Abstract
Purpose
Women of reproductive age who carry fragile X premutation (PM) alleles have 56 to 200 CGG repeats in the 5′-untranslated region of FMR1 gene are at increased risk for producing children with intellectual disabilities (ID) or autism spectrum disorders (ASD) due to expansion of PM alleles to full mutation alleles (> 200 repeats) during maternal transmission.
Methods
In present study fragile X PM carrier screening was performed in total 808 women who were consulting primary health care centers for preconception care in Khyber Pakhtunkhwa region of Pakistan between April, 2018 and December, 2020. Polymerase chain reaction (PCR) was performed for detection of PM carrier women and the CGG repeats number was confirmed by Southern blotting and capillary electrophoresis.
Results
The prevalence rate for PM carriers among preconception women was found to be 0.7% that was contributed by 0.5% women in risk group (RG1) with family history of ID and 0.2% in risk group 2 (RG2) with family history of ASD. PM carrier women had at least one affected child or sibling. In addition, the preconception women with FMR1 PM alleles were found to be at increased risk for primary ovary insufficiency (RG1: P = 0.0265, RG2: P = 0.0389), postpartum depression (RG1: P = 0.0240, RG2: P = 0.0501) and neuropsychiatric disorders (RG1: P = 0.0389, RG2: P = 0.0432).
Conclusions
Current study provides first evidence of fragile X PM carrier screening in Pakistani preconception women in primary care consultation. Findings of current study may help to improve preconception care and to reduce burden of fragile X associated disorders in our population.
Similar content being viewed by others
Introduction
Fragile X syndrome (FXS) is a rare neurodevelopmental disorder (MIM # 300,624) that affects approximately 1 in 4000 males and 1 in 6000–8000 females [1]. FXS is characterized by a wide range of inherited intellectual disabilities and autism spectrum disorders in children [2]. FXS is caused by the cysteine-guanine-guanine (CGG) repeat expansion mutations in 5′-untranslated region (UTR) of the FMR1 gene on chromosome X. The CGG repeat expansion mutations cause gene methylation and in turn inactivation of the FMR1 gene [3]. Importantly, the number of CGG repeats size is not constant among individuals. There are four allelic forms of the FMR1 gene based on CGG repeat length. They are known as normal alleles with less than 45 repeats, intermediate alleles with 45–54 repeats, permutation (PM) alleles with 55–200 repeats and full mutation (FM) alleles greater than 200 repeats [4]. Individuals with PM alleles are considered carriers and those with FM alleles are referred affected and exhibit FXS clinical phenotypes [5].
Notably, about 1 in 150–300 women carry PM alleles, however, PM carrier frequency may vary among women from different ethnic groups [6]. PM carrier women usually are at increased risk to develop fragile X associated primary ovarian insufficiency (FXPOI) [7] and fragile X associated diminished ovarian reserve (FXDOR) [8] in reproductive age as well as fragile X associated tremor/ataxia syndrome (FXTAS) in late age [9]. Women of reproductive age diagnosed with FXPOI may have symptoms of low minerals bone density, osteoporosis, bone fractures [10, 11] and fibromyalgia [12] PM carrier women may experience obstetric and perinatal difficulties like late pregnancy bleeding [13] and vestibular issues such as dizziness, spinning and not able to balance body [14]. PM carrier women may suffer from hypertension, central pain sensitivity syndrome, sleep problems, restless legs syndrome, migraine and gait issues [15]. Depression, anxiety and attention problems were more commonly observed psychiatric features in PM carriers [16]. These reported clinical features are also observed to be prevalent among non-PM carrier women, but the case of PM carrier women is more critical as they are at increased risk of producing affected children with FXS due to maternal PM alleles expansion to FM alleles during transmission [17].
Population based studies conducted in different countries have detected a significant number of PM carrier women, who transmitted PM alleles to fetus and delivered children with FXS [18, 19]. These studies strongly suggest counseling and screening of preconception women in primary care consultation for detection of PM carrier status that may be of paramount importance [8, 20]. In addition, the American College of Medical Genetics recommends FMR1 PM carrier screening for preconception women with a family history of fragile X associated disorders (FXD) under a clinical research protocol. According to guidelines of ACOG, Polymerase chain reaction (PCR) and Southern blotting are the most preferred molecular diagnostic methods for detection of FMR1 PM carrier status of preconception women [21, 22].
