Abstract
Background
Pregnancy has been considered a risk factor for the development of osteoporosis. Despite much research in this field, the relationship between parity and bone mineral density (BMD) is still controversial. Therefore, we conducted this study to investigate whether there was an association between parity and BMD of the femoral neck and lumbar spine in postmenopausal women.
Methods
Cross-sectional study was conducted using data from the National Health and Nutrition Examination Survey (NHANES). Three linear regression models, Model 1 (unadjusted), Model 2 (adjusted for age and body mass index (BMI)), and Model 3 (adjusted for all covariates), were established to evaluate the relationship between parity and BMD. In addition, the p value trend of BMD in the different parity groups was mutually verified with the results of multiple regression. Multiple logistic regression models were used to assess the relationship between parity and osteoporosis.
Results
In total, 924 postmenopausal women aged 45–65 years were eligible for this study. After adjustment for potential confounders, women with ≥ 6 parities had significantly lower lumbar spine BMD than women with 1–2 parities (β = − 0.072, 95% CI: − 0.125, − 0.018, P = 0.009). However, there was no correlation between parity and femoral neck BMD in any of the three regression models. Furthermore, ≥ 6 parities were associated with a significantly higher prevalence of lumbar spine osteoporosis compared with 1–2 parities (OR = 3.876, 95% CI: 1.637, 9.175, P = 0.002).
Conclusions
After adjustment for BMD-related risk factors, ≥ 6 parities were associated with decreased lumbar spine BMD but not femoral neck BMD in postmenopausal women. This suggests that postmenopausal women with high parity are at increased risk of lumbar osteoporotic fractures and should pay more attention to their bone health.
Similar content being viewed by others
Background
Osteoporosis is one of the most common chronic metabolic skeletal diseases, increasing the risk of bone fragility and fracture due to low bone mass and the destruction of bone microstructure [1]. Postmenopausal women are at high risk for osteoporosis because estrogen deficiency accelerates bone turnover with net bone loss [2]. A study from the National Health and Nutrition Examination Survey (NHANES) showed that among older US adults, the prevalence of osteoporosis and low bone mass in women was significantly higher than that in men, whether at the femoral neck or lumbar spine [3]. Bone mineral density (BMD), as an index to evaluate the mineral content in bone, is often used in the diagnosis of osteoporosis. Low BMD is strongly related to an increased risk of fracture, which increases the incidence rate and mortality for elderly women [4]. Therefore, it is very important to determine the possible risk factors for low BMD in postmenopausal women.
Pregnancy has been considered a risk factor for the development of osteoporosis [5, 6]. Theoretically, bone mass may decrease due to calcium requirements during pregnancy, while on the contrary, bone mass may increase due to higher estrogen levels in the third trimester of pregnancy and increased bone load caused by weight gain during pregnancy [5, 6]. Despite much research in this field, the relationship between parity and BMD is still controversial. Therefore, we conducted a cross-sectional study to investigate whether there was an association between parity and BMD of the femoral neck and lumbar spine in postmenopausal women.
Method
Study population
This study was conducted using data from the NHANES, a two-year-cycle cross-sectional survey conducted by the Centers for Disease Control and Prevention (CDC). Participants in each NHANES cycle were identified through stratified, multi-stage probability sampling of the non-institutionalized US population. The data from this survey have been widely used in epidemiological research, nutritional status assessments, and disease risk factor investigations [7,8,9,10]. The Ethics Review Board of the National Center for Health Statistics (NCHS) approved the survey protocols and written informed consent was obtained from all participants.
In this study, we merged the data of the three cycles (2005–2006, 2007–2008, and 2009–2010). From 2005 to 2010, a total of 31,034 individuals participated in the NHANES project. Among 2870 women aged 45–65 years, we excluded participants with missing BMD (n = 987) and parity (n = 289) data and women who had not given birth (n = 23). Among 1112 postmenopausal women, participants with cancer (n = 124) and missing covariate data (n = 64) were further excluded. Those who answered “yes” to the question “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” were defined as cancer participants. Finally, 924 postmenopausal women aged 45–65 years were included in our study (Fig. 1).
