratified multivariate logistic regression model was used to perform the subgroup analysis by SUA levels. The relationship between SUA levels and infertility was investigated by using SUA data as a continuous variable and in quartiles. The odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated. The following stratified multivariate logistic regression models were used to assess the effect of SUA levels on female infertility: Model 1: no adjustment; Model 2: adjusted for social demographic covariables (age, race/ethnicity, education, PIR) and health insurance coverage; Model 3: adjusted for the variables in Model 2 plus BUN, Cr, and eGFR; Model 4: adjusted for the variables in Model 3 plus BMI, DM, hypertension, and MetS.
All statistical analyses were carried out using the software tools R, version 4.1.1 (http://www.R-project.org, The R Foundation), and Free Statistics, version 1.5. In all tests, a statistically significant difference was defined as P < 0.05 (two-sided).
Results
Four cycles of NHANES (2013–2014, 2015–2016, 2017–2018, and 2017–2020) were used in this study. There were 44,960 eligible participants, and of these, 22,673 adult females completed the interview and 15,689 participants completed the reproductive health questionnaire. Participants with missing data in SUA or answering the fertility information for RHQ074 variables (n = 9,817) were excluded. Our analyses included the remaining 5,872 participants aged 18–49. Figure 1 shows the flowchart of the exclusion criteria.
Table 1 presents the descriptive characteristics of the study population according to their fertility status. Infertility was projected to affect 11.1% of women between the ages of 18 and 49. Infertile women were older (36.2 years vs. 32.9 years), their SUA mean was more significant (4.7 mg/dL vs. 4.5 mg/dL), they had higher PIR levels (2.6 vs. 2.3), and they had lower eGFR levels (108.5 mL/min/1.73 m2 vs. 111.0 mL/min/1.73 m2) than women with non-infertility. Female participants with infertility were more likely to have a regular partner (14.8% vs. 8.1%), have been pregnant at least once (13.3% vs. 7.4%), have obesity (14.3% vs. 8.6%), have hyperuricemia (13.8% vs. 10.7%), diabetes mellitus (8.8% vs. 4.6%), MetS (12.3% vs. 9.2%), and have hypertension (21.5% vs. 15.5%). There were no statistical differences in ethnicity, education, health insurance coverage, BUN, or Cr.
Table 2 shows the outcomes of unweighted multivariable logistic regression studies examining the association between SUA levels and the likelihood of infertility. In the initial model, SUA levels were positively associated with female infertility (OR = 1.19; 95% CI: 1.11–1.288). In adjusted models, the connection between SUA levels and the risk of female infertility in women was still positive (Model 2: OR = 1.19, 95% CI: 1.1–1.28; Model 3: OR = 1.22, 95% Cl 1.13–1.32; Model 4: OR = 1.16, 95% Cl 1.06–1.27). Female infertility was 76% more likely for women with SUA in the highest quartile [OR = 1.76, P < 0.001], compared to 59% more likely in Model 4 [aOR = 1.59, P = 0.002].
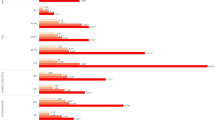
In Fig. 2, the outcomes of the subgroup analysis are displayed. Participants aged 18 to 35 (OR = 1.27, 95% CI: 1.01–1.61), married or cohabiting (OR = 1.18, 95% CI: 1.01–1.38), below the poverty line (OR = 1.23, 95% CI: 1–1.51), and those without obesity (OR = 1.37, 95% CI: 1.12–1.68) showed the connection between infertility and SUA levels. Participants between the ages of 35 and 49, those who were single, lived above the poverty line, had never given birth, or belonged to the obesity categories, did not show any correlation.
Discussion
In this nationally representative cross-sectional study, infertility was prevalent among women between the ages of 18 and 49 at an estimated 11.1%, which was within the range of the reported national prevalence (6.7%–15.5%) [36, 37]. There is a positive correlation between SUA and infertility among US female adults. In sensitivity studies, the magnitude and direction of this connection remained constant. The strength of the dose-dependent relationship between the SUA quartiles and infertility increased. The largest connection between infertility and SUA was seen among participants in the highest quartile (Q4). One of the interesting findings in this research is that the strength of the connection between SUA values and infertility grew in a dose-dependent manner. This was more notable in secondary infertility than primary infertility.
To the best of our knowledge, this is the first investigation into the relationship between SUA and infertility among American women of reproductive age who took part in the NHANES between 2013 and 2020. Studies on the connection between SUA and female infertility status are sparse and inconsistent. High SUA levels are associated with MetS, diabetes mellitus, cardiovascular disease, kidney disease, and female reproductive disorders [21, 22, 31, 38].
