Abstract
Background
Psychological distress is common in patients with cancer; interfering with physical and psychological wellbeing, and hindering management of physical symptoms. Our aim was to systematically review published evidence on non-pharmacological interventions for cancer-related psychological distress, at all stages of the disease.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review was registered on PROSPERO (CRD42022311729). Searches were made using eight online databases to identify studies meeting our inclusion criteria.
Data were collected on outcome measures, modes of delivery, resources and evidence of efficacy. A meta-analysis was planned if data allowed. Quality was assessed using the Mixed Methods Appraisal Tool (MMAT).
Results
Fifty-nine studies with 17,628 participants were included. One third of studies included mindfulness, talking or group therapies. Half of all studies reported statistically significant improvements in distress. Statistically significant intervention effects on distress were most prevalent for mindfulness techniques. Four of these mindfulness studies had moderate effect sizes (d = -0.71[95% CI: -1.04, -0.37] p < 0.001) (d = -0.60 [95% CI: -3.44, -0.89] p < 0.001) (d = -0.77 [CI: -0.146, -1.954] p < 0.01) (d = -0.69 [CI: -0.18, -1.19] p = 0.008) and one had a large effect size (d = -1.03 [95% CI: -1.51, -0.54] p < 0.001). Heterogeneity of studies precluded meta-analysis. Study quality was variable and some had a high risk of bias.
Conclusions
The majority of studies using a mindfulness intervention in this review are efficacious at alleviating distress. Mindfulness—including brief, self-administered interventions—merits further investigation, using adequately powered, high-quality studies.
Systematic review registration
This systematic review is registered on PROSPERO, number CRD42022311729.
Similar content being viewed by others
Background
Psychological distress is highly prevalent in patients living with cancer. Despite its identification and management being supported by a growing body of literature, there is still no universally shared understanding of the concept of distress [1]. The National Comprehensive Cancer Network (NCCN), in their recently revised guidance, define distress in cancer as ‘….a multifactorial unpleasant experience of a psychological (i.e., cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with one’s ability to cope effectively with cancer, its physical symptoms, and its treatment’ [2]. The American Medical Association (AMA) characterises psychological distress as an inability to cope with the disease or its treatment, a lack of control and a condition distinct from anxiety and depression [3]. Distress has been proposed as the 6th vital sign in cancer care by the International Psycho-Oncology Society [4, 5] and the AMA recommend that screening for, and treating, distress should become an integral part of care plans. They propose that ‘…. novel interventions to address distress must be developed and rigorously tested…’ [3].
Huda, et al. [1] and Ridner [6] attempted to produce conceptual models for psychological distress in advanced cancer using an adaptation of the Walker and Avant model [7]. They identified defining attributes of cancer-related distress, such as anxiety, depression, loss of hope and having to come to terms with a potential life-limiting disease. The resulting consequences of these attributes are on a continuum from positive to negative, but are frequently negative. These range from mild and infrequent mood disturbances, through to situations where friends and family become affected, symptoms are exacerbated and the patient experiences a loss of coping strategies [1, 6]. Cancer-related psychological distress may be complex and can be a barrier to effective management of symptoms such as fatigue, pain and breathlessness [8]. It is also detrimental to health-related behaviours which can result in an exacerbation of mental health issues such as stress, anxiety and depression [9, 10]. Distress also affects relationships between cancer patients, family members and carers [11,12,13].
Approximately 40% of patients with cancer suffer symptoms related to distress, with higher rates reported (58%) amongst patients receiving palliative care [14]. Alternatively, 52% of patients with cancer are reported to have high levels of psychological distress when defined as ≥ 5 on the Distress Thermometer (DT), accompanied by fatigue, sadness and sleep problems [15]. Despite this high prevalence, 71% of patients with ‘significant distress’ decline help; most commonly because they consider their condition was not severe enough or because they prefer to manage it themselves [16]. Patients with cancer who are distressed frequently refuse treatment for it [17], even though alleviating distress might facilitate more effective symptom management [8]. This might be due, in part, to the stigma associated with having a mental illness which can lead to social disapproval or diminished self-esteem at a time when it is possibly most needed [18].
The importance of screening for distress is increasingly recognised as important in cancer care [19]. However, as identified by Deshields, et al. [10] there is a lack of detail or consistency in currently available guidance. A systematic review published in 2018 by McCarter, et al., revealed a lack of robust evidence for effective strategies to improve the routine implementation of distress screening and referral for patients with cancer [20]. The review also identified a lack of training in distress screening amongst clinical staff. Importantly, it has been identified that distress changes significantly at key stages during the cancer trajectory [21], and suggested that screening measures at each key stage of the disease should be ongoing for patients at the time of diagnosis, during initial treatment, following treatment and at the time of recurrence [22].
More recently a new clinical pathway has been developed and tested for the screening, assessment and management of anxiety and depression in adult cancer patients (ADAPT CP), and this might also provide a useful tool for identifying psychological distress at key disease stages [23, 24].
It has been suggested that patients with cancer might benefit physically, as well as psychologically, from appropriate interventions for distress. Improvements in psychological and physical symptoms and in overall well-being were achieved in patients who were routinely screened for distress and received appropriate interventions [25]. Distress and physical symptoms, particularly fatigue and pain, have been shown to be interrelated in patients with malignant myelodysplastic syndromes [26].
A great deal of literature on the alleviation of distress, anxiety and depression in cancer has focused on the use of cognitive behavioural therapy (CBT) or combinations of therapies including CBT techniques, such as mindfulness-based cognitive therapy (MBCT) or acceptance and commitment therapy (ACT) [27]. However, systematic reviews often reveal small effect sizes and methodological shortcomings [28] and a review of reviews of psychological interventions for distress stated that there was a lack of systematically reviewed evidence of good quality [29].
A systematic review by Warth, et al., investigated the use of brief psychological interventions (four sessions or less, over fewer than 21 days) for improving psychological well-being in palliative care. Patients reported that these were effective in improving quality of life and in reducing emotional distress and existential suffering [30]. The most commonly reported techniques in this review were life review techniques and music therapy. Although the study was in patients nearing end of life, it is likely that such interventions will be relevant for cancer patients at earlier stages of the disease too. Another systematic review by Xunlin, et al. [31] looked at mindfulness-based stress reduction techniques for a variety of psychological symptoms and quality of life in breast cancer patients and found promising improvements in distress. Other reviews have focussed on mindfulness interventions alone and found some evidence of efficacy but clinical evidence was lacking [32, 33].
The available evidence suggests that there are many potential benefits in providing effective screening for cancer-related distress and implementing interventions to alleviate it. However, the systematic reviews and meta-analyses conducted to date have not considered distress in all types of cancer and at all stages of the disease and their inclusion criteria has been relatively narrow. Therefore, the research question, which provided the basis for our methodology, was to investigate what interventions were specifically used to manage cancer-related distress at all stages of active disease. The primary aim of our systematic review was to identify and synthesise randomised controlled trials (RCTs) and non-randomised controlled clinical trials (CCTs) investigating interventions specifically targeting cancer-related psychological distress in patients with any type or stage of the disease.
Methods
This systematic review was registered on PROSPERO, number CRD42022311729.
Criteria for considering studies for this review
For the purposes of this review, the definition of psychological distress is taken from the NCCN Guidelines [2].
