Abstract
Background
Oral cancer is a life-threatening malignancy, which affects the survival rate and quality of life of patients. The aim of this systematic review was to review deep learning (DL) studies in the diagnosis and prognostic prediction of oral cancer.
Methods
This systematic review was conducted following the PRISMA guidelines. Databases (Medline via PubMed, Google Scholar, Scopus) were searched for relevant studies, from January 2000 to June 2023.
Results
Fifty-four qualified for inclusion, including diagnostic (n = 51), and prognostic prediction (n = 3). Thirteen studies showed a low risk of biases in all domains, and 40 studies low risk for concerns regarding applicability. The performance of DL models was reported of the accuracy of 85.0–100%, F1-score of 79.31 - 89.0%, Dice coefficient index of 76.0 - 96.3% and Concordance index of 0.78–0.95 for classification, object detection, segmentation, and prognostic prediction, respectively. The pooled diagnostic odds ratios were 2549.08 (95% CI 410.77–4687.39) for classification studies.
Conclusions
The number of DL studies in oral cancer is increasing, with a diverse type of architectures. The reported accuracy showed promising DL performance in studies of oral cancer and appeared to have potential utility in improving informed clinical decision-making of oral cancer.
Similar content being viewed by others
Background
Oral cancer is one of the major causes of death globally, the 17th most common worldwide and the 11th most common in Asia. According to the World Health Organization, more than 370,000 new cases of oral cancer were reported and caused over 170,000 deaths in 2020 [1]. There are various types of oral cancer depending on its origin (carcinoma and sarcoma), but the most common type is oral squamous cell carcinoma (OSCC), which is mostly transformed from oral potentially malignant disorders (OPMDs). The definitive gold standard diagnostic tool of oral cancer and OPMDs is surgical biopsy and histopathologic evaluation [2, 3]. The treatment modalities for oral cancer were surgery, radiotherapy, and chemotherapy either alone or in combination, which is generally determined according to the stage of the disease. The treatment outcomes, especially in advanced stages, have resulted in high morbidity, affecting the masticatory function, facial esthetics, and quality of life of oral cancer patients [2]. Currently, advances in oral cancer treatment have not improved the prognosis of oral cancer over the past decade [4]. Oral cancer prognosis has been based on cancer staging [5], which decreases significantly in advanced stages compared to early stages of oral cancer or in the stage of OPMDs. Therefore, the early diagnosis of oral cancer is the crucial step to increase the survival rate of oral cancer patients.
Deep learning (DL), a subset of artificial intelligence (AI), is built based on neural networks, which are biologically inspired programming algorithms that have the ability to learn complex representations to improve pattern recognition from raw data [6]. These algorithms are composed of multiple layers, which transform input data (such as medical images) into outputs (such as diagnostic or prognostic recommendations) while automatically learning higher-level features [6, 7]. DL has been proven capable of analyzing complex data and is widely applied in the medical field, including diagnostics, detecting abnormalities in medical images, etc. [7]. Integrating DL technology into routine clinical practice relies on achieving diagnostic accuracy that is not inferior to professional healthcare. In addition, it must provide other benefits, such as speed, efficiency, reduced cost, enhanced accessibility, and ethical conduct [8].
Nowadays, DL research in oral cancer is highly dynamic and keeps increasing due to its feasibility and many advantages to improve the cancer survival rate in the aspect of detection, prevention, and prognostic prediction [8,9,10]. There are studies that developed a mobile phone-based application for the oral cancer screening as an alternative method for early detection of oral cancer with a high accuracy to distinguish oral lesions from clinically suspicious lesions, which showed the potential of the application of computer-assisted visualization in the clinical practice [11, 12]. Application of DL to oral cancer data can assist clinicians in the diagnosis, detection, and prognostic prediction of oral cancer in clinical practice for early diagnosis and selection of the most appropriate treatment to increase the survival rate of patients with oral cancer.
There have been some previous systematic reviews on AI and machine learning in oral cancer [13, 14]. This study, therefore, mainly focused on the application of DL, which is the neural network-based architecture that has an ability to learn complex features, on oral cancer data. The main objective of this study is to systematically analyze evaluation studies of the application of DL in oral cancer data to aid in the diagnosis, detection, and prognostic prediction of oral cancer, and compare their results regarding the reported performance measures. In addition, this study further aimed to synthesize the results and assess the robustness of the body of evidence of DL-based diagnostic and prognostic predictive models on oral cancer data.
Methods
This is a systematic review of diagnostic and prognostic prediction studies. Reporting of this study follows the PRISMA guideline [15]. The study protocol was registered at the international prospective register of systematic reviews (PROSPERO) (CRD42023425992).
