Abstract
Objective
To explore the efficacy of progestin-primed ovarian stimulation (PPOS) combined with clomiphene citrate (CC) versus PPOS protocol used alone on cycle characteristics and pregnancy outcomes for women with the poor ovarian response (POR).
Methods
We performed a retrospective cohort study and a total of 578 POR patients who underwent IVF/ICSI cycles were collected and divided into Group A (HMG 300 IU/d + MPA 10 mg/d) and Group B (HMG 300 IU/d + MPA 10 mg/d + CC 50 mg/d). The primary outcome measure was the number of oocytes retrieved, other outcome measures were cycle characteristics and clinical pregnancy rate.
Results
The baseline information between the two groups were not statistically significant (P > 0.05). Compared with Group A, Group B had a lower total dose of human menopausal gonadotrophin (HMG) (2998.63 ± 1051.09 vs. 3399.18 ± 820.75, P < 0.001) and the duration of stimulation (10.21 ± 3.56 vs. 11.27 ± 2.56, P < 0.001). Serum luteinizing hormone level was higher in Group B on human chorionic gonadotrophin injection day (P < 0.001). The number of oocyte for retrieval, maturation, and fertilization were significantly lower in Group B than that in Group A (P < 0.001). However, the oocyte retrieval rate, maturation rate, fertilization rate, and viable embryo rate showed no statistical difference in the two groups (P > 0.05). After adjusting for confounders, the clinical pregnancy rate (OR 1.286; 95% CI 0.671–2.470) and live birth rate (OR 1.390; 95% CI 0.478–3.990) were comparable between the two groups.
Conclusions
PPOS protocol combined with CC reduces the total dose of HMG and the duration of stimulation, and can also achieve similar oocyte yields and clinical pregnancy rate compared with the PPOS protocol used alone in poor ovarian responders.
Similar content being viewed by others
Introduction
Poor ovarian response (POR) indicates that the diminished ovarian reserve or poor response to ovarian stimulation, remains a challenge for both clinicians and patients in IVF/ICSI, which occurs in 5.6–35.1% of ovarian induction cycles [1]. These patients often face the problems of premature luteinizing hormone (LH) surge, lower oocyte yields, and higher cycle cancellation rate which caused emotional, physical, and financial burdens for infertile couples [2]. Due to the lack of consensus on the definition of POR in some previous clinical trials, the Bologna criteria was proposed by ESHRE and has been widely accepted for the definition of POR since 2011 [3]. To further improve the clinical diagnostic accuracy and management of POR patients, the POSEIDON criteria were established in 2016. According to this criteria, poor responders were divided into 4 groups based on age, ovarian response markers [antral follicle count (AFC) and anti-Müllerian hormone (AMH)], and response to induction [4].
Many studies are being devoted to finding a suitable and effective treatment to improve oocyte yields and clinical outcomes in women with POR. However, regardless of the mild or conventional ovarian stimulation strategies chosen, or co-treatment with clomiphene citrate (CC) or letrozole were found to be non-significant on oocyte yields and pregnancy outcomes, therefore, the most appropriate management of POR patients is still controversial, which needs further studies [1, 5]. Later, progestin-primed ovarian stimulation (PPOS) protocol was revealed to be effective and safe for suppressing premature LH surge and also showed similar oocyte yields and pregnancy outcomes compared with conventional short protocol [6, 7], which has been applied in normally ovulating women, poor responders, and polycystic ovarian syndrome (PCOS) [6, 8,9,10].
CC as the first drug to be used for the development of multiple follicles in ovarian stimulation regimens [11], is a selective estrogen receptor modulator, interfering with the negative feedback of endogenous estrogen on the hypothalamus-pituitary axis, and resulting in higher circulating concentrations of gonadotropin-releasing hormone (GnRH) [12]. PPOS protocol combined with CC was previously used in women with normal ovulatory and PCOS and reported that the addition of CC was beneficial in reducing human menopausal gonadotrophin (HMG) consumption and the duration of stimulation, alleviating the profound pituitary suppression caused by progesterone (P) to some extent, whereas did not conclusively improve oocyte yields and pregnancy outcomes [13, 14]. Data on the efficacy of CC in the PPOS protocol for POR patients are limited. Therefore, we conducted a retrospective cohort study in POR women to investigate the effectiveness of CC supplementation in the PPOS protocol during ovarian induction.