Inclusion criteria
Using the PICOS framework, the following criteria were used:
-
Population
-
i.
adults (age ≥ 18 years) of whom > 50% have any type/stage of cancer, currently with active disease, in any setting
-
i.
-
Interventions
-
i.
non-pharmacological interventions aimed at alleviating psychological distress
-
i.
-
Comparators
-
i.
no treatment, usual care, treatment-as-usual, waiting list or active comparators
-
i.
-
Outcome Measures
-
i.
psychological distress as a primary outcome
-
i.
-
Study design
-
i.
RCTs and CCTs
-
ii.
Studies with primarily quantitative data, or studies with mixed-methodologies.
-
i.
Exclusion criteria
Types of studies
-
i.
Qualitative studies with no quantitative data
-
ii.
Case studies, surveys, audits, and uncontrolled studies
-
iii.
Protocols
-
iv.
Systematic reviews or narrative reviews
-
v.
Grey literature
-
vi.
Letters, editorials, and conference abstracts.
Study populations
-
i.
Animal studies
-
ii.
Studies including > 50% of persons under the age of 18 years.
-
iii.
Populations stated to be ‘cancer survivors’ or having undergone curative treatment (i.e., has either had cancer and is deemed to be cured, or has completed treatment and has no evidence of active disease).
We also excluded any studies not written in, or translated into English.
Data sources
The following electronic databases were searched for articles published from 2002 to the present (2022):
-
(a)
MEDLINE (via OVID)
-
(b)
Web of Science
-
(c)
Scopus
-
(d)
CINAHL (via EBSCO)
-
(e)
PubMed
-
(f)
APA PsycINFO (OVID)
-
(g)
AMED (OVID)
-
(h)
CENTRAL (Cochrane)
Additional references were included from an initial scoping review if not identified during the main searches.
Search strategy
All online search strategies are included in Appendix A (Supplementary information).
Reference lists of other systematic reviews were also screened against inclusion criteria.
The results of searches and screening were reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [34, 35] (PRISMA checklist: Appendix B (Supplementary information)).
Data management and synthesis
Management of data was achieved using the Covidence systematic review software [36]. Two independent reviewers (CP and EC) screened studies which met the eligibility criteria by title and abstract. Full-text review was carried out if studies were deemed eligible or where eligibility was unclear. Where reviewers disagreed on inclusion/exclusion, a third author acted as arbiter. Data collection was completed using a template created which was specifically designed for this review (Appendix C (Supplementary information)).
A narrative synthesis was planned. Clinically and statistically significant differences in distress due to the intervention would be reported for included studies. Where effect sizes and confidence intervals were not included in the study reports, these were calculated provided the necessary data were available. If data allowed, meta-analysis would be utilised to examine change in distress outcomes (effect size (Standard Mean Difference)) for different interventions. Further subgroup analysis was not planned.
Quality and risk of bias assessment
Quality was assessed using the Mixed Methods Appraisal Tool (MMAT) [37] (Appendix D(a) (Supplementary information)). Three additional questions were added to enable further appraisal of overall methodological quality and risk of bias. These were: ‘Was attrition/exclusion data reported?’, ‘Were adverse events reported?’ and ‘Was an appropriate sample size calculation carried out?’ Reporting of attrition and adverse event are elements of risk of bias from selective reporting, as outlined by Higgins, et al. in the Cochrane Handbook [38]. The issue of sample size is the subject of much debate and for the purposes of meta-analysis it has been stated that individual studies should have arms of ≥ 200 participants, or pooled events of ≥ 500 otherwise they are at high risk of bias and likely to produce imprecise effect estimates [39, 40].
Results
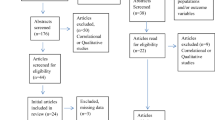
One thousand one hundred sixteen records were screened and fifty-nine studies with 17,628 participants were included. The literature screening process was recorded and illustrated according to PRISMA guidelines in the flow diagram below (Fig. 1).
PRISMA flow diagram [34]
Characteristics of included studies
Table 1 summarises study characteristics, interventions and comparators, measures of distress used and whether the results were statistically significant. Effect sizes are included where these were given or could be calculated from the available data.
Of the 59 included studies, 45 (78%) were randomised controlled trials (RCTs) two were CCTs and 12 were classed as ‘others’ and included cluster designs, pragmatic trials and quasi-experimental controlled studies. None of the trials were described as mixed-methodology studies although some did contain minimal qualitative data. Twenty-four studies (41%) were based in the USA or Europe. Participant characteristics between studies were variable by gender, type and stage of cancer, including patients in the early stages of cancer through to those in palliative care. Across all included studies the total number of participants randomised (in RCTs) or consented (in CCTs) was 17,628. The number of participants per study ranged from 30 to 3133 (including cluster studies and dyads) and the mean number of participants per study was 298.1 (median 122).
A high degree of heterogeneity was evident across the included studies in relation to the interventions, dose, the outcome measures used and follow-up times. Seventeen different measures of distress were used in the included studies. Not all these measures have been specifically validated for cancer populations.
Criteria suggested by Borenstein, et al., was used to decide whether pooling data for a meta-analysis was appropriate [101]. These criteria include a subjective assessment of the similarity of studies in terms of patients, inclusion criteria and baseline characteristics, and comparing studies with the same interventions, comparators and outcomes. Only three RCTs met the criteria for similarity of patients, inclusion criteria, baseline characteristics and outcome measures [43, 44, 47] and all were higher quality studies as evaluated by MMAT [37]. However, Liu, et al. [44] had less than 80% adherence to the intervention and Park, et al. [47] aborted recruitment before the target was reached. There was also uncertainty/doubt as to the method of randomisation used in Compen, et al. [43]. These 3 studies also looked at different cancer types and stages. Patients were recruited in different ways with notable differences in gender proportions. Also, the total number of pooled events from these 3 studies would have been < 500 which, according to Moore, et al. [39, 102] would be insufficient. Heterogeneity and small numbers of studies therefore precluded meta-analysis.
Quality analysis and risk of bias
The MMAT tool for quality assessment was used independently by two authors (EC and CP). Any disparities were discussed and agreed (Appendix D(b) (Supplementary information)). Of the 59 included studies, 35 (59.3%) lacked outcome assessor blinding and 27 (45.8%) studies had < 80% adherence to the intervention. An important finding was that 33 (55.9%) studies did not report any sample-size calculation so there was no indication of statistical power. Also, 53 (89.8%) of studies did not record the presence or absence of adverse events. Failure to record and report adverse events is an important omission, especially in advanced cancer, because some interventions may result in greater distress due to an increased focus and attention of the patient on their disease and its associated problems (Paley CA: Investigations into the use of acupuncture for treating cancer-induced bone pain in adults, unpublished).
Other study design and quality issues included a lack of explanation regarding randomisation methods and some did not report whether study arms had comparable demographics and baseline measurements.
In terms of methodological quality, only three studies, Araújo, et al., [88] Compen, et al. [43] and Semple, et al. [71] met all the basic MMAT criteria but did not positively meet the additional questions added regarding reporting adverse events, and although Semple et al. did calculate sample size, this study was not an RCT and patients self-selected their study arm, thus introducing bias. Only the study by Araújo, et al. [88] positively met all the MMAT criteria and additional questions but was still a relatively small study with only 50 participants in total. Semple et al. [71] was also a small study with 54 participants. Compen, et al. [43] had a larger sample of 245 participants, but these were randomised to 3 arms: face-to-face- group MBCT, internet-based eMBCT and treatment as usual (TAU). Overall, the methodological quality of studies included in this review was low, mainly due to small sample-sizes and a lack of outcome assessor blinding in more than one third of studies. Unclear reporting and baseline differences in study groups were also prevalent.