Inclusion criteria and exclusion criteria
The eligible studies must have evaluated the diagnostic or prognostic significance of oral cancer using DL algorithms. Publications were selected for review if they satisfied the following inclusion criteria: full texts available in English language; studies using DL (of any class) to provide diagnostic and prognostic prediction information of oral cancer and OPMDs; studies providing outcomes of model performance (diagnostic and prognostic prediction accuracy) and/or compared to a human diagnostic performance. For DL-based diagnostic studies in clinical and radiographic images (classification, detection, or segmentation), ground truth of captured images was identified by histopathologic result as the gold standard diagnosis of oral cancer and OPMDs.
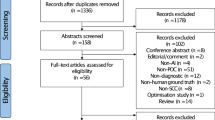
Studies with the following criteria were excluded: studies where ground truth of DL-based diagnostic studies was not explicitly confirmed; studies of machine learning (ML) applications without DL algorithms; studies without sufficient details on the data used for training and testing (e.g., dataset size, data modality, etc.); studies without a clear explanation of the DL model; studies that examined DL applications for normal oral mucosa, oral lesions (without cancer or OPMDs), periodontal disease, or dental caries, DNA and RNA microarray genes, proteomics, fluorescence spectroscopy, and genetic programming; articles in languages other than English. The details of the inclusion and exclusion criteria are presented in Fig. 1.
Information sources and search
An electronic search was conducted in the following electronic databases up to 14th June 2023: Medline via PubMed, Google Scholar, and Scopus. The search was conducted from January 2000 through June 2023. Each database was searched with adapted keywords. The search query for each database is described in Table 1.
Study selection
For managing the citations, Endnote 20 (Clarivate, Philadelphia, USA) was used. Two independent reviewers performed title and abstract screening after removing duplicate papers (K.W. and S.S.). Then, the reviewers evaluated full texts of eligible studies based on inclusion and exclusion criteria. Any disagreements or discrepancies were resolved by discussion and consensus of the two reviewers.
Data collection and extraction
Two reviewers (K.W. and S.S.) independently collected data from the included studies. Any disagreements or discrepancies were resolved by discussion and consensus of the two reviewers. The following data items were extracted: bibliographic details (name of authors, the year of publication and country), data modality, dataset size (train/valid/test, if given), augmentation, DL algorithms examined in the study, the definition of the study objective (diagnostic or prognostic), ground truth identification and annotation and task (classification, detection, segmentation) in the DL based diagnostic study, hyperparameters of the DL models, hardware used, performance metrics reported, including precision, recall, accuracy, sensitivity, specificity, F1-score, average precision (AP), Dice index, area under receiving operating characteristics curve (AUC), Concordance index (c-index) and Integrated Brier score (IBS). If more than one model was used, this study only reported on the best performance model.
Risk of bias and applicability
The methodological quality of the included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [16] for risk of bias assessment. The QUADAS-2 checklist consists of four risks of bias domains, including patient selection, index test, reference standard, and flow and timing. Any disagreements between the two reviewers were resolved by discussion and consensus. Some questions were slightly modified to specifically assess studies on DL [17]. In “patient selection”, limited information about the presented dataset as well as unclear data allocation strategies were considered to indicate a high risk of bias. For “index test”, insufficient information on model construction, including hyperparameters, and lack of model robustness analyzes were considered to indicate high risk of bias. For “reference standard”, the lack of information on the definition of the reference standard and the use of a single examiner to establish the reference test were considered to indicate a high risk of bias. Finally, in “flow and timing”, the indicators used different reference standards in the same study and inappropriate intervals between the index test and the reference standard. Details of the modified QUADAS-2 tool are provided in the supplemental information (Table 1S).
Statistical analysis
All statistical analyses were performed using R software, version 3.6.3 (Vienna, Austria) and IBM SPSS Statistics version 26. Because a few studies reported the number of true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN). This study used the diagnostic odds ratios (DOR) as pooled outcome from the reported sensitivity and specificity to determine the diagnostic accuracy of the deep learning system [18], calculated as follows:
Results
Study selection and study characteristics
The search results and process of selecting articles are shown in Fig. 1. After the literature search, a total of 905 articles were identified. Articles were excluded for the following reasons: studies that were duplicated (n = 328), studies focusing on other topics (n = 461), and studies that were not written in English language (n = 2). A total of 112 studies were assessed in full text. Fifty-eight of these studies, including studies that did not use DL models, studies that did not report the desired outcomes and dataset size, and studies on clinical images that did not use biopsy as the gold standard, were excluded after full text assessment.
Characteristics of relevant studies
The individual studies are summarized in Tables 2, 3, 4 and 5 with each table showing studies using DL in diagnostic studies, including classification (Table 2), object detection (Table 3), segmentation (Table 4), and prognostic prediction studies (Table 5).