Methods
Data sources and patient selection
Data from this retrospective study were collected in women who underwent in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) during the period January 2019 to June 2021 in our reproductive medicine center of the First Hospital of Lanzhou University. The study was approved by the First Hospital of Lanzhou University review boards. The inclusion criteria were that POR patients met the criteria of POSEIDON group 3 (Age < 35 years, AFC < 5 and AMH < 1.2 ng/ml) and group 4 (Age ≥ 35 years, AFC < 5 and AMH < 1.2 ng/ml), and the starting dose of HMG was limited to 300 IU. The exclusion criteria were patients with a history of pelvic tuberculosis, chromosome abnormality, severe endometriosis (meet the revised American Fertility Society (r-AFS) classification [15] III-IV stage during laparoscopic surgery), intrauterine adhesion, and other endocrine and metabolic diseases (poorly controlled diabetes or thyroid dysfunction). All patient details regarding diagnosis and treatment were available from our computer system. Finally, eligible patients were divided into two groups with or without CC supplementation during ovulation induction: 304 women in Group A (HMG 300 IU + MPA 10 mg), and 274 women in Group B (HMG 300 IU + MPA 10 mg + CC 50 mg).
Ovarian stimulation procedures
All patients were scheduled to evaluate baseline hormones and do a transvaginal ultrasound on the third day of the menstrual cycle to determine ovarian stimulation protocols. All the patients received HMG (Lizhu Pharmaceutical Trading Co., China) 300 IU daily and Medroxyprogesterone acetate (MPA) (Zhejiang Xianju Pharmaceutical Co., Ltd.) 10 mg/d, starting from the third day of menstrual bleeding. Daily administration of CC (Fertilan; Codal-Synto Ltd., France) 50 mg was added in Group B and started from cycle day 3 onward. Transvaginal ultrasound was first performed on cycle days 7–8 to monitor the follicular growth, and serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), progestin (P) were assayed for the same day. These checks were scheduled every two days, and when one or more dominant follicles reached a diameter of > 18 mm, trigger medicine 0.1 mg triptorelin (Decapeptyl, Ferring GmbH, Germany) and 2000 or 4000 IU human chorionic gonadotrophin (HCG) (Lizhu Pharmaceutical Trading Co., China) were administered. Oocyte aspiration was performed by transvaginal ultrasound guidance 34–36 h later.
Oocytes were fertilized in vitro by IVF or ICSI, depending on semen parameters on the day of oocyte aspiration. Embryos were evaluated on day 3 by the number and size of blastomeres and the degree of embryonic fragmentation [16], grade I and grade II embryos were considered as high-quality embryos that were cryopreserved by vitrification for frozen-thawed cycles. The spare non-high-quality embryos were further cultured until the blastocyst-stage was reached. At this stage, on days 5 or 6, only blastocysts with good morphology were frozen.
Frozen embryo transfer (FET) cycle preparation
The embryos transferred in this FET cycle all came from the same ovulation induction cycle. There were 109 FET cycles in Group A and 88 FET cycles in Group B. Hormone replacement treatment was used in the present study for endometrial preparation. Oral estradiol valerate tablets (Bayer Healthcare Co., Ltd.) 2 mg were given three times a day starting from cycle day 3 for 10–14 days. Once endometrial thickness reached > 8 mm, oral dydrogesterone tablets (Abbott Biologicals B.V.) 30 mg/d and vaginal progesterone soft capsule (Cyndea Pharma, S.L.) 600 mg/d were started. The embryos were transferred on day 3 (cleavage stage) and day 5/6 (blastocyst stage). If clinical pregnancy was achieved, the progesterone supplementation was continued until 10 weeks of gestation.
Hormone measurement
All hormone levels were determined by the chemiluminescence technique (Abbott Biologicals B.V.). Lower limits of sensitivity for detection were defined as follows: FSH = 0.06 mIU/ml, LH = 0.09 mIU/ml, E2 = 10 pg/ml, and P = 0.1 ng/ml. The upper limit for serum E2 detection was 5000 pg/ml. If the E2 concentration was higher than the upper limit on the trigger day or the day after, the E2 level was recorded as 5000 pg/ml.