Previous studies have linked decreased fertility to increasing age, obesity, diabetes, MetS, hypertension, and gout [39,40,41,42]. Because SUA levels and female fertility are both associated with a variety of disease states, we were curious to see if there was a link between the two. Subfertility and infertility are terms that can be used interchangeably, and infertility is a disease that causes disability by impairing function [43]. Numerous plausible processes may underlie the link between SUA and infertility, according to earlier investigations. Previous studies have demonstrated that the antioxidant effect of physiological levels of SUA serves as an in vivo protective function [7]. When antioxidants like ascorbic acid are scarce, SUA can become an oxidant and participate in a variety of pathological processes caused by oxidative stress [6]. An imbalance between pro-oxidants and antioxidants can contribute to female reproductive difficulties like endometriosis, PCOS, and unexplained infertility [44]. In the reproductive system, excessive SUA levels are also linked to PCOS, endometriosis, pregnancy difficulties, adverse fatal outcomes, and other diseases [14, 16, 17, 22, 45]. As a result, we believe there is a link between SUA levels and female infertility.
Multiple body organs and systems can become damaged by an excessive buildup of SUA [38]. There is substantial evidence that uric acid levels can directly cause inflammation and abnormal lipid metabolism [8, 9]. Inflammatory pathways and hormonal aberrations are shared by reproductive illnesses (adenomyosis, endometriosis, uterine fibroids, and PCOS) and unexplained infertility and may reduce pregnancy success through shared processes [46]. Such processes could also contribute to the decreased fecundity of patients with endometriosis or POCS [47, 48]. Some infertility reasons have been connected to ovarian inflammation. According to one study, SUA levels have an impact on the quality of semen and male infertility [49]. It seems biologically plausible to have excessive SUA levels as a risk factor for excessive SUA levels and more studies are needed to confirm our findings and investigate the underlying mechanisms.
It has been proposed that dietary treatments provide a secure, economical means of controlling hyperuricemia. The Dietary Approaches to Stop Hypertension (DASH) diet and the Mediterranean diet, are well-known to help obtain optimal SUA levels, have differing SUA-lowering effects, and have been mentioned in previous reports on dietary styles restricting the consumption of fats and meats [50, 51]. Insulin resistance impairs glycolysis and the kidneys' ability to remove SUA, causing the increased synthesis of SUA and decreased urine SUA clearance [52]. According to a review, women had improved fertility when they followed healthy diets that prioritised fish, poultry, whole grains, fruits, and vegetables [24]. Excluding drug control, therapeutic lifestyle changes, appropriate weight loss, and adequate physical activity are beneficial to overall health while also improving hyperuricemia and infertility [53, 54]. For women who would like to become pregnant, paying attention to their nutrition and lifestyle choices, as well as their SUA levels, will help improve fertility.
This study had several strengths and limitations. The use of NHANES data is advantageous because it offers a nationally representative data set, extensive and detailed information on nutritional, demographic, and lifestyle factors, objective cognitive performance tests, and biological samples to control for known major confounders. The current study contains several drawbacks. First, because this research was cross-sectional, we were unable to draw any conclusions about the cause of the link between SUA and infertility in women of reproductive age. Second, women might not be able to recall exactly how long they attempted to get pregnant because infertility was measured through self-reporting. Thirdly, we could have missed infertile women who haven't attempted to become pregnant yet. Lastly, the study did not include data on concomitant gynaecological diseases (such as endometriosis, PCOS, fibroids, polyps, etc.), and we did not treat serum lipids as a covariate [55]. However, we did explore the potential influence of obesity and MetS on the association between SUA and infertility.
Conclusions
According to the results of this cross-sectional investigation, SUA is positively correlated with infertility in the US adult female population. This discovery assists clinicians in consciously controlling SUA levels to decrease the rate of infertility.
Availability of data and materials
The datasets generated or analysed during the current study are included in this published article. Any further inquiries can be directed to the corresponding author.
Abbreviations
- AOR:
-
Adjusted odds ratio.