Here, we report first study for fragile X PM carrier screening among Pakistani preconception women of reproductive age in primary care consultation and also determined health risks associated with the FMR1 PM carrier status of women.
Materials and methods
Study subjects
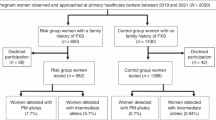
The approval of present study was obtained from the Ethical Committee and Advanced Studies Research Board (ASRB) of Kohat University of Science and Technology (KUST), Kohat, Khyber Pakhtunkhwa, Pakistan. The women of reproductive age who were consulting primary health care centers in Khyber Pakhtunkhwa region of Pakistan between April-2018 and December-2020 for preconception care. Before recruitment informed written consent was obtained from each participating woman. Women who fulfilled the criteria as described by ACOG for fragile X carrier screening of preconception women were recruited at primary health care centers for this study as shown in Fig. 1. Preconception women with a family history of either intellectual disability (ID) or autism spectrum disorder (ASD) were recruited in risk group 1 (RG1) and risk group 2 (RG2) respectively. To determine prevalence rate of FMR1 PM alleles, women in preconception care with obstetric/gynecologic problems but had no family history of FXD and provided consent for participation in study were included in control group. A significant number of women with or without family history of FXD did not provide written informed consent or did not want to be counseled or couldn’t be counseled for participation in this study were excluded. Information on demographics, family medical history, health status, clinical investigations were collected from recruited women with help of obstetricians and gynecologists at primary health care centers.
Fragile X carrier screening
The standard phenol–chloroform method was used to extract genomic DNA from peripheral blood of women participating in study. Polymerase Chain Reaction (PCR) was used as initial screening method for detection of fragile X carrier women. For amplification of FMR1 alleles in the first step PCR, primers forward 5′-TCAGCTCCGTTTCGGTTTC-3′ and reverse 5′-CCTTGTAGAAAGCGCCATTG-3′ were designed. PCR amplifications were performed in a 25 μl reaction containing 2X DreamTaq Green PCR master mix (Thermo Scientific), 0.5 μM of each primer, 100 ng/μL of template DNA and 3% DMSO and 2.5 M Betaine. The cycling conditions for PCR were initial denaturation for 5 min at 95 °C, followed by 30 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 59 °C, extension for 30 s at 72 °C, and a final extension for 8 min at 72 °C. In second step PCR, DNA of women amplified as single bands in first step PCR were subsequently analyzed by using primers (c primer: 5′-GCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3′ and CGG-chimeric primer:5′-AGCGTCTACTGTCTCGGCACTTGCCCGCCGCCGCCG-3′ for the random amplification of CGG repeats by optimizing PCR amplification conditions as described previously [23]. Furthermore, the CGG repeats number in FMR1 PM alleles in carrier women and in their family members were determined by Southern blotting and capillary electrophoresis and for this purpose services of commercial diagnostic laboratories in Islamabad were utilized.
Statistical analysis
The characteristics of the participating preconception women were described or summarized using the SPSS 21.0., software. To compare qualitative variables, the chi-square statistic was used and a P value < 0.05 was considered significant to find potential risk factors for FMR1 PM carrier women.