Statistical analysis
The primary outcome measure was the number of oocytes retrieved. Other outcome measures were cycle characteristics and clinical pregnancy rate. Clinical pregnancy was defined as the detection of an intrauterine gestational sac with a positive heartbeat on transvaginal ultrasound after 30 days of embryo transfer (ET). The miscarriage rate was defined as the proportion of patients with spontaneous loss of pregnancy before viability during early- and mid-pregnancy. Biochemical pregnancy was defined as a positive HCG test without visualization of an intrauterine gestational sac. Ongoing pregnancy was defined as a pregnancy beyond 12 weeks gestation. The gestational week was calculated according to the date of embryos were transferred.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 23.0) for Windows. Descriptive statistics of continuous variables of normal distribution were presented as mean ± standard and Student t test was used to compare two independent groups. The Mann–Whitney U-test for continuous variables of non-normal distribution. Categorical variables were expressed as numbers with percentages and compared using the χ2 test or Fishers exact test. P < 0.05 was considered statistically significant. Logistic regression analysis was performed to estimate the odds ratio (OR) and 95% confidence interval (CI) of pregnancy outcomes between the two groups. Accordingly, logistic regression analysis was also used to adjust the confounders, including age, AMH, BMI, AFC, E2 level on the day of HCG, retrieved oocytes, high-quality embryos, number of transferred embryos, and endometrial thickness.
Results
Patient characteristics
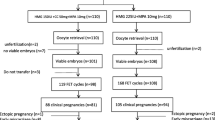
Between January 2019 and June 2021 at our center, 4322 cycles received the PPOS protocol for ovarian stimulation. Of these cycles, 20.8% (901/4332) fulfilled the POSEIDON group 3 and group 4 criteria for POR, and 578 patients of these were eventually eligible for inclusion. The flowchart of the study selection is shown in Fig. 1.
The data on baseline characteristics are summarized in Table 1. In this study, 65.1% (376/578) of the POR patients met the POSEIDON group 4 criterion, and the baseline information between the two groups was not statistically significant (P > 0.05). The average age was 37.25 ± 5.10 years old in Group A and 36.93 ± 6.21 years old in Group B.
Ovarian stimulation and embryo performance
The clinical and cycle characteristics of the two ovarian stimulation groups are presented in Table 2. The baseline serum hormone levels were comparable between the two groups. The serum E2 level in Group B on the day of HCG was lower than that in Group A (P < 0.001), but the level of LH and P were significantly higher in Group B (P < 0.001). The total dose of HMG and duration of stimulation in Group B were significantly lower than those in Group A (P < 0.001). The number of oocyte for retrieval, maturation, and fertilization were significantly lower in Group B than that in Group A (P < 0.001). However, the oocyte retrieval rate, maturation rate, fertilization rate, and viable embryo rate showed no statistical difference in the two groups (P > 0.05).
Pregnancy outcomes after FET cycles
FET pregnancy outcomes in the two groups are presented in Table 3. Due to the poor ovarian reserve and a low oocyte retrieval rate in POR patients, which means available embryos require gradually accumulate, led the embryos transferred in the FET cycle may come from different ovulation cycles, and this condition was not eligible for inclusion in our study. Finally, two and a half years of follow-up was completed for only 197 FET cycles in the present study (109 in Group A and 88 in Group B). The mean number of transferred embryos and endometrial thickness were comparable between the two groups. Comparison of Group A and Group B revealed a similar rate of clinical pregnancy (37.61% vs. 32.95%, respectively; OR 1.230; 95% CI 0.680–2.211; adjusted OR 1.286; 95% CI 0.671–2.470), an identical rate of live birth (41.46% vs. 34.48, respectively; OR 1.353; 95% CI 0.496–3.611; adjusted OR 1.390; 95% CI 0.478–3.990). Similarly, the miscarriage rate and biochemical pregnancy rate were not significantly different between the two groups. The mean gestational age and birth weights were 37.9 ± 1.5 weeks and 3014.4 ± 357.3 g in Group A, and 37.9 ± 2.3 weeks and 2895.8 ± 393.4 g in Group B, respectively. For pregnancy outcomes in FET cycles, data were available only until June 2021. Thus, the ongoing pregnancy rate was 17.07% in Group A and 17.24% in Group B.