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- CDC:
-
The centrescenters for disease control and prevention
- CI:
-
Confidence intervals
- Cr:
-
Creatinine
- DBP:
-
Diastolic blood pressure
- EGFR:
-
Estimated glomerular filtration rate
- FPG:
-
Fasting plasma glucose
- HDL:
-
High-density lipoprotein
- HUA:
-
Hyperuricemia
- MEC:
-
The mobile examination centrecenter
- MetS:
-
Metabolic syndrome
- NCHS:
-
National centrecenter for health statistics
- NHANES:
-
National health and nutrition examination survey
- OR:
-
Odds ratio
- PCOS:
-
Polycystic ovary syndrome
- PIR:
-
Ratio of family income to poverty
- SBP:
-
Systolic blood pressure
- SE:
-
Standard errors
- SUA:
-
Serum uric acid
- TG:
-
Triglycerides
- TG:
-
Triglycerides
- UA:
-
Uric acid
- WC:
-
Waist circumference
- XOR:
-
Xanthine oxidoreductase
References
Diagnostic evaluation of the infertile female. A committee opinion. Fertil Steril. 2015;103(6):e44-50.
Infertility Workup for the Women’s Health Specialist. ACOG committee opinion, number 781. Obstet Gynecol. 2019;133(6):e377–84.
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–4.
Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril. 2010;93(1):16.e1.
Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: results from a global burden of disease study, 2017. Aging (Albany NY). 2019;11(23):10952.
Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145–51.
Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):608–19.
Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep. 2011;13(2):160–6.
Lima WG, Martins-Santos ME, Chaves VE. Uric acid as a modulator of glucose and lipid metabolism. Biochimie. 2015;116:17–23.
Joo HJ, Kim GR, Choi DW, Joo JH, Park EC. Uric acid level and kidney function: a cross-sectional study of the Korean national health and nutrition examination survey (2016–2017). Sci Rep. 2020;10(1):21672.
Sansone A, Reisman Y, Jannini EA. Relationship between hyperuricemia with deposition and sexual dysfunction in males and females. J Endocrinol Invest. 2022;45(4):691–703.
Cassano E, Tosto L, Balestrieri M, Zicarelli L, Abrescia P. Antioxidant defense in the follicular fluid of water buffalo. Cell Physiol Biochem. 1999;9(2):106–16.
Wen X, Perrett D, Jones N, Tozer AJ, Docherty SM, Iles RK. High follicular fluid adenosine levels may be pivotal in the metabolism and recycling of adenosine nucleotides in the human follicle. Metabolism. 2010;59(8):1145–55.
Mu L, Pan J, Yang L, Chen Q, Chen Y, Teng Y, et al. Association between the prevalence of hyperuricemia and reproductive hormones in polycystic ovary syndrome. Reprod Biol Endocrinol. 2018;16(1):104.
Li J, Guan L, Zhang H, Gao Y, Sun J, Gong X, et al. Endometrium metabolomic profiling reveals potential biomarkers for diagnosis of endometriosis at minimal-mild stages. Reprod Biol Endocrinol. 2018;16(1):42.
Bellos I, Pergialiotis V, Loutradis D, Daskalakis G. The prognostic role of serum uric acid levels in preeclampsia: a meta-analysis. J Clinic Hyper. 2020;22(5):826–34.
Laughon SK, Catov J, Provins T, Roberts JM, Gandley RE. Elevated first-trimester uric acid concentrations are associated with the development of gestational diabetes. Am J Obstet Gynecol. 2009;201(4):402.e1-5.
Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25(1):3–8.
Petrikova J, Janicko M, Fedacko J, Drazilova S, Madarasova Geckova A, Marekova M, et al. Serum uric acid in roma and non-roma-its correlation with metabolic syndrome and other variables. Int J Environ Res Public Health. 2018;15(7):1412.
El Din UA, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: a review. J Adv Res. 2017;8(5):537–48.
Cortese F, Scicchitano P, Cortese AM, Meliota G, Andriani A, Truncellito L, et al. Uric acid in metabolic and cerebrovascular disorders: a review. Curr Vasc Pharmacol. 2020;18(6):610–8.
Hu J, Xu W, Yang H, Mu L. Uric acid participating in female reproductive disorders: a review. Reprod Biol Endocrinol. 2021;19(1):65.
Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, et al. Female subfertility. Nat Rev Dis Primers. 2019;5(1):7.
Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218(4):379–89.
National health and nutrition examination survey (NHANES). laboratory data protocol. U.S. In: department of health and human services, centers for disease control and prevention. Available at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2007. Accessed 20 Jun 2022.
Centers for disease, control, and prevention. National Health and Nutrition Examination Survey. Survey methods and analytic guidelines. Availabe online: https://www.cdc.gov/nchs/nhanes/index.htm (Accessed on June 28th, 2022).
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations 1999–2010. Vital Health Stat. 2013;56:1–37.