Study variables
The independent variable of our study was parity. Parity information was obtained from the question of reproductive health in the module of questionnaire data: “How many deliveries live birth result?” We divided parity into three groups: 1–2, 3–5 and ≥ 6, of which the 1–2 parity group was the reference group.
The dependent variables in our study were the femoral neck and lumbar spine BMD. From 2005 to 2010, dual-energy X-ray absorptiometry (DXA) scans of the proximal femur and lumbar spine were performed at the NHANES mobile examination center (MEC). The radiation exposure from DXA for both the femur or spine scan is extremely low at less than 20 uSv. The DXA examinations were performed by trained and certified radiographers. In addition, we converted BMD to T-scores, and according to established criteria, T-scores ≤ − 2.5 was classified as osteoporosis.
The selection of covariates was based on previous literature [11,12,13]. Age, family poverty income ratio (PIR), race, education level, smoking behavior, alcohol consumption, physical activity (PA), reproductive health, hormone replacement therapy (HRT), ever treated for osteoporosis, drug (e.g., corticosteroid, anticoagulant, anticonvulsive drug and immunosuppressive) use and disease history were obtained by self-reports. The family PIR was categorized as <1.3 (low income), 1.3–3.5 (middle income), and ≥3.5 (high income). Alcohol consumption was divided into three categories: non-drinkers, moderate drinkers, and heavy drinkers. Female drinkers, on average, have less than 2 drinks per day as moderate drinkers and 2 or more drinks as heavy drinkers [14]. PA was divided into two types according to the metabolic equivalent of task (MET): active and non-active. The total MET-minutes/week was calculated based on participants’ self-reported activity types and time. The MET-minutes/week was calculated by multiplying the MET value of each activity by the total number of minutes of each activity per week. Finally, by summing the MET-minutes/week of each activity, the total MET-minutes/week of all activities was calculated. Participants were defined as non-active with MET-minutes/week < 500 and ≥ 500 as active [15]. Referring to previous study [16], HRT was divided into currently using, previously used and never used. Menopausal status was defined as not having a menstruation in the past 12 months, with the exception of pregnancy and breastfeeding. Years since menopause were calculated by subtracting the age at the last menstrual period from the age at the time of the survey. Ever treated for osteoporosis, drug use and disease history are only shown in the table as “yes”. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Details for total calcium and serum phosphorus are provided in the standard biochemical profile under the laboratory data section of the NHANES website.
Statistical analyses
NHANES sample weights were considered in the analysis to represent the noninstitutionalized civilian population of the United States. EmpowerStats statistical software (X&Y Solutions, Boston, MA) and R software (version 3.4.3) were used for all analyses, and a p < 0.05 was considered statistically significant.
The data are reported as mean ± SD and Min-Max for continuous variables and percentages for categorical variables. The p values of continuous variables and categorical variables were calculated by weighted linear regression model and weighted chi-square test, respectively.
Three linear regression models, Model 1 (unadjusted), Model 2 (adjusted for age and BMI), and Model 3 (adjusted for all the covariates listed in Table 1), were established to evaluate the relationship between parity and BMD of the femoral neck and lumbar spine. In addition, the p value trend of BMD in the different parity groups was mutually verified with the results of multiple regression. Multiple logistic regression models were used to assess the relationship between parity and osteoporosis.
Result
In total, 924 postmenopausal women aged 45–65 years were eligible for this study (Fig. 1). The features of the participants based on parity are shown in Table 1. There were significant differences in age, BMI, years since menopause, family PIR, race, education level, HRT, and alcohol consumption among the different parity groups (1–2; 3–5; ≥ 6).
Table 2 shows the associations between parity and femoral neck BMD. There was no correlation between parity and femoral neck BMD in any of the three regression models (Table 2).
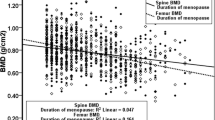
Table 3 shows the associations between parity and lumbar spine BMD. After adjustment for potential confounders, ≥ 6 parities had significantly lower lumbar spine BMD than 1–2 parities (β = − 0.072, 95% CI: − 0.125, − 0.018, P = 0.009) (Table 3). This significant trend of lumbar spine BMD was further verified and is shown in Table 4.