Discussion
The present study here aimed to evaluate the efficacy of CC supplementation in PPOS protocol for patients with POR. In our study, all included patients used the PPOS protocol, and such a choice was suitable for poor responders [17]. Our current results showed that the PPOS protocol combined with CC reduced the total dose of HMG and the duration of stimulation, which was related to the negative feedback mechanism of CC. The synergistic action by CC and HMG during the early follicular phase, made CC supplementation have significant advantages in terms of shorter duration and lower HMG consumption, and these findings were in agreement with other studies. A prospective study published in 2015 made a comparison of HMG with or without CC supplementation in ovarian stimulation for PCOS patients [18] and found that CC significantly reduced both the HMG dose and the duration of treatment. Similar results have been previously reported in another study for 320 normal ovulatory women that were divided into the HMG + MPA + CC group and the HMG + MPA group [13]. A recent meta-analysis of adjuvant treatment with CC in POR has reached the same conclusion [1]. Moreover, it must be emphasized that our study population is relevant for women with POR, who need repeating ovarian stimulation and oocyte retrieval to accumulate embryos, therefore, this advantage of CC mentioned above could relieve patients from the pain of daily injection and financial burden to some extent.
Moreover, although the AFC is similar between the two groups in baseline characteristics, the number of ovarian follicles > 14 mm on the day of HCG, oocyte retrieved, matured oocyte, and fertilized oocyte were lower in the group treated with CC. Indeed, similar results have been reported earlier, a retrospective study found that adding CC to the PPOS protocol in PCOS women showed a lower number of the retrieved and fertilized oocytes [10], they hypothesized that exogenous HMG promoted more development of medium-sized follicles, however, CC plays a role mainly by leading endogenous gonadotropins secretion in the early stage of ovarian stimulation, and this effect of CC may not be as direct as HMG, thus the use of less HMG in the CC group results in less medium-sized follicles. Their study also indicated that the number of follicles (diameters > 14 mm) in the CC group was lower while having a greater number of small-sized follicles (10–14 mm), which could also explain their hypotheses above. Ye H et al. in 2018 made a comparison of CC co-treatment with milder PPOS protocol (HMG 150 IU/d + CC 50 mg/d + MPA 10 mg/d) and PPOS (HMG 225 IU/d + MPA 10 mg/d) alone in PCOS patients and reached a result that CC led to lower oocyte yields, they interpret the results as the characteristics of milder stimulation [14]. In contrast to these studies, a prospective cohort trial included 12 POR patients that met the Bologna criteria and underwent 27 stimulation cycles of GnRH antagonist protocol and indicated that the addition of CC increased the number of oocytes retrieved and available embryos [19]. In our study, although the number of the oocyte for retrieval, maturation, and fertilization were significantly lower in Group B than that in Group A, the oocyte retrieval rate, maturation rate, fertilization rate, and viable embryos rate showed no statistical difference in the two groups.
Notably, our findings revealed that the LH level on the day of HCG was the highest in the CC supplementation group. Similarly, Jiang et al. in 2017 found that obese PCOS patients who were treated with CC had a higher LH level on HCG day [10], and the reason for this can be explained as there were some gene mutations of the LH and LH receptors in women with PCOS that made them LH-dependent during follicle development [20]. Same results have been reported in another research for PCOS patients who used the PPOS protocol with CC [14]. It is worth mentioning that previous studies have indicated that CC acts directly on the hypothalamus to increase the pulse frequency of GnRH release with normally ovulating women [21] while enhancing the amplitude of GnRH release in PCOS women [22]. It is generally known that LH plays a major role in the development and maturation of follicles [23], levels of LH that are too low or too high are associated with an increased risk of adverse clinical outcomes [24, 25]. Some studies have found that the high LH levels might be correlated with adverse ovarian stimulation outcomes such as lower rate of oocytes maturation and fertilization, impaired embryos quality [26, 27]. Shoham et al. proposed a window for LH in ovarian stimulation and believed that higher LH levels contribute to impaired folliculogenesis [28]. These may explain our results that the decreased oocytes maturation rate and fertilization rate in Group B treated with CC had a nearly three-fold higher LH level than that in Group A. On the other hand, although the LH level increased in the group added with CC, no premature LH surge occurred in the groups, and the FET pregnancy outcomes were similar between the two groups which revealed the embryos from the groups had the same developing potential.