Of the 54 included studies, 51 studies examined the use of DL applications in the diagnostic performance on medical images and 3 studies evaluated the prognostic prediction of DL applications. Most studies on the application of DL techniques in oral cancer were published recently, i.e. in 2019 and 2023 (n = 52) (Fig. 2). With regards to the regions of relevant articles, 37 of the studies were carried out entirely in Asia, 9 in Europe, 2 in Africa and 6 in the United States.
Seven different types of imagery data were employed to the DL applications on diagnostic studies, including histopathological images (n = 30), CT images (n = 8), clinical oral images (n = 9), and other types of image (n = 4), including confocal laser endomicroscopy images, optical coherence tomography images, and endoscopic videos. Clinicopathological and treatment data (n = 3) were incorporated in the DL applications on prognostic prediction studies. In addition, types of oral cancer data which were used in the development of DL models included OSCC (n = 41), non-specific type of oral cancer (n = 5), OPMDs (n = 5), and multiclass analysis of OSCC and OPMDs (n = 3). In diagnostic studies, some studies used expert annotation to set the reference test (n = 19). Specifically, one human expert (n = 7), two (n = 3), three or more (n = 9) experts were involved in defining the reference test.
Regarding the DL task, the most often chosen task was classification (n = 40), followed by segmentation (n = 10) and object detection (n = 5). Various DL models were used. In classification studies, most of the studies used multiple DL models (n = 25), including transfer learning models and multi-layer perceptron, followed by customized CNN structures (n = 8), LeNet-5 (n = 2), AlexNet (n = 2), DenseNet121 (n = 1), EfficientNet B0 (n = 1), and Swin-Transformer (n = 1). Regarding segmentation, most of the studies used multiple DL models, including auto-encoders models (n = 5), customized CNN structures (n = 3), and single auto-encoders models (e.g., U-Net) (n = 2). Regarding object detection, one-stage object detectors (e.g., YOLO) or two-stage object detectors (e.g., Faster R-CNN) were used in the majority of studies (n = 5). Classification studies mainly reported on precision, recall (sensitivity), F1-score, accuracy, and specificity; other outcome measures were the area-under-the receiver-operating-characteristics curve (AUC). In object detection studies, most studies were focused on precision, recall, F1-score, average precision (AP) and the AUC. Segmentation studies were more heterogeneous but additionally reported the Dice coefficient index and the mean Intersection over Union (mIoU). Furthermore, studies in prognostic prediction consistently reported the Concordance index (c-index) and Integrated Brier score (IBS) in all studies (Tables 3, 4 and 5).
Risk of bias and applicability
Detailed information about modified leading questions of QUADAS-2 for critical appraisal and the risk of bias are presented in Table S1–S5. Among the included studies, 13 (24.1%) were found to have low risk of biases in all four domains. Moreover, 40 studies (74.1%) were evaluated as low risk for concerns regarding applicability. The most problematic domain was “Reference Standard”, where only 22 studies (40.7%) were classified as low risk of bias followed by “Patient selection” where 32 studies (59.3%) were classified as low risk of bias.
Findings of the studies
In diagnostic studies, classification studies reported accuracies ranging from 85.0 to 100%, 78.2 to 93.62%, and 76.0 to 98.58% for classifying oral cancer on histopathological images, CT images and oral clinical images, respectively. The detection performance of object detection studies reported the F1-score ranging from 79.31 to 89.0%. In addition, the model performance of segmentation studies reported the Dice coefficient index ranging from 76.0 to 96.3%. In prognostic prediction studies, the prediction performance of DL models reported the c-index and IBS ranging from 0.78 to 0.95 and 0.04 to 0.12, respectively.
As outlined, classification and segmentation studies of oral cancer were used for further synthesis. Of these, 23 studies could be pooled, including classification of 20 studies and segmentation of 3 studies. The pooled sensitivity, specificity, and DOR of classification studies were 0.92 (95% CI 0.87–0.97), 0.92 (95% CI 0.88–0.96), and 2549.08 (95% CI 410.77–4687.39), respectively (Fig. 3). The pooled sensitivity, specificity, and DOR of segmentation studies were 0.87 (95% CI 0.72–1.02), 0.96 (95% CI 0.86–1.06), and 340.68 (95% CI -414.87 – 1096.22), respectively (Fig. 4). In addition, the majority of studies used histopathological data to develop the DL-based image classification with a high sensitivity and specificity of 0.99 (95% CI 0.98–0.99), and 0.97 (95% CI 0.94–0.99), respectively.