The evidence for reductions in cancer-related distress
For ease of reference, the included studies were divided into broad intervention groups: mindfulness, talking/communication/CBT/group therapies, screening/assessment only, expressive/creative writing, psychological/psychosocial therapies, dignity therapy, web-based/mobile app, life review, problem-solving/education, couples (dyadic) therapies, physical therapies, art/music and others (uncategorised) (Table 1).
Of the 59 included studies, 29 (54.2%) reported statistically significant reductions in psychological distress at follow-up. The remaining 30 studies did not find that the interventions made any significant changes in distress. Within the studies reporting significant changes were three anomalies: the study by Sandgren, et al. [85] used telephone therapy, but both intervention groups and control group showed a similar decline in levels of distress; Mahendran, et al. [70] compared a brief psychosocial intervention with a control condition, but the level of distress in the intervention group was significantly higher at baseline so the results were skewed in favour of the intervention; and Clark, et al., [69] had a small sample size (n = 35) with a 26% attrition rate, leaving a small and under-powered study.
Of the remaining 26 studies showing statistically significant intervention effects, not all included effect sizes or provided data from which these could be calculated. Where data was available, effect sizes were calculated using Cohen’s d, (standardised mean difference), however, it is important to acknowledge that effect sizes are only meaningful for comparison if there is certainty that compared studies are similar in study design [103]. Cohen’s d is conventionally regarded as small at 0.2 or less, 0.5 as medium, and 0.8 as large [104], although these definitions are somewhat arbitrary [105, 106].
The mindfulness category was the largest group comprising variations on one specific approach (mindfulness) and included nine studies. All nine were RCTs and six out of nine studies showed positive effects, reaching statistical significance. Only one of these studies by Park, et al., had a large effect size (d = -1.03; [CI: -1.51 to -0.54] (p < 0.001)) at 12 weeks post-intervention when mindfulness-based cognitive therapy (MBCT) was compared with a waiting list control group in breast cancer patients [47], although recruitment was aborted before the target sample size was reached and there was no assessor blinding. Four studies had moderate effect-sizes [43, 44, 46, 48] and of these, Compen, et al. had the largest sample size and compared face to face mindfulness training (MBCT) and online MBCT (eMBCT) against a treatment-as-usual (TAU) control group. This was a relatively high-quality study, meeting all MMAT criteria and with a small effect size of d = -0.45 [95% CI: -0.83, -0.14 (p < 0.001)] for MBCT but reaching a moderate effect size d = -0.71 [95% CI: -1.04, -0.37 (p < 0.001)] for eMBCT. All mindfulness studies with a moderate or large effect-size used the Hospital Anxiety and Depression Scale (HADS) as a primary outcome measure, although variation in methodologies was still present, which precluded meta-analysis. One other study in the mindfulness category with statistically significant improvements in distress had a small effect size (d = -0.43) and did not provide data with which to calculate confidence intervals [49].
There were ten studies within the talking/communication/group therapies category. In this broad group, six studies out of ten reported significant intervention-related reductions in distress [50,51,52,53,54,55,56,57,58,59]. Mertz, et al. had a large effect size for screening and counselling [58], however this was a pilot study and had a small sample size (n = 50) with only 41 participants completing the intervention. Quality assessment using MMAT showed that the randomisation method for this study was unclear and there was no evidence of assessor blinding or of a sample-size calculation. Hejazi, et al. also demonstrated a large effect size for a communication programme in elderly cancer patients but again, the uncalculated sample size was small (n = 64), randomisation methodology was unclear and there was no outcome assessor blinding. They also omitted to report adverse events. A study by Chambers, et al. had a moderate effect size for web-based cognitive behavioural therapy (CBT) [55] but did not have comparable groups in the study arms, failed to report outcome assessor blinding and ≤ 80% of participants adhered to the intervention. Two other studies in this group showed no significant effects on distress. A pragmatic trial by Gregoire, et al. [95], which was categorised in the ‘others’ group, because it had multiple arms of different interventions, should be mentioned here because it had a CBT arm. This found no statistically significant changes in distress following the intervention, although it was significant for yoga and self-hypnosis. The CBT study arm also had very few participants (n = 10).
Within this category there were there were three statistically significant group interventions for distress [51, 52, 59]. The two studies by Andersen, et al. [51, 52] did not provide a full data set. The study by Taylor, et al. [59] described the effect size as “small”, with no figures provided. This study also had a small sample size and did not meet many of the MMAT quality standards, with no blinding, incomplete outcome data and fewer than 80% of participants completing the intervention.
Of all the other categories, there were two other studies with large effect sizes. Nezu, et al. [83] used problem-solving therapy with (PST) and without (PST-SO) a significant other accompanying the patient, compared with a waiting-list control. Both interventions were significant for reduction in distress immediately post-intervention, at 6 months and 1 year using POMS as an outcome measure. The effect sizes calculated for the purposes of this systematic review at post-intervention were large: d = 1.993 (PST) and d = 1.643 (PST-SO), and at 12 months: d = 2.17 and d = 2.04 respectively (all P < 0.001). Nevertheless, the study was of low quality as the authors did not report outcome assessor blinding or sample size calculation and the study had a small number of participants. Kovačič, et al. [89] used a yoga intervention compared with standard physiotherapy only but although the effect sizes were large the study was very small with only 32 patients in total. No sample-size calculation was carried out.
Other studies meeting MMAT criteria, coupled with significant improvements in distress, included a yoga intervention (Araújo, et al. [88]) and a psychosocial intervention (Semple, et al. [71]) but the sample sizes for both studies were very small. Han, et al. [96] compared Naikan and Morita therapy [107] against usual care (n = 130) and had a large effect size immediately post-treatment for improvements in distress amongst Chinese breast cancer patients. Naikan and Morita therapy has Japanese/Buddhist roots and requires absolute commitment from the patient who puts him/herself under the total direction of the therapist.
The remaining studies across categories used a variety of interventions in various combinations and in different settings, including face-to-face therapies and web-or app-based interventions. Patient groups varied from all cancers at all stages to specific cancer sites or specified stages; e.g., stages I-III or stage IV metastatic cancer.
Discussion
Summary of main findings
This systematic review has shown a wide variation in approaches to alleviating distress in patients with cancer. There was no definitive consensus on any one intervention or means of delivery, although therapies involving mindfulness-based approaches were the most frequently researched with some evidence of efficacy, followed by talking or communication-based therapies and interventions conducted in groups with weaker evidence of benefit. In this review, mindfulness interventions were generally of high methodological quality. However, as stated in a systematic review and meta-analysis by Faller, et al., many studies with large effect sizes mostly have small sample sizes which will tend to inflate effect size estimation and should therefore be interpreted with caution [28].