Results
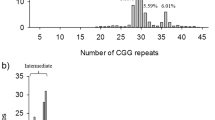
In total 808 women screened for FMR1 PM, majority of participating preconception women were in control group with no family history of FXD (77.35%). However, a substantial number of preconception women were in RG1 and RG2 with family history of either ID (14.35%) or ASD (8.3%) respectively (Fig. 1). Table 1 presents the frequencies of affected family members of recruited risk groups preconception women, however, the preconception women in RG1 and RG2 had at least one child or sibling affected with either ID or ASD respectively. The prevalence rate for PM carriers among preconception women was found to be 0.7% that was contributed by 0.5% women in RG1 and 0.2% in RG2 who were detected carries for PM alleles. However, PM alleles were not detected in any woman from control group. In addition, 21 (2.3%) women in RG1 and about 1.1% in RG2 were found to carry intermediate alleles. In addition, PM and FM alleles were also detected in family members of PM carrier women. Almost all PM carriers had low anti-müllerian hormone (AMH) levels (< 1 ng/mL) and high follicle stimulating hormone (FSH) levels (25.8 ≥ IU/L) as shown in Table 2 and Table 3. Additional characteristics of PM carrier women are summarized in Table 3 and health risks associated with PM carriers are shown in Table 4. Majority of PM carrier women (66.7%) were less than 33 years of age, suffered from irregular menstruation (83.3%) and hot splashes/ night sweats (66%). PM carriers’ women had a significant increased risk of developing FXPOI (RG1: P = 0.0265 and RG2: P = 0.0389). PM carrier women less frequently experienced obstetric and perinatal difficulties such as antepartum hemorrhage (33%), whereas more obviously experienced early onset osteoporosis (83.3%), however, no significant differences were found in PM carrier and non-PM carrier women with respect to these factors as shown in Table 4. Postpartum depression was more prevalent (83.3%) among PM carrier women and they were found at significantly increased risk for this health problem (RG1: P = 0.0240 and RG2 P = 0.0501). Although, a substantial number of PM carrier women suffered from hypertension (66%) and migraine (50%), however, PM carrier women were not found at increased risk for these health conditions (RG1: P = 0.1436 and RG2 P = 0.5384). Interestingly, all PM carrier women had normal intelligence quotient (IQ) levels. In addition, the most common neuropsychiatric features present in majority of PM carrier women were anxiety (83.3%), sleep disturbance (83.3%), aggression (83.3%), difficulty in concentrating (66%), hyperreactivity (66%), and language issues (50%). Importantly, PM carrier women were found at significantly increased risk for neuropsychiatric disorders (RG1: P = 0.0389 and RG2: P = 0.0432).
Discussion
Expansion of CGG repeats > 200 in the 5′-UTR of the FMR1 gene on the X chromosome and subsequent epigenetic modifications are the most common cause of inherited ID and monogenic cause of ASD usually in males [2, 6, 24]. Importantly, fragile X PM carrier women with < 200 repeats are at increased risk for producing affected children due to expansion of repeats to FM that occurs almost only in transmission from mother to children [25]. In addition, the family history of FXD of women increases significantly risk of having children with FXS [26, 27]. Fragile X PM carrier status is essentially silent in women of reproductive age unless they develop POI, thus family history of FXD may lead clinicians to diagnose PM carrier women in reproductive age in populations [28]. Therefore, ACOG has recommended FMR1 carrier screening of preconception women with a family history of FXD to determine PM carrier status of women [21, 22]. Primary health care is recognized as a setting where direct approach to preconception women for screening genetic disorders like FXS may be possible. Diagnosis of fragile X PM carrier status of preconception women may help in making reproductive decisions and family planning [26].
According to our knowledge, this is a first study in which fragile X PM carrier screening was performed in women who were consulting obstetricians and gynecologists for preconception care at primary health care centers in Khyber Pakhtunkhwa region of Pakistan. An important aspect of this study is participation of obstetricians and gynecologists’ other clinicians that make feasible this study in clinical setting as recommended by guidelines of the ACOG [21, 22]. In consistent with our study, fragile X carrier screening was preferentially offered to women before pregnancy in previously conducted population-based prevalence studies [29, 30]. In contrast to our study population-based prevalence studies conducted in China preferred pregnant women for fragile X carrier screening. However, some studies also considered both preconception and pregnant women for fragile X carrier screening in different populations of world [31, 32]. Interestingly, in Israel and some parts of USA, pregnant women are not considered for fragile X carrier screening [33, 34]. In addition, fragile X carrier screening is not offered to women at all in many countries as screening may usually pose significant counseling and educational difficulties [35].
Population-based studies have well investigated the prevalence of the fragile X PM carriers among preconception/pregnant women in different populations. The prevalence rates observed were 0.13% in Korean women [18] 0.08 to 0.13% in Chinese women [19, 36], 0.04 to 0.27% % in Australian women [31], 0.88% to 1.3% in Israeli women [29, 37], 0.9% in Spanish women [38]. The overall prevalence rate of fragile X PM carriers was observed 0.7% in this study. Interestingly, no PM carrier was detected in control group women and about 0.5% in RG1 and 0.2% in RG2 women were detected PM carriers thus the observed prevalence in this study was contributed solely by risk groups’ women who had family history of either ID or ASD. Importantly, all risk groups’ women who consulted for preconception care had at least one child or sibling affected with either ID or ASD. Thus, this study and previously reported studies provides evidence of association of positive family history of fragile X-associated disorders with greater risk of preconception carrier women of transmitting the FM to their children. This association has also been found in previous studies [36, 39]. The findings of this study also suggest that FXS could be a reason for the high prevalence of ID and ASD that have been reported in Pakistani population [7, 40, 41].