Discussion
Oral cancer is a life-threatening malignancy with frequent tumor metastasis and recurrence, which affects the survival rate and quality of life of patients [73,74,75]. The number of studies investigating the application of DL in oral cancer has increased in recent years. Most of the studies in this systematic review were published in 2019. This study compiled and assessed studies involving the DL for diagnosis and prognostic prediction of oral cancer by analyzing medical data including histopathological, CT, clinical image data, clinicopathological and treatment modality features data. Notably, however, the studies were of limited quality overall and comparison between studies was impeded by heterogeneity in conducting and reporting of the studies.
This systematic review found that most of the studies showed relatively high accuracy, sensitivity, and specificity of DL for the diagnosis of oral cancer (generally exceeding 80%). Nevertheless, heterogeneity in study conduct and reporting was high, precluding further comparisons between studies or quantitative synthesis. This review found that the included studies lacked details on the annotation process, did not mention the separation of the test dataset and the proportion between training, validation, and test dataset, which resulted in a high risk of bias in the reference test and patient selection. Additionally, seven diagnostic studies that mentioned the annotation process were annotated by one expert, resulting in these studies lacking inter-annotator agreement. To reduce the high risk of bias, future diagnostic studies should address minimum standard guidelines, such as Standards for Reporting of Diagnostic Accuracy Study-AI (STARD-AI); standards for diagnostic studies using AI models [76], Checklist for Artificial Intelligence in Medical Imaging (CLAIM); and a checklist for AI in medical imaging [77].
Regarding the heterogeneity in DL diagnostic studies of oral cancer, most studies did not report the value of TP, TN, FP, and FN; which caused a limitation for this systematic review of qualitative analysis of the results of oral cancer diagnostic study. Alternatively, the authors considered pooling sensitivity and specificity to calculate summary DORs as a single accuracy parameter. Moreover, the hyperparameter of DL models is essential for the explanation of tuning DL models to achieve the best performance from the model. This study found that several studies did not report the hyperparameters of DL models. This had a significant impact on the reliability and explainability of DL model performance, leading to a high risk of bias in the index test. To the best of our knowledge, there are no guidelines on reporting the hyperparameter tuning outcome/procedure for DL as models for medical diagnosis and prediction. This could explain why the hyperparameters reported in DL studies were heterogeneous.
Only three prognostic prediction studies applied DL algorithms, such as DeepSurv and DeepHit, in clinicopathologic and treatment modality data. The number of studies on DL was even less than studies in the era of machine learning (ML) [13, 14]. Nevertheless, the predictive performance of DL also yielded high accuracy for this task, achieving a c-index of 0.78–0.95 [70,71,72]. The predicted parameters were still the same as those of the ML era, which was interested in using clinicopathological and treatment modalities data to predict the prognosis and survival rate of oral cancer patients [13, 14]. Furthermore, there are no prognostic prediction studies of oral cancer in DL using molecular, cytological, and genomic data as a predictor, especially during preoperative evaluation. Combining various types of oral cancer data with the AI model could develop future prognostic prediction models allowing clinicians to decide on the most appropriate treatment plan to increase the survival rate of oral cancer patients.
All the studies included in this systematic review highlighted that DL techniques provide an increased precision approach for clinicians in making informed decisions. It should be emphasized that almost all the included studies only determined the accuracy performance of the DL model, in a few cases comparing it against the clinicians or experts. Furthermore, a fundamental element in achieving safe and efficient deployment of DL models in clinical practices is that the models achieve reliable generalizability. That is, the performance of the model when it is applied to external cases outside of the data for which it was trained [8, 10]. Therefore, the international collaboration among multiple healthcare centers could collect the data from multiple sources to develop the DL-based medical diagnosis and prognostic prediction system with the potential to be used in clinical practice. Nowadays, there are no standard guidelines for the appropriate accuracy of AI for clinical practice. Clinicians should understand that AI models are a decision support tool to improve treatment effectiveness and efficiency, but management options are based on the clinician’s decision.
This study has a number of strengths and limitations of the included studies and the review analysis. First, this review comprehensively and systematically appraised studies on DL for the diagnosis and prognostic prediction of oral cancer, and thus allows a narrative synthesis of the calculated DOR. Second, for limitation, this study selected only the scope of DL in oral cancer and found that studies reported heterogeneity, including various types of data and different reported outcome parameters, which was limited in qualitative analysis. In addition, this systematic review did not analyze the diagnostic performance of classification studies with the receiver operating characteristic (ROC) curve, which is one of the most widely used to analyze the diagnostic accuracy of classification models [78]. Future studies should critically determine reference tests and patient selection by addressing the checklist for AI in medical diagnostic and prognostic studies [76, 77, 79], which could improve utility to assess potential impact and clinical utility. Furthermore, many DL-based clinical image studies used image data from a public database and did not report diagnostic biopsy of lesions, which is an important ground truth that shows the reliability of the data for pathological AI research. Therefore, the future study should address the method to verify the reliability of clinical image from public database apart from biopsy proven to verify the ground truth of clinical image data for the medical AI study.