Implications of this review
Only three studies from the 26 showing statistically significant intervention-related reductions in distress were rated positive on all MMAT quality standards, and of these, the largest study (Compen, et al. [43]) used MBCT compared with eMBCT and TAU, was the most promising. However, both intervention groups were resource-heavy, requiring input from trained therapists; particularly the face-to-face group which included 8 weekly group sessions along with home practice. The other two high quality studies with significant benefits for distress involved a yoga intervention (Araújo, et al. [88]) and a psychosocial intervention (Semple, et al. [71]); again, both resource-heavy in terms of staff training and time. In a recent unpublished survey of hospices in England, it was identified that healthcare professionals perceive there to be substantial gaps in training and supervision for meeting the psychological assessment and treatment needs of patients [108]. The survey identified wide variations and major gaps in provision of psychological screening/assessment and intervention across this sector, which suggests that resource-heavy interventions do not represent a practical way forward.
Taking resources into consideration, brief interventions for distress, especially those which can be self-administered after training, are likely to be the most feasible in practice. In 2020, Compen, et al., looked at the cost-effectiveness of mindfulness-based cognitive therapy, either online or face to face, compared with treatment as usual [109]. They revealed positive findings, especially for an internet-based intervention, which also has the advantage of convenience for patients and staff. Mindfulness interventions can be very brief (as little as 5 min) and can be taught relatively quickly to patients and their families or carers so that the techniques can be used flexibly, as required. Such interventions which allow self-management of symptoms have potential to be widely incorporated into routine care. Mindfulness techniques have also been found to improve motivation for the adoption of healthy lifestyle changes and enhancement of interpersonal relationships [110]. Evidence suggests that patients prefer self-help strategies to manage their distress when needed [111], but there is little evidence supporting self-guided interventions and further evidence is needed to either support the efficacy of current strategies or suggest new ones [112].
Of the studies showing benefit, nine interventions were under 1 h duration and sessions continued for 1 month or less, but only three of these demonstrated statistically significant improvements in distress [46, 56, 62]. One of these studies used an intervention that was described as ‘brief’ (5 min of mindfulness) [46]. In the studies showing no significant effect on psychological distress there was another 5 min mindful breathing intervention which demonstrated significant and rapid reductions in ‘perceived stress’, rather than distress [42]. Six other interventions were delivered on an as-needed basis or self-managed. These findings were similar to another systematic review by Xunlin, et al., [31] which included 29 randomised controlled trials (RCTs) of mindfulness interventions to improve quality of life in patients with cancer. Their review showed that mindfulness techniques are effective in reducing anxiety, depression, and stress in cancer patients and survivors.
Comparison with other systematic reviews
Other meta-analyses have specifically examined the effects of mindfulness-based stress reduction techniques, and these show significant effects on distress [28, 33, 113]. Cillessen, et al. included 29 RCTs with a total of 3274 patients [33]. It demonstrated small, but significant treatment effects for follow-up of up to 6 months when a manual for the intervention was followed and when patient groups were younger (mean age 55 years), compared with a passive control group [33]. A meta-analysis conducted by Haller, et al., also found significant effects of mindfulness-based interventions on health-related quality of life, fatigue, sleep, stress, anxiety, and depression in women with breast cancer although the effect sizes were small [32]. Faller, et al., conducted a systematic review and meta-analysis of psycho-oncologic interventions for emotional distress and quality of life in adults with cancer and concluded that there were small to medium effect sizes for individual, group, and couples psychotherapy, psychoeducation, and relaxation training, but there were methodological shortcomings including study quality and risk of bias [28]. Most studies incorporated CBT techniques, usually in combination with other techniques, such as coping strategies, but none used mindfulness to alleviate distress.
It has been suggested that because CBT techniques are commonly used for distress in cancer patients and have found to be effective [114], it might be useful to conduct research using both techniques in combination [33]. Our review did not reveal many studies using CBT as a single technique, although it was used in combination with mindfulness in three studies, as MBCT and Park, et al. reported large effect sizes for the intervention and Compen et al. demonstrated moderate effects [41, 43, 47]. As discussed above, implementation of CBT is more resource-dependent, usually requiring face-to-face contact.
As previously described, Warth, et al., conducted a similar review but only included patients in the advanced stages of terminal illness (not necessarily cancer) with a prognosis of < 3 months [30], which precluded direct comparisons. Also, the interventions included were only those with ≤ 4 sessions and < 21 days. Four of the papers met the inclusion criteria for our review [46, 74, 76, 82]. Warth, et al. demonstrated significant effects on emotional and existential distress and quality of life. However, the authors acknowledged a number of limitations, including baseline differences, a generally low methodological quality and possibly an underpowered meta-regression analysis. The authors did not examine follow-up data. They concluded that that psychosocial techniques are effective, and that these include interventions such as mindfulness, dignity therapy, life review, and creative-based therapies.
It was interesting that none of our included studies investigated acceptance and commitment therapy (ACT), as it has recently become more widely used and this was highlighted in a cross-sectional survey of therapeutic approaches used in UK hospices [115]. A systematic review investigating the use of ACT for psychological and physical symptoms amongst cancer patients revealed large effect sizes on psychological distress in cancer patients, although this was predominantly in younger patients who lived in eastern countries and received therapy for longer [27].
An important consideration is the country of origin for each study. Those carried out in regions where healthcare has to be paid for (e.g., the USA or parts of the far east) might be biased in favour of patients who had access to cancer treatment and were therefore recruited into research studies during clinic appointments. In addition, cultural differences make comparisons of interventions problematic.
High attrition rates are frequently a problem in cancer-related studies, particularly where patients are in the advanced stages of the disease with a high symptom burden or where patients lack social support from family and friends [116, 117]. Some studies have recorded drop-out rates of up to 50% [116]. This might suggest that brief interventions for distress, and particularly those which can be self-administered as needed, would be more practical and have better adherence, especially in patients who are in the advanced stages of cancer.
Limitations of this review
This review had a number of limitations. The inclusion criteria restricted the review to studies published in the English language and our searches only included published literature. The inclusion criteria were very broad across study methodologies and populations to enable identification of as many relevant studies as possible. This added complexity when comparing the efficacy of studies. The resulting heterogeneity of studies precluded meta-analysis. Restricting our searches to RCTs would have enabled us to use the Cochrane Risk of Bias tool (ROB2) and the GRADE quality assessment rather than the MMAT tool which did not provide as much sensitivity for RCTs. However, this would have narrowed the scope of the review and not given such a wide picture of the range of interventions being used for psychological distress and how they were delivered. Also, excluding studies involving cancer survivors, according to our narrower definition of survivors as those not undergoing active treatment may have resulted in some relevant interventions, such as ACT, being missed; although each title, abstract and full text was examined by two authors. Nevertheless, the review has revealed some interesting and useful information which has allowed us to suggest some implications for clinical practice and possible directions for future research.
Implications for clinical practice
Mindfulness interventions appear to be effective and appropriate for people with cancer, particularly those with advanced disease. Mindfulness techniques are relatively quick to teach, and can be self-administered outside medical settings by patients and carers. They can be taught face to face, via the internet and practised at home by patients or carers who have had some instruction. We suggest that brief mindfulness interventions, might also be suitable for use when needed in palliative and end-of-life care when patients are often unable to cope with more lengthy interventions or activities requiring sustained concentration.
Implications for research
Further and more robust evidence is required to support the findings of this review. A clear international consensus of psychological distress needs to be established, along with core, validated outcome measures. Studies should be adequately powered and of high methodological quality to reduce bias and provide reliable evidence-based guidance for those working with this patient group. There is a growing body of evidence to indicate that mindfulness interventions are beneficial to patients, and feasible to implement and utilise. Future studies should focus on the efficacy of self-administered, brief mindfulness interventions for psychological distress in patients with advanced disease.