In this study, we also determined the most significant clinical characteristics among PM carrier women. The risk of FXPOI is increased in PM carrier women and findings of this study supported this fact as PM carriers demonstrated significantly low AMH (< 1 ng/mL) and increased FSH (> 25 IU/L) levels, irregular menstruation, hot splashes and night sweats before age of 40 years compared to non-PM carriers. The observed prevailing FXPOI symptoms act as predictors for early ovarian dysfunction or menopause among PM carriers and the average age for early menopause in FXPOI has already been reported 5 years earlier in PM carrier women than women in general population [42, 43]. Many studies have also noted significantly low AMH and increased FSH levels among PM carriers [42, 44,45,46]. Similarly, other studies observed irregular, skipped or shorter menstrual cycles and subfertility as prominent features of PM carriers [43, 46]. Hypoestrogenism in PM carrier women may cause variety of clinical conditions [47]. Hypoestrogenism causing osteoporosis has been reported at a high frequency among PM carriers previously [43] as well as in this study, however, it was also found that PM carrier women have no significantly increased risk of osteoporosis.
Previous studies revealed that PM carriers experience significantly antepartum hemorrhage and preeclampsia at least in one pregnancy [13, 47], however, PM carriers were not found at risk for any of these obstetric or perinatal difficulties in the present study. Similarly, in contrast to the study conducted by Wheeler et al. [43], we in this study and Obadia et al. [47] in a previous study examined an increased risk for postpartum depression in PM carrier women who had either one or two children with FXS. Therefore, having more than one child with FXS could be a reason of increased risk of women for postpartum depression, rather than their PM carrier status [47].
Interestingly, in present study all PM carriers showed normal IQ scores compared to their children and siblings with either ID or ASD. The normal IQ scores were also reported in adult PM carriers in previous studies [48, 49]. In contrast, few studies have reported lower IQ scores among PM carriers [50, 51]. Moreover, PM carrier women were at significantly increased risks for various neuropsychiatric features such as anxiety, sleep disturbances, aggression, difficulty in concentrating, hyperreactivity and language issues than non-PM carrier women as these features were present at high frequencies in PM carriers in this study. Previous studies have also reported significantly higher rates of sleep disturbances [52, 53], depression, stress and anxiety [31, 54], language issues [16, 55], attention deficits [16, 48], and memory impairment [56, 57] in PM carriers with or without diagnosis of Fragile X associated disorders compared with controls. Hartley et al. [58] in a study noted predominantly aggression, inattentive, behavior problems and irritability, whereas Chonchaiya et al. [59] observed high prevalence of balance problem, memory loss, dizziness among PM carriers with history of FXD. Prevalence rates of hypertension and migraine were high; however, PM carriers were not found at significantly increased risk for these conditions in this study. In contrast, observed significantly high prevalence of hypertension [60] and migraine [61] in PM carriers with history of FXTAS. Moreover, PM carriers had not an increased risk for diabetes as found in this and previous studies [1, 60].
It is the first study that determined prevalence of fragile X PM alleles in Pakistani women who consulted primary health care centers for preconception care. The PM carrier women had at least one child or sibling affected with either ID or ASD, therefore, this study also provides evidence that FXS could be a reason for the high prevalence of ID and ASD that have been reported in Pakistani population. In addition, the preconception women with FMR1 PM alleles were found to be at increased risk for FXPOI, postpartum depression and neuropsychiatric disorders. The findings of current study may be used to improve preconception care, direct future screening strategies and educate women about implications of fragile X associated health, reproductive and neuropsychiatric conditions that may greatly help in reducing burden of FXD in our population.
Availability of data and materials
All the data used to support the findings of this study are included within the article and are available on request from corresponding author.
References
Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet. 2010;77(4):374–81.
Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB, Moine H, Kooy RF, Tassone F, Gantois I, Sonenberg N, Mandel JL, Hagerman PJ. Fragile X syndrome. Nat Rev Dis Primers. 2017;3(1):1–9.
Monaghan KG, Lyon E, Spector EB. ACMG Standards and Guidelines for fragile X testing: a revision to the disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genet Med. 2013;15(7):575–86.
Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, Hagerman PJ, Tassone F. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn. 2009;11(4):324–9.
Schaefer GB, Mendelsohn NJ. Response to letter by Chodirker and Chudley. Genet Med. 2008;10(11):845.
Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am J Med Genet A. 2014;164(7):1648–58.
Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402.
Retamal JS, Ramírez-García PD, Shenoy PA, Poole DP, Veldhuis NA. Internalized GPCRs as potential therapeutic targets for the management of pain. Front Mol Neurosci. 2019;12:273.
Biancalana V, Glaeser D, McQuaid S, Steinbach P. EMQN best practice guidelines for the molecular genetic testing and reporting of fragile X syndrome and other fragile X-associated disorders. Eur J Hum Genet. 2015;23(4):417–25.
Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14(3):567–71.
Nguyen HH, Milat F, Vincent A. Premature ovarian insufficiency in general practice: meeting the needs of women. Aust Fam Phys. 2017;46(6):360–6.
Hagerman RJ, Protic D, Rajaratnam A, Salcedo-Arellano MJ, Aydin EY, Schneider A. Fragile X-associated neuropsychiatric disorders (FXAND). Front Psychiatry. 2018;9:564.
Kallinen J, Korhonen K, Kortelainen S, Heinonen S, Ryynänen M. Pregnancy outcome in carriers of fragile X. BJOG Int J Obstet Gynaecol. 2000;107(8):969–72.
Smith LE, Barker ET, Seltzer MM, Abbeduto L, Greenberg JS. Behavioral phenotype of fragile X syndrome in adolescence and adulthood. Am J Intellect Dev Disabil. 2012;117(1):1–7.
Zafarullah M, Tassone F. Fragile X-associated tremor/ataxia syndrome (FXTAS). In: Ben-Yosef D, Mayshar Y, editors. Fragile-X syndrome 2019. New York: Humana Press; 2019. p. 173–89.
Bailey DB Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146(16):2060–9.
Hagerman R, Au J, Hagerman P. FMR1 premutation and full mutation molecular mechanisms related to autism. J Neurodev Disord. 2011;3(3):211–24.
Kim MJ, Kim DJ, Kim SY, Yang JH, Kim MH, Lee SW, Jeong SO, Park SY, Ryu HM. Fragile X carrier screening in Korean women of reproductive age. J Med Screen. 2013;20(1):15–20.
Hung CC, Lee CN, Wang YC, Chen CL, Lin TK, Su YN, Lin MW, Kang J, Tai YY, Hsu WW, Lin SY. Fragile X syndrome carrier screening in pregnant women in Chinese Han population. Sci Rep. 2019;9(1):1–7.
Zhao J, Hu Q, Chen Y, Luo S, Bao L, Xu Y. A novel missense mutation in the paired domain of human PAX9 causes oligodontia. Am J Med Genet A. 2007;143(21):2592–7.
Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7(8):584–7.
on Genetics GC. ACOG Committee opinion no. 469: carrier screening for fragile X syndrome. Obstet Gynecol. 2010;116(4):1008–10.
Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10(1):43–9.
Zeesman S, Zwaigenbaum L, Whelan DT, Hagerman RJ, Tassone F, Taylor SA. Paternal transmission of fragile X syndrome. Am J Med Genet A. 2004;129(2):184–9.
Archibald AD, Hickerton CL, Wake SA, Jaques AM, Cohen J, Metcalfe SA. “It gives them more options”: preferences for preconception genetic carrier screening for fragile X syndrome in primary healthcare. J Community Genet. 2016;7(2):159–71.
Nolin SL, Brown WT, Glicksman A, Houck GE Jr, Gargano AD, Sullivan A, Biancalana V, Bröndum-Nielsen K, Hjalgrim H, Holinski-Feder E, Kooy F. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72(2):454–64.
Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19(R1):R83–9.
Pesso R, Berkenstadt M, Cuckle H, Gak E, Peleg L, Frydman M, Barkai G. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn. 2000;20(8):611–4.