Conclusions
This systematic review reveals the increasing number of DL studies in oral cancer with a diverse type of architectures. The reported accuracy showed promising performances for diagnostic and prognostic analyses in studies of oral cancer, Furthermore, this systematic review found that different oral cancer data modalities in diagnostic studies impacted the sensitivity and specificity results of DL. This presents researchers with opportunities to investigate DL algorithms to various data modalities. Finally, the application of DL in oral cancer appeared to have potential utility in improving informed clinical decision-making and providing better diagnosis and prognosis of oral cancer. Future work to improve the explainability and interpretability of DL models and the use of clinically applicable performance measures would be needed to translate these models for use in clinical practice.
Availability of data and materials
The data of this study is available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Shah JP, Gil Z. Current concepts in management of oral cancer--surgery. Oral Oncol. 2009;45:394–401.
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16.
Chen SW, Zhang Q, Guo ZM, Chen WK, Liu WW, Chen YF, et al. Trends in clinical features and survival of oral cavity cancer: fifty years of experience with 3,362 consecutive cases from a single institution. Cancer Manag Res. 2018;10:4523–35.
Amin MB, Edge S, Greene FL, Schilsky RL, Byrd DR, Gaspar LE, et al. AJCC Cancer staging manual. 8th ed. New York: Springer Nature; 2017.
Min S, Lee B, Yoon S. Deep learning in bioinformatics. Brief Bioinform. 2017;18:851–69.
Fourcade A, Khonsari RH. Deep learning in medical image analysis: a third eye for doctors. J Stomatol Oral Maxillofac Surg. 2019;120:279–88.
Kim M, Yun J, Cho Y, Shin K, Jang R, Bae HJ, et al. Deep learning in medical imaging. Neurospine. 2020;17:471–2.
Aggarwal R, Sounderajah V, Martin G, Ting DSW, Karthikesalingam A, King D, et al. Diagnostic accuracy of deep learning in medical imaging: a systematic review and meta-analysis. NPJ Digit Med. 2021;4:65.
Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019;25:24–9.
Song B, Sunny S, Li S, Gurushanth K, Mendonca P, Mukhia N, et al. Mobile-based oral cancer classification for point-of-care screening. J Biomed Opt. 2021;26:065003.
Haron N, Zain RB, Ramanathan A, Abraham MT, Liew CS, Ng KG, et al. M-health for early detection of Oral Cancer in low- and middle-income countries. Telemed J E Health. 2020;26:278–85.
Alabi RO, Youssef O, Pirinen M, Elmusrati M, Makitie AA, Leivo I, et al. Machine learning in oral squamous cell carcinoma: current status, clinical concerns and prospects for future-a systematic review. Artif Intell Med. 2021;115:102060.
Adeoye J, Tan JY, Choi SW, Thomson P. Prediction models applying machine learning to oral cavity cancer outcomes: a systematic review. Int J Med Inform. 2021;154:104557.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Mohammad-Rahimi H, Motamedian SR, Rohban MH, Krois J, Uribe SE, Mahmoudinia E, et al. Deep learning for caries detection: a systematic review. J Dent. 2022;122:104115.
Simundic AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203–11.
Aubreville M, Knipfer C, Oetter N, Jaremenko C, Rodner E, Denzler J, et al. Automatic classification of cancerous tissue in Laserendomicroscopy images of the Oral cavity using deep learning. Sci Rep. 2017;7:11979.
Ariji Y, Fukuda M, Kise Y, Nozawa M, Yanashita Y, Fujita H, et al. Contrast-enhanced computed tomography image assessment of cervical lymph node metastasis in patients with oral cancer by using a deep learning system of artificial intelligence. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127:458–63.
Xu S, Liu C, Zong Y, Chen S, Lu Y, Yang L, et al. An early diagnosis of Oral Cancer based on three-dimensional convolutional neural networks. IEEE Access. 2019;7:158603–11.
Ariji Y, Sugita Y, Nagao T, Nakayama A, Fukuda M, Kise Y, et al. CT evaluation of extranodal extension of cervical lymph node metastases in patients with oral squamous cell carcinoma using deep learning classification. Oral Radiol. 2020;36:148–55.
Panigrahi S, Swarnkar T, editors. Automated Classification of Oral Cancer Histopathology images using Convolutional Neural Network. 2019 IEEE international Conference on Bioinformatics and Biomedicine (BIBM). San Diego: IEEE; 2019. p. 18–21. https://doi.org/10.1109/BIBM47256.2019.8982979.