Conclusions
The majority of studies using mindfulness interventions in this review are efficacious at alleviating distress. We suggest that brief mindfulness interventions might be appropriate for clinical implementation in advanced disease and palliative care. Our review suggests that therapist-guided or online interventions show greater efficacy in reducing distress but self-directed mindfulness interventions have merit in by allowing patients to use these techniques when needed. In conclusion, mindfulness interventions merit further investigation using adequately powered, high-quality studies.
Availability of data and materials
Review data are available from corresponding author; C. A. Paley (c.a.paley@leeds.ac.uk).
Abbreviations
- ACT:
-
Acceptance and commitment therapy
- AMA:
-
American Medical Association
- CBT:
-
Cognitive behavioural therapy
- CCT:
-
Controlled Clinical Trial
- DT:
-
Distress thermometer
- eMBCT:
-
Online (electronic) mindfulness-based cognitive therapy
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluations
- HADS:
-
Hospital Anxiety and Depression Scale
- MBCT:
-
Mindfulness-based cognitive therapy
- MBSR:
-
Mindfulness-based stress reduction
- MMAT:
-
Mixed methods appraisal tool
- NCCN:
-
National Comprehensive Cancer Network
- PST:
-
Problem-solving therapy with a significant other
- PST-SO:
-
Problem-solving therapy without a significant other
- POMS:
-
Profile of mood states
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT:
-
Randomised controlled trial
- RoB2:
-
Cochrane risk of bias tool
- TAU:
-
Treatment as usual
- UC:
-
Usual care
References
Huda N, Shaw MK, Chang HJ. Psychological Distress Among Patients With Advanced Cancer: A Conceptual Analysis. Cancer Nurs. 2022;45:E487-e503. https://doi.org/10.1097/ncc.0000000000000940.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management V.2.2023. © National Comprehensive Cancer Network, Inc. 2023. NCCN.org. Accessed 10 May 2023.
LeBlanc TW, Kamal AH. Assessing psychological toxicity and patient-reported distress as the sixth vital sign in cancer care and clinical trials. AMA J Ethics. 2017;19:460–6. https://doi.org/10.1001/journalofethics.2017.19.5.stas1-1705. 20170501.
IPOS. IPOS international standard of quality cancer care, https://www.ipos-society.org/about/quality (2010). Accessed 19 July 2022.
Watson M, Bultz BD. Distress, the 6th vital sign in cancer care. Psycho-Oncologie. 2010;4:159–63. https://doi.org/10.1007/s11839-010-0269-z.
Ridner SH. Psychological distress: concept analysis. J Adv Nurs. 2004;45:536–45. https://doi.org/10.1046/j.1365-2648.2003.02938.x.
Walker L, Avant K. Concept analysis. Strategies for theory construction in nursing. 6th ed. NY: Pearson; 2019.
Chapman EJ, Pini S, Edwards Z, et al. Conceptualising effective symptom management in palliative care: a novel model derived from qualitative data. BMC Palliative Care. 2022; 21. https://doi.org/10.1186/s12904-022-00904-9.
Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life care: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036–44. https://doi.org/10.1016/j.ejca.2010.03.033. 20100504.
Deshields TL, Wells-Di Gregorio S, Flowers SR, et al. Addressing distress management challenges: recommendations from the consensus panel of the american psychosocial oncology society and the association of Oncology social work. CA Cancer J Clin. 2021;71:407–36. https://doi.org/10.3322/caac.21672. 20210524.
Kaye J, Gracely E. Psychological distress in cancer patients and their spouses. J Cancer Educ. 1993;8:47–52. https://doi.org/10.1080/08858199309528207.
Gröpper S, van der Meer E, Landes T, et al. Assessing cancer-related distress in cancer patients and caregivers receiving outpatient psycho-oncological counseling. Support Care Cancer. 2016;24:2351–7. https://doi.org/10.1007/s00520-015-3042-9.
Segrin C, Badger TA. Psychological distress in different social network members of breast and prostate cancer survivors. Res Nurs Health. 2010;33:450–64. https://doi.org/10.1002/nur.20394.
Bultz BD, Carlson LE. Emotional distress: the sixth vital sign–future directions in cancer care. Psychooncology. 2006;15:93–5. https://doi.org/10.1002/pon.1022.
Mehnert A, Hartung TJ, Friedrich M, et al. One in two cancer patients is significantly distressed: prevalence and indicators of distress. Psycho-oncology (Chichester, England). 2018;27:75–82. https://doi.org/10.1002/pon.4464.
Clover KA, Mitchell AJ, Britton B, et al. Why do oncology outpatients who report emotional distress decline help? Psychooncology. 2015;24:812–8. https://doi.org/10.1002/pon.3729. 20141211.
Carolan CM, Smith A, Davies GR, et al. Seeking, accepting and declining help for emotional distress in cancer: A systematic review and thematic synthesis of qualitative evidence. Eur J Cancer Care. 2018;27:e12720. https://doi.org/10.1111/ecc.12720.
Corrigan P. How stigma interferes with mental health care. Am Psychol. 2004;59:614–25. https://doi.org/10.1037/0003-066x.59.7.614.
Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4–12.
McCarter K, Britton B, Baker AL, et al. Interventions to improve screening and appropriate referral of patients with cancer for psychosocial distress: systematic review. BMJ Open. 2018;8:e017959. https://doi.org/10.1136/bmjopen-2017-017959.
Holland J. NCCN practice guidelines for the management of psychosocial distress. Natl Comprehen Cancer Network Oncol. 1999;13(5A):113–47.
Ziegler L, Hill K, Neilly L, et al. Identifying psychological distress at key stages of the cancer illness trajectory: a systematic review of validated self-report measures. J Pain Symptom Manage. 2011;41:619–36. https://doi.org/10.1016/j.jpainsymman.2010.06.024.
Butow P, Shaw J, Shepherd HL, et al. Comparison of implementation strategies to influence adherence to the clinical pathway for screening, assessment and management of anxiety and depression in adult cancer patients (ADAPT CP): study protocol of a cluster randomised controlled trial. BMC Cancer. 2018;18:1077. https://doi.org/10.1186/s12885-018-4962-9.
Butow P, Shepherd HL, Cuddy J, et al. Acceptability and appropriateness of a clinical pathway for managing anxiety and depression in cancer patients: a mixed methods study of staff perspectives. BMC Health Serv Res. 2021;21:1243. https://doi.org/10.1186/s12913-021-07252-z.
Watson L, Groff S, Tamagawa R, et al. Evaluating the impact of provincial implementation of screening for distress on quality of life, symptom reports, and psychosocial well-being in patients with cancer. J Natl Compr Canc Netw. 2016;14:164–72. https://doi.org/10.6004/jnccn.2016.0019.
Troy JD, de Castro CM, Pupa MR, et al. Patient-reported distress in myelodysplastic syndromes and its association with clinical outcomes: a retrospective cohort study. J Natl Compr Canc Netw. 2018;16:267–73. https://doi.org/10.6004/jnccn.2017.7048.
Zhao C, Lai L, Zhang L, et al. The effects of acceptance and commitment therapy on the psychological and physical outcomes among cancer patients: A meta-analysis with trial sequential analysis. J Psychosom Res. 2021;140:110304. https://doi.org/10.1016/j.jpsychores.2020.110304.
Faller H, Schuler M, Richard M, et al. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol. 2013;31:782–93. https://doi.org/10.1200/jco.2011.40.8922. 20130114.