Dick ML, Bain CJ, Purdie DM, Siskind V, Molloy D, Green AC. Self-reported difficulty in conceiving as a measure of infertility. Hum Reprod. 2003;18(12):2711–7.
Centers for disease control and prevention. National Health and nutrition examination survey (NHANES) MEC laboratory procedures manual [Internet]. 2016. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_MEC_Laboratory_Procedures_Manual.pdf.
Definitions of infertility and recurrent pregnancy loss. A committee opinion. Fertil Steril. 2020;113(3):533–5.
Gaubert M, Bardin T, Cohen-Solal A, Diévart F, Fauvel JP, Guieu R, et al. Hyperuricemia and hypertension, coronary artery disease, kidney disease: from concept to practice. Int J Mol Sci. 2020;21(11):4066.
Azeez O, Kulkarni A, Kuklina EV, Kim SY, Cox S. Hypertension and diabetes in non-pregnant women of reproductive age in the United States. Prev Chronic Dis. 2019;24(16):E146.
Obesity N. The practical guide identification , evaluation , and treatment of overweight and obesity in adults.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–52.
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–49.
Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324-31.e1.
Key statistics from the national survey of family growth. Available from:. Accessed Feb 4, 2019.; Available from: https://www.cdc.gov/nchs/nsfg/key_statistics/i.htm#infertility. .
Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;7(357): j2376.
Thong EP, Codner E, Laven JSE, Teede H. Diabetes: a metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 2020;8(2):134–49.
Lotti F, Marchiani S, Corona G, Maggi M. Metabolic syndrome and reproduction. Int J Mol Sci. 2021;22(4):1988.
Vollenhoven B, Hunt S. Ovarian ageing and the impact on female fertility. F1000Res. 2018;7:F1000 Faculty Rev-1835. https://doi.org/10.12688/f1000research.16509.1.
Yü TF. Some unusual features of gouty arthritis in females. Semin Arthritis Rheum. 1977;6(3):247–55.
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406.
Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;14(3):28.
Weissgerber TL, Milic NM, Turner ST, Asad RA, Mosley TH Jr, Kardia SL, et al. Uric acid: a missing link between hypertensive pregnancy disorders and future cardiovascular disease? Mayo Clin Proc. 2015;90(9):1207–16.
Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22(1):104–15.
Sensky TE, Liu DT. Endometriosis: associations with menorrhagia, infertility and oral contraceptives. Int J Gynaecol Obstet. 1980;17(6):573–6.
Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-a systematic review, meta-analysis, and meta-regression. Obes Rev. 2019;20(5):659–74.
Ma J, Han R, Cui T, Yang C, Wang S. Effects of high serum uric acid levels on oxidative stress levels and semen parameters in male infertile patients. Medicine (Baltimore). 2022;101(3):e28442.
Chatzipavlou M, Magiorkinis G, Koutsogeorgopoulou L, Kassimos D. Mediterranean diet intervention for patients with hyperuricemia: a pilot study. Rheumatol Int. 2014;34(6):759–62.
Gao Y, Cui Lf, Sun Yy, Yang Wh, Wang Jr, Wu Sl, et al. Adherence to the dietary approaches to stop hypertension diet and hyperuricemia: a cross‐sectional study. Arthritis Care & Res 2021;73(4):603–11.
Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–11.
Chalès G. How should we manage asymptomatic hyperuricemia? Joint Bone Spine. 2019;86(4):437–43.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
Peng TC, Wang CC, Kao TW, Chan JY, Yang YH, Chang YW, et al. Relationship between hyperuricemia and lipid profiles in US adults. Biomed Res Int. 2015;2015: 127596.
Acknowledgements
We sincerely appreciate Dr. Jie Liu's help and support with the study design, statistical support, and manuscript responses from the Department of Vascular and Endovascular Surgery at the Chinese PLA General Hospital.
Funding
The authors received no funding for this work.
Author information
Authors and Affiliations
Contributions
All the authors made a significant contribution to this work. KD designed the study and critically revised the manuscript for important intellectual content. JL classified and analysed the data and drafted the manuscript. XC analysed the data. JH, WN, QY, and QH collected data. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Each participant gave written informed consent, and the survey protocol was approved by the National Centre for Health Statistics (NCHS) Ethics Review Board. All the methods carried out in the study were in accordance with relevant regulations & guidelines.
Consent for publication
Not applicable.
Competing interests
The researchers state to have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liang, J., Chen, X., Huang, J. et al. Implications of serum uric acid for female infertility: results from the national health and nutrition examination survey, 2013–2020. BMC Women's Health 23, 103 (2023). https://doi.org/10.1186/s12905-023-02234-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02234-1