Jeyaraj PR, ERS N, Panigrahi BK, editors. ResNet Convolution Neural Network Based Hyperspectral Imagery Classification for Accurate Cancerous Region Detection. 2019 IEEE conference on information and communication technology; 2019. p. 6–8.
Kiruthika S, Nisha SR. Automated Oral Cancer detection and classification using very deep convolutional neural network algorithm. TEST Eng Manag. 2020;83:20019–27.
Ramalingam A, Aurchana A, Dhanalakshmi P, Vivekananadan K, Venkatachalapathy V. Analysis of Oral squamous cell carcinoma into various stages using pre-trained convolutional neural networks. IOP Conference Series: Materials Science and Engineering. 2020;993:012058.
Chinnaiyan R, Shashwat M, Shashank S, Hemanth P, editors. Convolutional nNeural nNetwork mModel based aAnalysis and pPrediction of Oral Cancer. 2021 International Conference on Advancements in Electrical, Electronics, Communication. Computing and Automation (ICAECA); 2021. p. 8–9.
Heidari AE, Pham TT, Ifegwu I, Burwell R, Armstrong WB, Tjoson T, et al. The use of optical coherence tomography and convolutional neural networks to distinguish normal and abnormal oral mucosa. J Biophotonics. 2020;13:e201900221.
Das N, Hussain E, Mahanta LB. Automated classification of cells into multiple classes in epithelial tissue of oral squamous cell carcinoma using transfer learning and convolutional neural network. Neural Netw. 2020;128:47–60.
Fu Q, Chen Y, Li Z, Jing Q, Hu C, Liu H, et al. A deep learning algorithm for detection of oral cavity squamous cell carcinoma from photographic images: a retrospective study. E Clin Med. 2020;27:100558.
Musulin J, Štifanić D, Zulijani A, Šegota SB, Lorencin I, Anđelić N, et al. Automated Grading of Oral Squamous Cell Carcinoma into Multiple Classes Using Deep Learning Methods. 2021 IEEE 21st international conference on bioinformatics and bioengineering (BIBE); 2021. p. 25–7.
Alosaimi W, Uddin MI. Efficient data augmentation techniques for improved classification in limited data set of Oral squamous cell carcinoma. Comput Model Eng Sci. 2022;131:1387–401.
Tomita H, Yamashiro T, Heianna J, Nakasone T, Kobayashi T, Mishiro S, et al. Deep learning for the preoperative diagnosis of metastatic cervical lymph nodes on contrast-enhanced computed ToMography in patients with Oral squamous cell carcinoma. Cancers (Basel). 2021;13:600.
Camalan S, Mahmood H, Binol H, Araujo ALD, Santos-Silva AR, Vargas PA, et al. Convolutional neural network-based clinical predictors of Oral dysplasia: class activation map analysis of deep learning results. Cancers (Basel). 2021;13:1291.
Musulin J, Stifanic D, Zulijani A, Cabov T, Dekanic A, Car Z. An enhanced histopathology analysis: an AI-based system for multiclass grading of Oral squamous cell carcinoma and segmenting of epithelial and stromal tissue. Cancers (Basel). 2021;13:1784.
Warin K, Limprasert W, Suebnukarn S, Jinaporntham S, Jantana P. Automatic classification and detection of oral cancer in photographic images using deep learning algorithms. J Oral Pathol Med. 2021;50:911–8.
Kavyashree C, Vimala HS, Shreyas J, editors. Improving Oral Cancer Detection Using Pretrained Model. 2022 IEEE 6th conference on information and communication technology (CICT); 2022. p. 18–20.
Aljuaid A, Almohaya M, Anwar M, editors. An early detection of Oral epithelial dysplasia based on GoogLeNet inception-v3. 2022 IEEE/ACM conference on connected health: applications, systems and engineering technologies (CHASE); 2022. p. 17–9.
Sandhya Krishna P, Lavanya J, Kavya G, Prasamya N, Swapna, editors. Oral Cancer Diagnosis using Deep Learning for Early Detection. 2022 International Conference on Electronics and Renewable Systems (ICEARS); 2022. p. 16–8.
Sharma D, Kudva V, Patil V, Kudva A, Bhat RS. A convolutional neural network based deep learning algorithm for identification of Oral precancerous and cancerous lesion and differentiation from Normal mucosa: a retrospective study. Eng Sci. 2022;18:278–87.
Shetty S, Patil A. Duck pack optimization with deep transfer learning-enabled Oral squamous cell carcinoma classification on histopathological images. Int J Grid High Perform Comput. 2023;15:1–21.
Jubair F, Al-Karadsheh O, Malamos D, Al Mahdi S, Saad Y, Hassona Y. A novel lightweight deep convolutional neural network for early detection of oral cancer. Oral Dis. 2022;28:1123–30.