However, in our study, the MPA was used in the PPOS protocol which can suppress pituitary LH levels. Several investigators have demonstrated the negative effects of low endogenous LH concentrations on follicle development [29,30,31]. Westergaard et al. showed that a low LH level (< 0.5 IU/l) on day 8 of the ovarian stimulation had a negative impact on the pregnancy outcome, and led to a significantly higher incidence of abortion in the early phase [29]. Esposito et al. also demonstrated that low levels of LH (< 3 mIU/ml) in the late phase of oocyte development may significantly cause low fertilization rates [30]. Liu et al. also reported the same phenomena for women with normal ovarian reserve applying the PPOS protocol with CC and indicated that the MPA’s application may result in stronger LH suppression, the addition of CC can alleviate this suppression without affecting the effect of MPA [13]. In other words, it may reach a balance between CC and MPA in the collective effect action to the change in LH trend during ovulation induction. Additionally, our studies did not show a detrimental effect on pregnancy outcomes that adding CC to the PPOS protocol, similar results have also occurred in women with normal ovarian reserve or PCOS who used the same stimulation regimens [13, 14]. Furthermore, a meta-analysis in 2016 by Song et al. included four randomized controlled trials and indicated that the mild stimulation protocol with CC in poor responders had the same pregnancy outcomes compared with the conventional GnRH agonist protocol [5]. Nevertheless, the findings published by Siristatidis et al. and Pilehvari et al. reported a negative effect of CC on pregnancy outcomes in POR patients. Both of the researchers compared the addition of CC to a mild stimulation protocol with conventional long-agonist or antagonist protocols [2, 32].
The limitation of this paper is the inclusion of POR patients who met only the group 3 and group 4 criteria for POSEIDON, which caused it cannot be comprehensively assessed the treatment efficacy of CC supplementation in PPOS protocol in all POR patients. Another potential limitation is that it is a retrospective study and because our study subjects were POR patients who required multiple ovulation inductions to obtain the limited number of embryos, thus the number of FET cycles was relatively small and further studies with larger sample sizes are needed to verify our findings about the different pregnancy outcomes of the two protocols.
Conclusion
We demonstrate that the PPOS protocol combined with CC reduces the total dose of HMG and the duration of stimulation, can also achieve similar oocyte yields and clinical pregnancy rate compared with the PPOS protocol used alone in poor ovarian responders, which is more cost-saving. Our research may provide some ideas for further exploring the most suitable and patient-friendly treatment regimens for patients with poor ovarian response.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Zhang Y, Zhang C, Shu J, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum Reprod Update. 2020;26(2):247–63.
Siristatidis C, Salamalekis G, Dafopoulos K, et al. Mild versus conventional ovarian stimulation for poor responders undergoing IVF/ICSI. In vivo (Athens, Greece). 2017;31(2):231–7.
Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (Oxford, England). 2011;26(7):1616–24.
Alviggi C, Andersen CY, Buehler K, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105(6):1452–3.
Song D, Shi Y, Zhong Y, et al. Efficiency of mild ovarian stimulation with clomiphene on poor ovarian responders during IVF\ICSI procedures: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;204:36–43.
Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104(1):62-70.e63.
Cui L, Lin Y, Wang F, et al. Effectiveness of progesterone-primed ovarian stimulation in assisted reproductive technology: a systematic review and meta-analysis. Arch Gynecol Obstet. 2021;303(3):615–30.
Wang N, Wang Y, Chen Q, et al. Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol. 2016;84(5):720–8.
Chen Q, Wang Y, Sun L, et al. Controlled ovulation of the dominant follicle using progestin in minimal stimulation in poor responders. Reprod Biol Endocrinol:RB&E. 2017;15(1):71.
Jiang S, Kuang Y. Clomiphene citrate is associated with favorable cycle characteristics but impaired outcomes of obese women with polycystic ovarian syndrome undergoing ovarian stimulation for in vitro fertilization. Medicine. 2017;96(32): e7540.
Marrs RP, Vargyas JM, Shangold GM, et al. The effect of time of initiation of clomiphene citrate on multiple follicle development for human in vitro fertilization and embryo replacement procedures. Fertil Steril. 1984;41(5):682–5.
Dickey RP, Holtkamp DE. Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update. 1996;2(6):483–506.
Liu Y, Chen Q, Yu S, et al. Progestin-primed ovarian stimulation with or without clomiphene citrate supplementation in normal ovulatory women undergoing in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized controlled trial. Clin Endocrinol. 2018;88(3):442–52.
Ye H, Tian H, He W, et al. Progestin-primed milder stimulation with clomiphene citrate yields fewer oocytes and suboptimal pregnancy outcomes compared with the standard progestin-primed ovarian stimulation in infertile women with polycystic ovarian syndrome. Reprod Biol Endocrinol: RB&E. 2018;16(1):53.
Brosens IA, Cornillie F, Koninckx P, et al. Evolution of the revised American fertility society classification of endometriosis. Fertil Steril. 1985;44(5):714–6.
Cummins JM, Breen TM, Harrison KL, et al. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fertil Embryo Transf: IVF. 1986;3(5):284–95.