Lepore SJ, Coyne JC. Psychological interventions for distress in cancer patients: a review of reviews. Ann Behav Med. 2006;32:85–92. https://doi.org/10.1207/s15324796abm3202_2.
Warth M, Kessler J, Koehler F, et al. Brief psychosocial interventions improve quality of life of patients receiving palliative care: A systematic review and meta-analysis. Palliat Med. 2019;33:332–45. https://doi.org/10.1177/0269216318818011.
Xunlin NG, Lau Y, Klainin-Yobas P. The effectiveness of mindfulness-based interventions among cancer patients and survivors: a systematic review and meta-analysis. Support Care Cancer. 2020;28:1563–78. https://doi.org/10.1007/s00520-019-05219-9. 20191213.
Haller H, Winkler MM, Klose P, et al. Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Acta Oncol. 2017;56:1665–76. https://doi.org/10.1080/0284186x.2017.1342862. 20170707.
Cillessen L, Johannsen M, Speckens AEM, et al. Mindfulness-based interventions for psychological and physical health outcomes in cancer patients and survivors: a systematic review and meta-analysis of randomized controlled trials. Psychooncology. 2019;28:2257–69. https://doi.org/10.1002/pon.5214. 20190911.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
Veritas. Covidence systematic review software. 2022. www.covidence.org. Accessed 24 Mar 2022.
Hong QN, Gonzalez-Reyes A, Pluye P. Improving the usefulness of a tool for appraising the quality of qualitative, quantitative and mixed methods studies, the Mixed Methods Appraisal Tool (MMAT). J Eval Clin Pract. 2018;24:459–67. https://doi.org/10.1111/jep.12884. 20180221.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane; 2022. Available from www.training.cochrane.org/handbook.
Moore AR, Gavaghan D, Tramèr RM, et al. Size is everything–large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78:209–16. https://doi.org/10.1016/s0304-3959(98)00140-7.
Moore RA, Derry S, McQuay HJ, et al. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain. 2010;149:173–6. https://doi.org/10.1016/j.pain.2009.08.007. 20090911.
Chambers SK, Occhipinti S, Foley E, et al. Mindfulness-Based Cognitive Therapy in Advanced Prostate Cancer: A Randomized Controlled Trial. J Clin Oncol. 2017;35:291–7. https://doi.org/10.1200/jco.2016.68.8788.
Chui PL, Wai S, Lai LL, et al. Mindful Breathing: Effects of a Five-Minute Practice on Perceived Stress and Mindfulness Among Patients With Cancer. Clin J Oncol Nurs. 2021;25:174–80. https://doi.org/10.1188/21.Cjon.174-180.
Compen F, Bisseling E, Schellekens M, et al. Face-to-face and internet-based mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2018;36:2413–21. https://doi.org/10.1200/jco.2017.76.5669.
Liu Z, Li M, Jia Y, et al. A randomized clinical trial of guided self-help intervention based on mindfulness for patients with hepatocellular carcinoma: effects and mechanisms. Jpn J Clin Oncol. 2022;52:227–36. https://doi.org/10.1093/jjco/hyab198.
Milbury K, Li Y, Durrani S, et al. A mindfulness-based intervention as a supportive care strategy for patients with metastatic non-small cell lung cancer and their spouses: results of a three-arm pilot randomized controlled trial. Oncologist. 2020;25:e1794–802. https://doi.org/10.1634/theoncologist.2020-0125.
Ng CG, Lai KT, Tan SB, et al. The effect of 5 minutes of mindful breathing to the perception of distress and physiological responses in palliative care cancer patients: a randomized controlled study. J Palliat Med. 2016;19:917–24. https://doi.org/10.1089/jpm.2016.0046.
Park S, Sato Y, Takita Y, et al. Mindfulness-based cognitive therapy for psychological distress, fear of cancer recurrence, fatigue, spiritual well-being, and quality of life in patients with breast cancer-a randomized controlled trial. J Pain Symptom Manage. 2020;60:381–9. https://doi.org/10.1016/j.jpainsymman.2020.02.017. 20200224.
Schellekens MPJ, van den Hurk DGM, Prins JB, et al. Mindfulness-based stress reduction added to care as usual for lung cancer patients and/or their partners: A multicentre randomized controlled trial. Psychooncology. 2017;26:2118–26. https://doi.org/10.1002/pon.4430.
Wurtzen H, Dalton SO, Christensen J, et al. Effect of mindfulness-based stress reduction on somatic symptoms, distress, mindfulness and spiritual wellbeing in women with breast cancer: Results of a randomized controlled trial. Acta Oncol. 2015;54:712–9. https://doi.org/10.3109/0284186x.2014.997371.
Acevedo-Ibarra JN, Juárez-García DM, Espinoza-Velazco A, et al. Cognitive Behavioral Stress Management intervention in Mexican colorectal cancer patients: Pilot study. Psycho-Oncology. 2019;28:1445–52. https://doi.org/10.1002/pon.5094.
Andersen BL, Farrar WB, Golden-Kreutz D, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;21:953–61. https://doi.org/10.1016/j.bbi.2007.03.005.
Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004;22:3570–80. https://doi.org/10.1200/jco.2004.06.030.
Boesen EH, Karlsen R, Christensen J, et al. Psychosocial group intervention for patients with primary breast cancer: a randomised trial. Eur J Cancer. 2011;47:1363–72. https://doi.org/10.1016/j.ejca.2011.01.002.
Boesen EH, Ross L, Frederiksen K, et al. Psychoeducational intervention for patients with cutaneous malignant melanoma: a replication study. J Clin Oncol. 2005;23:1270–7. https://doi.org/10.1200/jco.2005.05.193.
Chambers SK, Ritterband LM, Thorndike F, et al. Web-delivered cognitive behavioral therapy for distressed cancer patients: randomized controlled trial. J Med Internet Res. 2018;20:e42. https://doi.org/10.2196/jmir.8850.
Hejazi F, Bahrami M, Keshvari M, et al. The effect of a communicational program on psychological distress in the elderly suffering from cancer. Iran J Nurs Midwifery Res. 2017;22:201–7. https://doi.org/10.4103/1735-9066.208158.
Manne SL, Virtue SM, Ozga M, et al. A comparison of two psychological interventions for newly-diagnosed gynecological cancer patients. Gynecol Oncol. 2017;144:354–62. https://doi.org/10.1016/j.ygyno.2016.11.025. 20161123.
Mertz BG, Dunn-Henriksen AK, Kroman N, et al. The effects of individually tailored nurse navigation for patients with newly diagnosed breast cancer: a randomized pilot study. Acta Oncol. 2017;56:1682–9. https://doi.org/10.1080/0284186x.2017.1358462.
Taylor KL, Lamdan RM, Siegel JE, et al. Psychological adjustment among African American breast cancer patients: One-year follow-up results of a randomized psychoeducational group intervention. Health Psychol. 2003;22:316–23. https://doi.org/10.1037/0278-6133.22.3.316.
Braeken AP, Kempen GI, Eekers DB, et al. Psychosocial screening effects on health-related outcomes in patients receiving radiotherapy A cluster randomised controlled trial. Psychooncology. 2013;22:2736–46. https://doi.org/10.1002/pon.3340.