Table 5 shows the associations between parity and osteoporosis of femoral neck and lumbar spine. After adjustment for potential confounders, ≥ 6 parities were associated with a significantly higher prevalence of lumbar spine osteoporosis compared with 1–2 parities (OR = 3.876 , 95% CI: 1.637, 9.175, P = 0.002).
Discussion
This study investigated the relationship between parity and BMD in postmenopausal women aged 45–65 years. In our study, we divided parity into three groups and characterized the study population accordingly. Our results showed that parity was negatively correlated with lumbar spine BMD in all three regression models, but not with femoral neck BMD. These results were verified by the P for trend of BMD based on parity. Consistent with the above results, the prevalence of lumbar spine osteoporosis was significantly higher in the highest parity group.
Previous research has reported conflicting conclusions. A study of postmenopausal women in Morocco showed results that were consistent with ours. The authors found that patients with 6 or more parities had significantly lower lumbar spine BMD values than patients with other numbers of parities, but there was no significant difference in the femoral neck BMD values [17]. Gur et al. [18] also found that there was a significant negative correlation between the number of pregnancies and spine BMD but no significant correlation with the femoral neck BMD. Heidari et al. [19] had similar findings. They reported an independent association between parity and lumbar spine osteoporosis but not for the femoral neck and a 13% increased risk of lumbar spine osteoporosis per parity. In addition, Demir et al. [20] and Seo et al. [11] observed that high parity was a risk factor for low BMD in postmenopausal women. However, other studies have shown no relationship between parity and BMD or osteoporosis [21, 22], while another study suggested a protective effect of high parity on postmenopausal osteoporosis [23]. The results of these conflicts may be due to different ethnic groups, lifestyles, nutritional status and so on.
Calcium is in high demand during pregnancy and breastfeeding due to the growth of fetal and newborn bones. The physiological requirement of a full-term singleton for calcium is about 30 g [24]. If the mother’s bone minerals are the only source of calcium, the mother’s bones will lose approximately 3% (30 g/1000 g) of the mineral with each pregnancy [18]. However, there are some adaptive changes during pregnancy and breastfeeding. During pregnancy, the efficiency of intestinal calcium absorption doubles to meet the need of the fetus for calcium, while during lactation, skeletal resorption increases to provide calcium for milk production. These hormone-mediated adaptations usually meet the daily mineral needs of the fetus and infant without adverse long-term effects on the maternal bones [25]. Fokter et al. [26] reported a case of proximal femoral fracture caused by osteoporosis in the third trimester of first pregnancy, with excellent healing effect after surgery, suggesting that transient osteopenia during pregnancy has the potential for normal healing. However, it is not clear whether the bone loss from multiple pregnancies is fully compensated. Animal studies have shown that as the number of litters increases, the decrease in trabecular bone density becomes irreversible [27, 28]. Therefore, when the number of parities increases, bone loss may not be fully recovered [29]. The mechanisms underlying the effects of parity on the skeleton are complex, and more basic and clinical studies are needed in the future to clarify this relationship.
In our study, we found differences in the influence of parity on lumbar spine and femoral neck BMD. This may be due to the difference in bone structure between the lumbar spine, which is dominated by trabecular bone, and the femoral neck, which is dominated by cortical bone. The increased calcium demand during pregnancy and lactation leads to increased bone absorption. Due to the larger surface area of bone trabeculae, osteoclasts reabsorb more rapidly [13]. MicroCT studies have confirmed that calcium restriction leads to greater reductions in bone volumes, trabecular number and thickness, and tissue density [30]. These results support our finding that parity is negatively correlated with lumbar spine BMD.
In this study, we designed three models to observe the correlation between parity and BMD by adjusting for different confounding factors. However, there are some limitations to this study. First, although the questionnaire data were obtained from face-to-face interviews, there were still some recall biases. Second, the disease history was based on self-reports and was not cross-checked with medical records. Third, due to lack of reproductive information such as twins, abortions, stillbirths, and breastfeeding duration, the impact of these factors on BMD was not considered. Finally, although we combined data from three cycles, the number of eligible nulliparous women was too small to be included from the analysis, so we did not compare the BMD values of nulliparous women with those of parous women. Future studies with a larger sample size and involving nulliparous women are needed.