Acharya K, Ross LF. Fragile X screening: attitudes of genetic health professionals. Am J Med Genet A. 2009;149(4):626–32.
Metcalfe SA, Martyn M, Ames A, Anderson V, Archibald AD, Couns GD, Carter R, Cohen J, Cotter M, GenCouns M, Dang W. Informed decision making and psychosocial outcomes in pregnant and nonpregnant women offered population fragile X carrier screening. Genet Med. 2017;19(12):1346–55.
Taber KJ, Beauchamp K, Lazarin G, Muzzey D, Arjunan A, Goldberg J. 893: Clinical utility of expanded carrier screening: Results-guided actionability and outcomes. Am J Obstet Gynecol. 2019;220(1):S578–9.
Berkenstadt M, Ries-Levavi L, Cuckle H, Peleg L, Barkai G. Preconceptional and prenatal screening for fragile X syndrome: Experience with 40 000 tests. Prenat Diagn. 2007;27(11):991–4.
Cronister A, DiMaio M, Mahoney MJ, Donnenfeld AE, Hallam S. Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genet Med. 2005;7(4):246–50.
Finucane B, Abrams L, Cronister A, Archibald AD, Bennett RL, McConkie-Rosell A. Genetic counseling and testing for FMR1 gene mutations: practice guidelines of the national society of genetic counselors. J Genet Couns. 2012;21(6):752–60.
Cheng YK, Lin CS, Kwok YK, Chan YM, Lau TK, Leung TY, Choy KW. Identification of fragile X pre-mutation carriers in the Chinese obstetric population using a robust FMR1 polymerase chain reaction assay: implications for screening and prenatal diagnosis. Hong Kong Med J. 2017;23(2):110–6.
Toledano-Alhadef H, Basel-Vanagaite L, Magal N, Davidov B, Ehrlich S, Drasinover V, Taub E, Halpern GJ, Ginott N, Shohat M. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69(2):351–60.
Alfaro Arenas R, Rosell Andreo J, Heine Suñer D, Group for the study of FXS in the Balearic Islands. Fragile X syndrome screening in pregnant women and women planning pregnancy shows a remarkably high FMR1 premutation prevalence in the Balearic Islands. Am J Med Genet Part B Neuropsychiatr Genet. 2016;171(8):1023–31.
Niu M, Han Y, Dy AB, Du J, Jin H, Qin J, Zhang J, Li Q, Hagerman RJ. Fragile X syndrome: prevalence, treatment, and prevention in China. Front Neurol. 2017;8:254.
Bashir A, Yaqoob M, Ferngren H, Gustavson KH, Rydelius PA, Ansari T, Zaman S. Prevalence and associated impairments of mild mental retardation in six-to ten-year old children in Pakistan: a prospective study. Acta Paediatr. 2002;91(7):833–7.
Imran N, Bhatti MR, Anwar A, Najmi F, Haider II. Children's Mental Health: pattern of referral, distribution of disorders and service use in child psychiatry outpatient setting. Pak J Med Sci. 2012;28(1):22-26
Tareen A, Mirza I, Minhas A, Minhas F, Rahman A. Developing a child and adolescent mental health service in a low-income country: a global partnership model. Psychiatr Bull. 2009;33(5):181–3.
Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. In: Seminars in reproductive medicine 2011 Jul, vol 29, no. 04, pp 299–307. © Thieme Medical Publishers.
Wheeler AC, Bailey DB Jr, Berry-Kravis E, Greenberg J, Losh M, Mailick M, Milà M, Olichney JM, Rodriguez-Revenga L, Sherman S, Smith L. Associated features in females with an FMR1 premutation. J Neurodev Disord. 2014;6(1):1–4.
Spath MA, Feuth TB, Allen EG, Smits AP, Yntema HG, van Kessel AG, Braat DD, Sherman SL, Thomas CM. Intra-individual stability over time of standardized anti-Müllerian hormone in FMR1 premutation carriers. Hum Reprod. 2011;26(8):2185–91.
Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol. 2008;68(4):499–509.
Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Charen K, He W, Taylor KC, Sherman SL. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22(8):2142–52.
Obadia RW, Iosif AM, Seritan AL. Postpartum depression in women with the FMR1 premutation. Curr Psychiatry Rev. 2013;9(1):72–7.