Carlson LE, Waller A, Groff SL, et al. Online screening for distress, the 6th vital sign, in newly diagnosed oncology outpatients: randomised controlled trial of computerised vs personalised triage. Br J Cancer. 2012;107:617–25. https://doi.org/10.1038/bjc.2012.309. 20120724.
Carlson LE, Groff SL, Maciejewski O, et al. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. J Clin Oncol. 2010;28:4884–91. https://doi.org/10.1200/jco.2009.27.3698. 20101012.
Oerlemans S, Arts LPJ, Kieffer JM, et al. Web-based return of individual patient-reported outcome results among patients with lymphoma: randomized controlled trial. J Med Internet Res. 2021;23:e27886. https://doi.org/10.2196/27886.
O’Hea E, Kroll-Desrosiers A, Cutillo AS, et al. Impact of the mental health and dynamic referral for oncology (MHADRO) program on oncology patient outcomes, health care utilization, and health provider behaviors: A multi-site randomized control trial. Patient Educ Counsel. 2020;103:607–16. https://doi.org/10.1016/j.pec.2019.10.006. Health & Mental Health Services 3370.
de Moor C, Sterner J, Hall M, et al. A pilot study of the effects of expressive writing on psychological and behavioral adjustment in patients enrolled in a Phase II trial of vaccine therapy for metastatic renal cell carcinoma. Health Psychol. 2002;21:615–9. https://doi.org/10.1037//0278-6133.21.6.615.
Mosher CE, DuHamel KN, Lam J, et al. Randomised trial of expressive writing for distressed metastatic breast cancer patients. Psychol Health. 2012;27:88–100. https://doi.org/10.1080/08870446.2010.551212. Behavioral & Psychological Treatment of Physical Illness 3361.
Nesterova D, Zhu J, Kramer C, et al. Group-led creative writing and behavioural health in cancer: a randomised clinical trial. BMJ Support Palliat Care. 2021. https://doi.org/10.1136/bmjspcare-2020-002463
Stanton AL, Danoff-Burg S, Sworowski LA, et al. Randomized, controlled trial of written emotional expression and benefit finding in breast cancer patients. J Clin Oncol. 2002;20:4160–8.
Clark PG. Decreasing psychological distress in cancer inpatients using FLEX Care®: a pilot study. Soc Work Health Care. 2010;49:872–90. https://doi.org/10.1080/00981389.2010.499826.
Mahendran R, Lim HA, Tan JYS, et al. Efficacy of a brief nurse-led pilot psychosocial intervention for newly diagnosed Asian cancer patients. Support Care Cancer. 2015;23:2203–6. https://doi.org/10.1007/s00520-015-2771-0.
Semple CJ, Dunwoody L, Kernohan WG, et al. Development and evaluation of a problem-focused psychosocial intervention for patients with head and neck cancer. Support Care Cancer. 2009;17:379–88. https://doi.org/10.1007/s00520-008-0480-7.
Wang S, Huang H, Wang L, et al. A psychological nursing intervention for patients with thyroid cancer on psychological distress and quality of life: a randomized clinical trial. J Nerv Ment Dis. 2020;208:533–9. https://doi.org/10.1097/NMD.0000000000001157.
Chochinov HM, Kristjanson LJ, Breitbart W, et al. Effect of dignity therapy on distress and end-of-life experience in terminally ill patients: a randomised controlled trial. Lancet Oncol. 2011;12:753–62. https://doi.org/10.1016/s1470-2045(11)70153-x.
Hall S, Goddard C, Opio D, et al. A novel approach to enhancing hope in patients with advanced cancer: a randomised phase II trial of dignity therapy. BMJ Support Palliat Care. 2011;1:315–21. https://doi.org/10.1136/bmjspcare-2011-000054. 20111009.
Li YC, Feng YH, Chiang HY, et al. The effectiveness of dignity therapy as applied to end-of-life patients with cancer in Taiwan: a quasi-experimental study. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:189–95. https://doi.org/10.1016/j.anr.2020.04.003.
Vuksanovic D, Green HJ, Dyck M, et al. Dignity therapy and life review for palliative care patients: a randomized controlled trial. J Pain Symptom Manage. 2017;53:162-170.e161. https://doi.org/10.1016/j.jpainsymman.2016.09.005. 20161101.
Cinar D, Karadakovan A, Erdogan AP. Effect of mobile phone app-based training on the quality of life for women with breast cancer. Eur J Oncol Nurs. 2021;52:101960. https://doi.org/10.1016/j.ejon.2021.101960.
De Hosson LD, Bouma G, Stelwagen J, et al. Web-based personalised information and support for patients with a neuroendocrine tumour: Randomised controlled trial. Orphanet J Rare Dis. 2019;14:60. https://doi.org/10.1186/s13023-019-1035-3.
Salzer MS, Palmer SC, Kaplan K, et al. A randomized, controlled study of Internet peer-to-peer interactions among women newly diagnosed with breast cancer. Psychooncology. 2010;19:441–6. https://doi.org/10.1002/pon.1586.
Chen Y, Xiao H, Zheng J, et al. Effects of a mind map-based life review programme on psychospiritual well-being in cancer patients undergoing chemotherapy: a randomised controlled trial. Eur J Cancer Care (Engl). 2020;29:e13221. https://doi.org/10.1111/ecc.13221. 20200107.
Sun F-K, Hung C-M, Yao Y, et al. The effects of logotherapy on distress, depression, and demoralization in breast cancer and gynecological cancer patients: a preliminary study. Cancer Nurs. 2021;44:53–61. https://doi.org/10.1097/ncc.0000000000000740.
Xiao H, Kwong E, Pang S, et al. Effect of a life review program for Chinese patients with advanced cancer: a randomized controlled trial. Cancer Nurs. 2013;36:274–83. https://doi.org/10.1097/NCC.0b013e318268f7ba.
Nezu AM, Nezu CM, Felgoise SH, et al. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol. 2003;71:1036–48. https://doi.org/10.1037/0022-006x.71.6.1036.
Passalacqua R, Caminiti C, Campione F, et al. Prospective, multicenter, randomized trial of a new organizational modality for providing information and support to cancer patients. J Clin Oncol. 2009;27:1794–9. https://doi.org/10.1200/jco.2007.15.0615.
Sandgren AK, McCaul KD. Long-term telephone therapy outcomes for breast cancer patients. Psychooncology. 2007;16:38–47. https://doi.org/10.1002/pon.1038.
Manne SL, Kashy DA, Zaider T, et al. Couple-focused interventions for men with localized prostate cancer and their spouses: A randomized clinical trial. Br J Health Psychol. 2019;24:396–418. https://doi.org/10.1111/bjhp.12359.
Manne SL, Siegel SD, Heckman CJ, et al. A randomized clinical trial of a supportive versus a skill-based couple-focused group intervention for breast cancer patients. J Consult Clin Psychol. 2016;84:668–81. https://doi.org/10.1037/ccp0000110.
Araujo RV, Fernandes AFC, Campelo RCV, et al. Effect of raja yoga meditation on the distress and anxiety levels of women with breast cancer. Religions. 2021;12:590. https://doi.org/10.3390/rel12080590.
Kovačič T, Kovačič M. Impact of relaxation training according to Yoga In Daily Life® system on perceived stress after breast cancer surgery. Integr Cancer Ther. 2011;10:16–26. https://doi.org/10.1177/1534735410387418. 20101208.
Hanser SB, Bauer-Wu S, Kubicek L, et al. Effects of a music therapy intervention on quality of life and distress in women with metastatic breast cancer. J Soc Integr Oncol. 2006;4:116–24.