Warin K, Limprasert W, Suebnukarn S, Jinaporntham S, Jantana P. Performance of deep convolutional neural network for classification and detection of oral potentially malignant disorders in photographic images. Int J Oral Maxillofac Surg. 2022;51:699–704.
Xu Z, Peng J, Zeng X, Xu H, Chen Q. High-accuracy Oral squamous cell carcinoma auxiliary diagnosis system based on EfficientNet. Front Oncol. 2022;12:894978.
Fati SM, Senan EM, Javed Y. Early diagnosis of Oral squamous cell carcinoma based on histopathological images using deep and hybrid learning approaches. Diagnostics (Basel). 2022;12:1899.
Warin K, Limprasert W, Suebnukarn S, Jinaporntham S, Jantana P, Vicharueang S. AI-based analysis of oral lesions using novel deep convolutional neural networks for early detection of oral cancer. PLoS One. 2022;17:e0273508.
Deif MA, Attar H, Amer A, Elhaty IA, Khosravi MR, Solyman AAA. Diagnosis of Oral squamous cell carcinoma using deep neural networks and binary particle swarm optimization on histopathological images: an AIoMT approach. Comput Intell Neurosci. 2022;2022:6364102.
Yuan W, Yang J, Yin B, Fan X, Yang J, Sun H, et al. Noninvasive diagnosis of oral squamous cell carcinoma by multi-level deep residual learning on optical coherence tomography images. Oral Dis. 2022;29:3223–31.
Yang SY, Li SH, Liu JL, Sun XQ, Cen YY, Ren RY, et al. Histopathology-based diagnosis of Oral squamous cell carcinoma using deep learning. J Dent Res. 2022;101:1321–7.
Chang X, Yu M, Liu R, Jing R, Ding J, Xia J, et al. Deep learning methods for oral cancer detection using Raman spectroscopy. Vib Spectrosc. 2023;126:103522.
Afify HM, Mohammed KK, Ella HA. Novel prediction model on OSCC histopathological images via deep transfer learning combined with grad-CAM interpretation. Biomed Signal Process Control. 2023;83:104704.
Agarwal P, Yadav A, Mathur P, Pal V, Chakrabarty A. BID-net: an automated system for bone invasion detection occurring at stage T4 in Oral squamous carcinoma using deep learning. Comput Intell Neurosci. 2022;2022:4357088.
Oya K, Kokomoto K, Nozaki K, Toyosawa S. Oral squamous cell carcinoma diagnosis in digitized histological images using convolutional neural network. J Dent Sci. 2023;18:322–9.
Das M, Dash R, Mishra SK. Automatic detection of Oral squamous cell carcinoma from histopathological images of Oral mucosa using deep convolutional neural network. Int J Environ Res Public Health. 2023;20:2131.
Flugge T, Gaudin R, Sabatakakis A, Troltzsch D, Heiland M, van Nistelrooij N, et al. Detection of oral squamous cell carcinoma in clinical photographs using a vision transformer. Sci Rep. 2023;13:2296.
Ananthakrishnan B, Shaik A, Kumar S, Narendran SO, Mattu K, Kavitha MS. Automated detection and classification of Oral squamous cell carcinoma using deep neural networks. Diagnostics (Basel). 2023;13:918.
Panigrahi S, Nanda BS, Bhuyan R, Kumar K, Ghosh S, Swarnkar T. Classifying histopathological images of oral squamous cell carcinoma using deep transfer learning. Heliyon. 2023;9:e13444.
Yang Z, Pan H, Shang J, Zhang J, Liang Y. Deep-learning-based automated identification and visualization of Oral Cancer in optical coherence tomography images. Biomedicines. 2023;11:802.
Ariji Y, Fukuda M, Nozawa M, Kuwada C, Goto M, Ishibashi K, et al. Automatic detection of cervical lymph nodes in patients with oral squamous cell carcinoma using a deep learning technique: a preliminary study. Oral Radiol. 2021;37:290–6.
Xu X, Xi L, Wei L, Wu L, Xu Y, Liu B, et al. Deep learning assisted contrast-enhanced CT-based diagnosis of cervical lymph node metastasis of oral cancer: a retrospective study of 1466 cases. Eur Radiol. 2023;33:4303–12.
Das DK, Koley S, Bose S, Maiti AK, Mitra B, Mukherjee G, et al. Computer aided tool for automatic detection and delineation of nucleus from oral histopathology images for OSCC screening. Appl Soft Comput. 2019;83:105642.
Fraz MM, Khurram SA, Graham S, Shaban M, Hassan M, Loya A, et al. FABnet: feature attention-based network for simultaneous segmentation of microvessels and nerves in routine histology images of oral cancer. Neural Comput & Applic. 2020;32:9915–28.