Huang P, Tang M, Qin A. Progestin-primed ovarian stimulation is a feasible method for poor ovarian responders undergoing in IVF/ICSI compared to a GnRH antagonist protocol: a retrospective study. J Gynecol Obstetrics Hum Reprod. 2019;48(2):99–102.
Xi W, Liu S, Mao H, et al. Use of letrozole and clomiphene citrate combined with gonadotropins in clomiphene-resistant infertile women with polycystic ovary syndrome: a prospective study. Drug Des Dev Ther. 2015;9:6001–8.
Triantafyllidou O, Sigalos G, Gkoles L, et al. The addition of clomiphene citrate to ovarian stimulation protocols for poor responders. Eur J Obstet Gynecol Reprod Biol. 2020;251:136–40.
Liu N, Ma Y, Wang S, et al. Association of the genetic variants of luteinizing hormone, luteinizing hormone receptor and polycystic ovary syndrome. Reprod Biol Endocrinol: RB&E. 2012;10:36.
Kerin JF, Liu JH, Phillipou G, et al. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61(2):265–8.
Rebar R, Judd HL, Yen SS, et al. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Investig. 1976;57(5):1320–9.
O’Dea L, O’Brien F, Currie K, et al. Follicular development induced by recombinant luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in anovulatory women with LH and FSH deficiency: evidence of a threshold effect. Curr Med Res Opin. 2008;24(10):2785–93.
Lu X, Hong Q, Sun L, et al. Dual trigger for final oocyte maturation improves the oocyte retrieval rate of suboptimal responders to gonadotropin-releasing hormone agonist. Fertil Steril. 2016;106(6):1356–62.
Humaidan P, Bungum L, Bungum M, et al. Ovarian response and pregnancy outcome related to mid-follicular LH levels in women undergoing assisted reproduction with GnRH agonist down-regulation and recombinant FSH stimulation. Hum Reprod (Oxford, England). 2002;17(8):2016–21.
Adams J, Franks S, Polson DW, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet (London, England). 1985;2(8469–70):1375–9.
Urman B, Tiras B, Yakin K. Assisted reproduction in the treatment of polycystic ovarian syndrome. Reprod Biomed Online. 2004;8(4):419–30.
Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil Steril. 2002;77(6):1170–7.
Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod. 2000;15(5):1003–8.
Esposito MA, Barnhart KT, Coutifaris C, et al. Role of periovulatory luteinizing hormone concentrations during assisted reproductive technology cycles stimulated exclusively with recombinant follicle-stimulating hormone. Fertil Steril. 2001;75(3):519–24.
Meyer L, Murphy LA, Gumer A, Reichman DE, Rosenwaks Z, Cholst IN. Risk factors for a suboptimal response to gonadotropin-releasing hormone agonist trigger during in vitro fertilization cycles. Fertil Steril. 2015;104(3):637–42.
Pilehvari S, ShahrokhTehraninejad E, Hosseinrashidi B, et al. Comparison pregnancy outcomes between minimal stimulation protocol and conventional GnRH antagonist protocols in poor ovarian responders. J Family Reprod Health. 2016;10(1):35–42.
Acknowledgements
This research project was funded by the Innovation Fund Project for Colleges and Universities of Gansu Province. The views expressed are those of the authors and not necessarily those of the fundings.
Funding
This study was supported by the Innovation Fund Project for Colleges and Universities of Gansu Province in 2020 (No. 2020B-012).
Author information
Authors and Affiliations
Contributions
AHL contributed to data analysis and write the original manuscript. JL and HFS contributed to data collection and revision of the manuscript. LLZ contributed to data analysis and funding acquisition. QYL contributed to data collection and chart making. XHZ contributed to the design of the study, interpretation of results, and critically revised the manuscript. All the authors provided a critical review and approved the final submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics and Research Committee of the First Hospital of Lanzhou University, Lanzhou, Gansu, China. (No: LDYYSZLLKH2021-010); In our survey, all methods were carried out in accordance with relevant guidelines and regulations. Informed consent was waived by the Ethics and Research Committee of the First Hospital of Lanzhou University, for retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, A., Li, J., Shen, H. et al. Progestin-primed ovarian stimulation protocol with or without clomiphene citrate for poor ovarian responders: a retrospective cohort study. BMC Women's Health 22, 527 (2022). https://doi.org/10.1186/s12905-022-02126-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-02126-w