Radl D, Vita M, Gerber N, et al. The effects of Self-Book© art therapy on cancer-related distress in female cancer patients during active treatment: A randomized controlled trial. Psychooncology. 2018;27:2087–95. https://doi.org/10.1002/pon.4758.
Eychmuller S, Zwahlen S, Fliedner MC, et al. Single early palliative care intervention added to usual oncology care for patients with advanced cancer: A randomized controlled trial (SENS Trial). Palliat Med. 2021;35:1108–17. https://doi.org/10.1177/02692163211005340.
Ferrell B, Chung V, Hughes MT, et al. A palliative care intervention for patients on phase 1 studies. J Palliat Med. 2021;24:846–56. https://doi.org/10.1089/jpm.2020.0597.
Gregoire C, Nicolas H, Bragard I, et al. Efficacy of a hypnosis-based intervention to improve well-being during cancer: a comparison between prostate and breast cancer patients. BMC Cancer. 2018;18:677. https://doi.org/10.1186/s12885-018-4607-z.
Gregoire C, Bragard I, Jerusalem G, et al. Group interventions to reduce emotional distress and fatigue in breast cancer patients: a 9-month follow-up pragmatic trial. Br J Cancer. 2017;117:1442–9. https://doi.org/10.1038/bjc.2017.326.
Han XB, Fang YQ, Liu SX, et al. Efficacy of combined naikan and morita therapies on psychological distress and posttraumatic growth in Chinese patients with advanced cancer: A randomized controlled trial. Medicine (Baltimore). 2021;100:e26701. https://doi.org/10.1097/md.0000000000026701.
Schuurhuizen C, Braamse AMJ, Beekman ATF, et al. Screening and stepped care targeting psychological distress in patients with metastatic colorectal cancer: the TES cluster randomized trial. J Natl Compr Cancer Netw. 2019;17:911–20. https://doi.org/10.6004/jnccn.2019.7285.
Young JM, Butow PN, Walsh J, et al. Multicenter randomized trial of centralized nurse-led telephone-based care coordination to improve outcomes after surgical resection for colorectal cancer: the CONNECT intervention. J Clin Oncol. 2013;31(3585–3591):20130903. https://doi.org/10.1200/jco.2012.48.1036.
Young J, Harrison J, Solomon M, et al. Development and feasibility assessment of telephone-delivered supportive care to improve outcomes for patients with colorectal cancer: pilot study of the CONNECT intervention. Support Care Cancer. 2010;18:461–70. https://doi.org/10.1007/s00520-009-0689-0.
Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration; 2020.
Borenstein L, Hedges L, Higgins J, et al. Ch. 45. When does it make sense to perform a meta-analysis? In: Introduction to meta-analysis. 2nd ed: Wiley; 2021. p. 393–400.
Moore AR, Eccleston C, Derry S, et al. “Evidence” in chronic pain–establishing best practice in the reporting of systematic reviews. Pain. 2010;150:386–9. https://doi.org/10.1016/j.pain.2010.05.011. 20100602.
McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont). 2009;6:21–9.
Cohen J. Statistical power analysis for the behavioral sciences. Burlington: Elsevier Science; 2013. https://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=923159. Accessed July 10 2022
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. https://doi.org/10.3389/fpsyg.2013.00863. 20131126.
Thompson B. Effect sizes, confidence intervals, and confidence intervals for effect sizes. Psychol Sch. 2007;44:423–32. https://doi.org/10.1002/pits.20234.
Murase T, Johnson F. Naikan, Morita, and Western psychotherapy: A comparison. Arch Gen Psychiatry. 1974;31:121–8. https://doi.org/10.1001/archpsyc.1974.01760130091016.
Paley CA, Arango MF, Keshwala V, et al. Evaluating provision of psychological assessment and support in palliative care: A survey of hospices in England. University of Leeds; 2022.
Compen F, Adang E, Bisseling E, et al. Cost-utility of individual internet-based and face-to-face mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in cancer patients. Psychooncology. 2020;29:294–303. https://doi.org/10.1002/pon.5246. 20191226.
Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. Jama. 2008;300:1350–2. https://doi.org/10.1001/jama.300.11.1350.
Clover KA, Mitchell AJ, Britton B, et al. Why do oncology outpatients who report emotional distress decline help? Psycho-Oncology. 2015;24:812–8. 20141211. https://doi.org/10.1002/pon.3729.
Ugalde A, Haynes K, Boltong A, et al. Self-guided interventions for managing psychological distress in people with cancer—A systematic review. Patient Educ Couns. 2016;100:846–57. https://doi.org/10.1016/j.pec.2016.12.009.
Zhang Q, Zhao H, Zheng Y. Effectiveness of mindfulness-based stress reduction (MBSR) on symptom variables and health-related quality of life in breast cancer patients-a systematic review and meta-analysis. Support Care Cancer. 2019;27:771–81. https://doi.org/10.1007/s00520-018-4570-x. 20181128.
Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med. 2006;29:17–27. https://doi.org/10.1007/s10865-005-9036-1. 20060107.
McInnerney D, Candy B, Stone P, et al. Access to and adequacy of psychological services for adult patients in UK hospices: a national, cross-sectional survey. BMC Palliat Care. 2021;20:31. https://doi.org/10.1186/s12904-021-00724-3.
Gebert P, Schindel D, Frick J, et al. Characteristics and patient-reported outcomes associated with dropout in severely affected oncological patients: an exploratory study. BMC Med Res Methodol. 2021;21:77. https://doi.org/10.1186/s12874-021-01259-0.
Bennett MI, Johnson MI, Brown SR, et al. Feasibility study of Transcutaneous Electrical Nerve Stimulation (TENS) for cancer bone pain. J Pain. 2010;11:351–9. https://doi.org/10.1016/j.jpain.2009.08.002. 20091022.
Acknowledgements
N/A
Funding
The review is part of the RESOLVE study (NIHR CPMS IDs: 46350 and 41794). The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by Yorkshire Cancer Research programme grant L412, RESOLVE: “Improving health status and symptom experience for people living with advanced cancer”. The sponsor had no role in study design or the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Organisational affiliation: University of Leeds https://medicinehealth.leeds.ac.uk/faculty-/dir-record/research-groups/646/division-of-primary-care-palliativecare-and-public-health
Author information
Authors and Affiliations
Contributions
CP and EC were responsible for writing the protocol. LZ, FM and JB assisted with the initial planning. EC and MS were responsible for the initial scoping review. EC and CP conducted the screening and analysis. CP and EC prepared Fig. 1 and Table 1. All authors contributed to drafting and re-drafting the final publication. All authors approved the final submitted version of the publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
N/A.
Competing interests
FM is a National Institute for Health and Care Research (NIHR) Senior Investigator.
LZ is a RESOLVE research programme Chief Investigator.
All other authors declared no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix A.
Search strategies. Appendix B. PRISMA Checklist. Appendix C. Covidence Data Extraction Template QR Code. Appendix D(a). MMAT Criteria. Appendix D(b). MMAT Scores.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Paley, C.A., Boland, J.W., Santarelli, M. et al. Non-pharmacological interventions to manage psychological distress in patients living with cancer: a systematic review. BMC Palliat Care 22, 88 (2023). https://doi.org/10.1186/s12904-023-01202-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12904-023-01202-8