Abstract
Background
Inter-appointment medication of the root canals with appropriate intracanal medicaments has been advocated to improve root canal disinfection. Graphene oxide (GO) has shown promising antimicrobial activity against a wide range of microorganisms, besides the capability of carrying antibiotics. The current study aimed to compare the antibacterial activity of double antibiotic paste (DAP) and GO per se and in combination (GO-DAP) against Enterococcus faecalis (E. faecalis).
Methods
A total of 108 extracted human mandibular premolars were contaminated with three-week-old E. faecalis and subjected to a primary microbial assessment. The samples were categorized into 15 groups concerning the intracanal medicament (DAP, GO, GO-DAP, and control) and contact time (1, 7, and 14 days). Then, the root canals were medicated, incubated, and resubjected to a secondary antimicrobial evaluation. The colony-forming units (CFU) were counted to calculate the antimicrobial efficacy. The data were analyzed via the Kruskal–Wallis test (α = 0.05).
Results
GO-DAP was the only medicament that completely eradicated E. faecalis in 1 day. The percentage reduction of CFU/ml in the GO-DAP and DAP groups was higher than that in the GO group at all allocated contact times. Furthermore, a significant decrease of the CFU/ml was seen in the GO and DAP groups after 7 and 14 days of being medicated (P < 0.05).
Conclusion
Since GO-DAP improved root canal disinfection, this novel material can be introduced as a promising intracanal medicament against E. faecalis even in the short run.
Similar content being viewed by others
Introduction
Removal of microorganisms and their by-products from the root canals is the supreme objective of endodontic therapy [1, 2], which is impeded by anatomical complexities like lateral canals, dentinal tubules, and isthmuses [3, 4]. This goal can be best achieved by combining mechanical preparation with different irrigants and intracanal medicaments [5, 6] like calcium hydroxide, triple antibiotic paste (TAP), and double antibiotic paste (DAP), which are commonly used between the treatment sessions [1, 7, 8].
TAP (metronidazole, ciprofloxacin, and minocycline) is efficient against gram-positive, gram-negative, and anaerobic bacteria; however, its minocycline content may induce tooth discoloration [9]. This content is excluded in DAP (metronidazole and ciprofloxacin), which is still adequately efficient against E. faecalis and Porphyromonas gingivalis [9, 10], and indicated in case of failure of commonly-used medicaments [1]. However, while even short-term application of antibiotic combinations (as in TAP and DAP) can increase the risk of antibacterial resistance, long-term exposure to DAP may cause demineralization and negatively affect the mechanical properties of dental hard tissues [11].
Graphene is a two-dimensional monolayer of single carbon atoms with a honeycomb structure, whose outstanding properties have attracted researchers’ attention [12,13,14]. Although the exact mechanism of the broad antibacterial activity of graphene-based materials is still unclear, it is generally attributed to several modes of action like the disruption and entrapment of the bacterial cell membrane, annihilator extraction of phospholipid molecules, oxidative stress, and self-killing effect [15, 16].
Graphene oxide (GO) possesses a monolayer of atoms with epoxide, carboxylic acid, and hydroxyl groups on the surface, which results in satisfactory water-solubility and creates a wide surface for pharmaceutical incorporation and group functionalization for specific use [17, 18]. It is a favorable carrier of pharmaceuticals and biomolecules and enhances the mechanical performance and bioactivity of biomaterials [19]. This cost-effective antibacterial material is physically and chemically bactericidal and only mildly cytotoxic to mammalian cells in low doses, causes less bacterial resistance, interacts with pharmaceuticals, and easily modifies surfaces with desired functionalities [20,21,22]. GO has antimicrobial potential against gram-negative and gram-positive microorganisms [23] and is bactericidal on most common dental pathogenic microorganisms [24].
To promote endodontic antimicrobial agents, efforts have been focused on devising brand-new antibacterial delivery techniques like incorporating nanomaterials [25]. Nanosilver graphene oxide and graphene silver nanoparticles exhibited remarkable antibacterial potency as endodontic irrigants [26, 27]. Moreover, graphene nanoplates were reported as a novel biocompatible material for root canal obturation with improved adhesion and antibacterial efficacy [21]. GO has been recommended as an effective antibiotic carrier [28]. To the best of the authors’ knowledge, graphene and its derivatives have not been investigated as intracanal medicaments. This study aimed to compare the antibacterial efficacy of DAP and GO, per se and in combination, against E. faecalis. The null hypothesis was that these medicaments would not significantly reduce the CFU/ml of E. faecalis in the root canals.
Materials and methods
Materials
Graphite powder was purchased from Tanfeng Graphene Technology Co., Ltd., Jiangsu, China. Phosphoric acid (H3PO4), sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), Phosphate buffer saline (PBS, 10 × concentrate, BioPerformance Certified, suitable for cell culture, pH: 7.4), and hydrochloric acid were purchased from Sigma-Aldrich, Gillingham, UK. Ciprofloxacin and metronidazole were purchased from Alborz Darou, Qazvin, Iran. Ampicillin was purchased from Merck, Darmstadt, Germany. Mueller–Hinton broth (MHB) medium was bought from HiMedia (Mumbai, India) and double distilled water was used in each experiment. All other chemicals including 2.5% and 5.25% NaOCl were purchased from Sigma-Aldrich Corporation, St Louis, MO, USA. EDTA was purchased from Wizard; Rehber Chemical Co., Ltd, Istanbul, Turkey. K-file, ProTaper rotary system, hand pluggers, and spiral filler were purchased from Dentsply, Maillefer, Ballaigues, Switzerland. The 30-gauge needle was purchased from Cerkamed, Poland. Ethylene oxide was purchased from Acecil, Campinas, São Paulo, Brazil. Paper points were purchased from Gapadent Co. Ltd., Korea.