Conclusions
After adjustment for BMD-related risk factors, ≥ 6 parities were associated with decreased lumbar spine BMD but not femoral neck BMD in postmenopausal women. This suggests that postmenopausal women with high parity are at increased risk of lumbar osteoporotic fractures and should pay more attention to their bone health.
Availability of data and materials
All the data are publicly available online (https://www.cdc.gov/nchs/nhanes/).
Abbreviations
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- NHANES:
-
National health and nutrition examination survey
- CDC:
-
Centers for disease control and prevention
- NCHS:
-
National center for health statistics
- DXA:
-
Dual-energy X-ray absorptiometry
- MEC:
-
Mobile examination center
- PIR:
-
Poverty income ratio
- PA:
-
Physical activity
- HRT:
-
Hormone replacement therapy
- MET:
-
Metabolic equivalent of task
References
Yan G, Huang Y, Cao H, Wu J, Jiang N, Cao X. Association of breastfeeding and postmenopausal osteoporosis in Chinese women: a community-based retrospective study. BMC Womens Health. 2019;19(1):110.
Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44.
Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos Int. 2017;28(6):1979–1988.
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341(8837):72–75.
Alderman BW, Weiss NS, Daling JR, Ure CL, Ballard JH. Reproductive history and postmenopausal risk of hip and forearm fracture. Am J Epidemiol. 1986;124(2):262–267.
Cumming RG, Klineberg RJ. Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. Int J Epidemiol. 1993;22(4):684–691.
Lu J, Lan J, Li X, Zhu Z. Blood lead and cadmium levels are negatively associated with bone mineral density in young female adults. Arch Public Health. 2021;79(1):116.
Dong X, Li S, Chen J, Li Y, Wu Y, Zhang D. Association of dietary ω-3 and ω-6 fatty acids intake with cognitive performance in older adults: National Health and nutrition examination Survey (NHANES) 2011–2014. Nutr J. 2020;19(1):25.
King DE, Xiang J. Glucosamine/Chondroitin and Mortality in a US NHANES Cohort. J Am Board Fam Med. 2020;33(6):842–847.
Wright DM, McKenna G, Nugent A, Winning L, Linden GJ, Woodside JV. Association between diet and periodontitis: a cross-sectional study of 10,000 NHANES participants. Am J Clin Nutr. 2020;112(6):1485–1491.
Seo E, Lee Y, Kim HC. Association Between Parity and Low Bone Density Among Postmenopausal Korean Women. J Prev Med Public Health. 2021;54(4):284–292.
Peker N, Tosun ÖÇ. Is grand multiparity a risk factor for the development of postmenopausal osteoporosis? Clin Interv Aging. 2018;13:505–508.
Cooke-Hubley S, Gao Z, Mugford G, Kaiser SM, Goltzman D, Leslie WD, Davison KS, Brown JP, Probyn L, Lentle B, Prior JC, Kovacs CS. Parity and lactation are not associated with incident fragility fractures or radiographic vertebral fractures over 16 years of follow-up: Canadian Multicentre Osteoporosis Study (CaMos). Arch Osteoporos. 2019;14(1):49.
Tucker LA. Physical activity and telomere length in U.S. men and women: An NHANES investigation. Prev Med. 2017;100:145–151.
Li Y, Tong WD, Qian Y. Effect of Physical Activity on the Association Between Dietary Fiber and Constipation: Evidence From the National Health and Nutrition Examination Survey 2005–2010. J Neurogastroenterol Motil. 2021;27(1):97–107.
Crespo CJ, Smit E, Snelling A, Sempos CT, Andersen RE. Hormone replacement therapy and its relationship to lipid and glucose metabolism in diabetic and nondiabetic postmenopausal women: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care. 2002;25(10):1675–1680.