Martino F, Bloisi D, Pennisi A, Fawakherji M, Ilardi G, Russo D, et al. Deep learning-based pixel-wise lesion segmentation on Oral squamous cell carcinoma images. Appl Sci. 2020;10
dos Santos DFD, de Faria PR, Travençolo BAN, do Nascimento MZ. Automated detection of tumor regions from oral histological whole slide images using fully convolutional neural networks. Biomed Signal Process Control. 2021;69:102921.
Paderno A, Piazza C, Del Bon F, Lancini D, Tanagli S, Deganello A, et al. Deep learning for automatic segmentation of Oral and oropharyngeal Cancer using narrow band imaging: preliminary experience in a clinical perspective. Front Oncol. 2021;11:626602.
Pennisi A, Bloisi DD, Nardi D, Varricchio S, Merolla F, editors. Multi-encoder U-Net for Oral Squamous Cell Carcinoma Image Segmentation. 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA); 2022. p. 22–4.
Ariji Y, Kise Y, Fukuda M, Kuwada C, Ariji E. Segmentation of metastatic cervical lymph nodes from CT images of oral cancers using deep-learning technology. Dentomaxillofac Radiol. 2022;51:20210515.
Liu Y, Bilodeau E, Pollack B, Batmanghelich K. Automated detection of premalignant oral lesions on whole slide images using convolutional neural networks. Oral Oncol. 2022;134:106109.
Dos Santos DFD, de Faria PR, Travencolo BAN, do Nascimento MZ. Influence of data augmentation strategies on the segmentation of Oral histological images using fully convolutional neural networks. J Digit Imaging. 2023;36:1608–23.
Kim DW, Lee S, Kwon S, Nam W, Cha IH, Kim HJ. Deep learning-based survival prediction of oral cancer patients. Sci Rep. 2019;9:6994.
Adeoye J, Koohi-Moghadam M, Lo AWI, Tsang RK, Chow VLY, Zheng LW, et al. Deep learning predicts the malignant-transformation-free survival of Oral potentially malignant disorders. Cancers (Basel). 2021;13:6054.
Adeoye J, Hui L, Koohi-Moghadam M, Tan JY, Choi SW, Thomson P. Comparison of time-to-event machine learning models in predicting oral cavity cancer prognosis. Int J Med Inform. 2022;157:104635.
Chandu A, Adams G, Smith AC. Factors affecting survival in patients with oral cancer: an Australian perspective. Int J Oral Maxillofac Surg. 2005;34:514–20.
Loeffelbein D, Ritschl LM, Güll FD, Roth M, Wolff KD, Mücke T. Influence of possible predictor variables on the outcome of primary oral squamous cell carcinoma: a retrospective study of 392 consecutive cases at a single Centre. Int J Oral Maxillofac Surg. 2017;46:413–21.
Sklenicka S, Gardiner S, Dierks EJ, Potter BE, Bell RB. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome? J Oral Maxillofac Surg. 2010;68:1270–5.
Viknesh S, Hutan A, Robert MG, Shravya S, Jeffrey De F, Lotty H, et al. Developing a reporting guideline for artificial intelligence-centred diagnostic test accuracy studies: the STARD-AI protocol. BMJ Open. 2021;11:e047709.
Mongan J, Moy L, Kahn CE Jr. Checklist for artificial intelligence in medical imaging (CLAIM): a guide for authors and reviewers. Radiol Artif Intell. 2020;2:e200029.
Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98.
Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med. 2015;13:1.
Acknowledgements
We gratefully acknowledge Mr. Anuwat Pengput, from Sirindhorn College of Public Health, Khon Kaen, Thailand. We thank Mr. Terrance J. Downey, English Editor for Thammasat University Office of Research and Innovation for English language editing.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: KW, SS; Data curation: KW, SS; Formal analysis: KW, SS; Investigation: KW, SS; Methodology: KW, SS; Project administration: KW; Supervision: SS; Writing – original draft: KW, SS; Writing – review & editing: KW, SS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
None.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1S
. Modified leading questions of QUADAS-2 for critical appraisal. Table 2S. Quality assessment of included studies using QUADAS-2 (Classification studies). Table 3S. Quality assessment of included studies using QUADAS-2 (Object detection studies). Table 4S. Quality assessment of included studies using QUADAS-2 (Segmentation studies). Table 5S. Quality assessment of included studies using QUADAS-2 (Prognosis prediction studies).
Additional file 2.
PRISMA 2020 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Warin, K., Suebnukarn, S. Deep learning in oral cancer- a systematic review. BMC Oral Health 24, 212 (2024). https://doi.org/10.1186/s12903-024-03993-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-03993-5