Roberts JE, Bailey DB Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150(1):130–9.
Allen EG, Sherman S, Abramowitz A, Leslie M, Novak G, Rusin M, Scott E, Letz R. Examination of the effect of the polymorphic CGG repeat in the FMR1 gene on cognitive performance. Behav Genet. 2005;35(4):435–45.
Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology. 2007;69(9):851–9.
Hamlin A, Liu Y, Nguyen DV, Tassone F, Zhang L, Hagerman RJ. Sleep apnea in fragile X premutation carriers with and without FXTAS. Am J Med Genet B Neuropsychiatr Genet. 2011;156(8):923–8.
Summers SM, Cogswell J, Goodrich JE, Mu Y, Nguyen DV, Brass SD, Hagerman RJ. Prevalence of restless legs syndrome and sleep quality in carriers of the fragile X premutation. Clin Genet. 2014;86(2):181–4.
Winarni TI, Sumekar TA, Wibowo S, Hagerman RJ, Faradz SM. Premutation allele combined with caregiver distress factor increase the risk of depression in fragile X carriers: Indonesia setting. J Intellect Disabil Diagn Treat. 2019;7(4):200–8.
Sterling AM, Mailick M, Greenberg J, Warren SF, Brady N. Language dysfluencies in females with the FMR1 premutation. Brain Cogn. 2013;82(1):84–9.
Losh M, Klusek J, Martin GE, Sideris J, Parlier M, Piven J. Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. Am J Med Genet B Neuropsychiatr Genet. 2012;159(6):660–8.
Al-Hinti JT, Nagan N, Harik SI. Fragile X premutation in a woman with cognitive impairment, tremor, and history of premature ovarian failure. Alzheimer Dis Assoc Disord. 2007;21(3):262–4.
Yang JC, Simon C, Niu YQ, Bogost M, Schneider A, Tassone F, Seritan A, Grigsby J, Hagerman PJ, Hagerman RJ, Olichney JM. Phenotypes of hypofrontality in older female fragile X premutation carriers. Ann Neurol. 2013;74(2):275–83.
Hartley SL, Seltzer MM, Hong J, Greenberg JS, Smith L, Almeida D, Coe C, Abbeduto L. Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: a diathesis-stress model. Int J Behav Dev. 2012;36(1):53–61.
Chonchaiya W, Nguyen DV, Au J, Campos L, Berry-Kravis EM, Lohse K, Mu Y, Utari A, Hervey C, Wang L, Sorensen P. Clinical involvement in daughters of men with fragile X-associated tremor ataxia syndrome. Clin Genet. 2010;78(1):38–46.
Lozano R, Saito N, Reed D, Eldeeb M, Schneider A, Hessl D, Tassone F, Beckett L, Hagerman R. Aging in fragile X premutation carriers. Cerebellum. 2016;15(5):587–94.
Au J, Akins RS, Berkowitz-Sutherland L, Tang HT, Chen Y, Boyd A, Tassone F, Nguyen DV, Hagerman R. Prevalence and risk of migraine headaches in adult fragile X premutation carriers. Clin Genet. 2013;84(6):546–51.
Acknowledgements
The authors acknowledge all those women who participated in the study.
Funding
This study was financially supported by Higher Education Commission (HEC) under national research program for universities (NRPU) project # 5886.
Author information
Authors and Affiliations
Contributions
Clinical data collection, collation, and analysis: NM, MY, HT, RS, IZ, MZ, HJ, SN, MJ and SS; Genetic testing and data analysis: NM, MY, HT, RS, IZ, MZ, NK, AH, ZR and SS; Manuscript writing and revision: NM, NK, ZR and SS; Study supervision and coordination: ZR and SS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The approval of present study was obtained from the Research and Ethical Committee and Advanced Studies Research Board (ASRB) of Kohat University of Science and Technology (KUST), Kohat, Khyber Pakhtunkhwa, Pakistan. Written informed consent was obtained from all women for participation in the study.
Consent for publication
Not applicable.
Competing interests
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Meraj, N., Yasin, M., Rehman, Z.U. et al. Fragile X premutation carrier screening in Pakistani preconception women in primary care consultation. BMC Women's Health 22, 57 (2022). https://doi.org/10.1186/s12905-022-01632-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-01632-1