Allali F, Maaroufi H, Aichaoui SE, Khazani H, Saoud B, Benyahya B, Abouqal R, Hajjaj-Hassouni N. Influence of parity on bone mineral density and peripheral fracture risk in Moroccan postmenopausal women. Maturitas. 2007;57(4):392–398.
Gur A, Nas K, Cevik R, Sarac AJ, Ataoglu S, Karakoc M. Influence of number of pregnancies on bone mineral density in postmenopausal women of different age groups. J Bone Miner Metab. 2003;21(4):234–241.
Heidari B, Hosseini R, Javadian Y, Bijani A, Sateri MH, Nouroddini HG. Factors affecting bone mineral density in postmenopausal women. Arch Osteoporos. 2015;10:15.
Demir B, Haberal A, Geyik P, Baskan B, Ozturkoglu E, Karacay O, Deveci S. Identification of the risk factors for osteoporosis among postmenopausal women. Maturitas. 2008;60(3–4):253–256.
Hassa H, Tanir HM, Senses T, Oge T, Sahin-Mutlu F. Related factors in bone mineral density of lumbal and femur in natural postmenopausal women. Arch Gynecol Obstet. 2005;273(2):86–89.
Cavkaytar S, Seval MM, Atak Z, Findik RB, Ture S, Kokanali D. Effect of reproductive history, lactation, first pregnancy age and dietary habits on bone mineral density in natural postmenopausal women. Aging Clin Exp Res. 2015;27(5):689–694.
Okyay DO, Okyay E, Dogan E, Kurtulmus S, Acet F, Taner CE. Prolonged breast-feeding is an independent risk factor for postmenopausal osteoporosis. Maturitas. 2013;74(3):270–275.
Pitkin RM. Calcium metabolism in pregnancy and the perinatal period: a review. Am J Obstet Gynecol. 1985;151(1):99–109.
Kovacs CS. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol Rev. 2016;96(2):449–547.
Fokter SK, Vengust V. Displaced subcapital fracture of the hip in transient osteoporosis of pregnancy. A case report. Int Orthop. 1997;21(3):201–203.
Gu A, Sellamuthu R, Himes E, Childress PJ, Pelus LM, Orschell CM, Kacena MA. Alterations to maternal cortical and trabecular bone in multiparous middle-aged mice. J Musculoskelet Neuronal Interact. 2017;17(4):312–318.
de Bakker CM, Altman-Singles AR, Li Y, Tseng WJ, Li C, Liu XS. Adaptations in the Microarchitecture and Load Distribution of Maternal Cortical and Trabecular Bone in Response to Multiple Reproductive Cycles in Rats. J Bone Miner Res. 2017;32(5):1014–1026.
Yang L, Hu X, Wu W, Pan W, Chen S, Lin X, Gu X. The inverse association between parity and bone health is independent of lifestyle in postmenopausal Chinese women. Endocr J. 2020;67(10):1039–1045.
Ardeshirpour L, Dumitru C, Dann P, Sterpka J, VanHouten J, Kim W, Kostenuik P, Wysolmerski J. OPG Treatment Prevents Bone Loss During Lactation But Does Not Affect Milk Production or Maternal Calcium Metabolism. Endocrinology. 2015;156(8):2762–2773.
Acknowledgements
The authors thank the NHANES project for providing access to their database.
Funding
This study was supported by Jiangsu Provincial Funds for Six Categories of Top Talents (Program No. WS-066), the Research Project of Jiangsu Provincial Health and Family Planning Commission (Program No. H201526), and the Nantong Technology Project (Program No. MS12017008-1, MS12020007).
Author information
Authors and Affiliations
Contributions
YMY conducted data collection, the statistical analysis, and the drafting and completion of the manuscript. HC conceived this study and helped with the drafting and final revision of the manuscript. SSW participated in the data collection and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The survey protocol was approved by the Ethics Review Board of the National Center for Health Statistics (NCHS), and each participant provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Wang, S. & Cong, H. Association between parity and bone mineral density in postmenopausal women. BMC Women's Health 22, 87 (2022). https://doi.org/10.1186/s12905-022-01662-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-01662-9