The study design was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.DENTAL.REC.1400.021). It was conducted in full accordance with ethical principles including the World Medical Association Declaration of Helsinki (version 2008).
Preparation of the medicaments
Every single step of this study was conducted under strict aseptic conditions. For the preparation of DAP, 500 mg of metronidazole was combined with 500 mg of ciprofloxacin in equal proportions (1:1) [1, 29].
GO was synthesized from graphite flakes through a modified Hummer’s method [30] as follows.
Ten grams of graphite flakes and 110 cc of H3PO4 and 1 L of H2SO4 (98%) were mixed in a 1000-ml volumetric flask in an ice bath (0–6 °C) while continually stirred for 2 h. Meanwhile, 50 g of KMnO4 was gradually added to the suspension and the addition rate was precisely controlled to maintain the reaction temperature below 14 °C. Then, the ice bath was eliminated and the mixture was stirred at 30 °C till achieving a brown paste and kept under stirring for 2 h. The temperature was raised to 50 °C every half an hour. It was weakened by slowly adding 100 ml of water. The reaction temperature was quickly raised to 96 °C via effervescence and the color went brown. The solution was weakened by adding 100 ml of water while continuously stirred, and eventually treated with 10 ml of H2O2 to terminate the reaction by the formation of yellow color. For filtration, a Büchner funnel and Whatman filter paper were applied and the Erlenmeyer flask was connected to a vacuum pump. For purification, the mixture was washed by centrifugation and rinsed with 8% HCL, and then deionized water several times. After filtration, it was dried in a hot air oven at 100 °C and later in a humidity-absorbing chamber for 48 h, the GO was attained as a powder (Fig. 1).
Characterization of nanostructures
The nanostructures used in this study were characterized by transmission electron microscopy (TEM, Zeiss-EM10C-100 kV, Germany), Energy-dispersive X-ray spectroscopy (EDS) (FESEM, Sigma VP, ZEISS, Germany), and Fourier transform infrared (FTIR) spectrometer (Perkin Elmer, UK).
Antibacterial function against E. faecalis biofilms
Chemo-mechanical preparation
Sample size was calculated according to Abbaszadegan et al. [31] study and the following formula [32]:
A total of 108 extracted human mandibular premolars with mature apices, which were extracted due to orthodontic or periodontal reasons were used in this study. The teeth were single-rooted, non-carious, and without fractures. The sample teeth were stored in distilled water to prevent probable dehydration till used. To achieve a standardized root length of 15 mm, all sample teeth were decoronated from 2 to 3 mm below the cementoenamel junction with a safe-sided diamond disc under water cooling. A #15 k-file (Dentsply, Maillefer, Ballaigues, Switzerland) was used to ensure the teeth had only one canal. The working length was obtained by subtracting one mm from the length measured when the tip of #15 k-file was first observed at the apical foramen. The root canals were prepared to the working length by sequential use of the ProTaper rotary system to file #F3 (Dentsply, Maillefer Tulsa, OK, USA). Then, they were irrigated with 2 ml of 2.5% NaOCl (Sigma-Aldrich Corporation, St. Louis, MO, USA) by using a plastic syringe with a 30-gauge needle (Cerkamed, Poland) between each instrument change.
To remove the smear layer, the root canal was consecutively rinsed with 5 ml of 17% ethylenediaminetetraacetic acid (EDTA) (Wizard; Rehber Chemical Co., Ltd, Istanbul, Turkey) and 5 ml of 5.25% NaOCl (Sigma-Aldrich Corporation, St Louis, MO, USA) for 5 min each (total = 10 min). To prevent bacterial leakage, the root canal apices were sealed with resin composite. Besides the coronal access cavity, the external root surfaces were sealed with nail polish to prevent bacteria leakage from the accessory lateral canals.
Root canal contamination
A culture with 96 wells was used to mount and fix specimens. Experimental samples (each with 10 samples) were randomly distributed into nine 96-well cell culture microplates and 6 control microplates (each containing three samples). They were all sterilized with ethylene oxide (Acecil, Campinas, São Paulo, Brazil). The root canals were filled with brain heart infusion (BHI) broth and their sterilization efficacy was evaluated. The samples were incubated (Mart Microbiology BV, the Netherlands) for 48 h at approximately 95% humidity and 37 °C, and microbial evaluation was done on the samples taken from each root canal.
Under a laminar flow chamber, the root specimens were inoculated. By using a 5-ml BHI broth media, the 2-day isolated pure culture colonies of E. faecalis (grown on BHI agar plates) were conceded in a 5-ml BHI broth medium. They were adjusted to achieve spectrophotometric turbidity of 1.5 × 108 CFU/ml. The root canals were contaminated under a laminar air-flow cabinet with 10 μl inoculums of E. faecalis. The samples were incubated at 37 °C for 3 weeks. Meanwhile, BHI was applied to the root canals (except for the negative control) on alternate days (QOD) by 0.5-ml insulin syringes to have continuous bacterial feeding. Gram staining and catalase reaction evaluations were conducted to evaluate the purity of the bacterial culture.
After 21 days, the primary microbial assessment was conducted by flooding the canal with sterile saline while inserting a Hedström file #30 into the canal for scraping the dentin throughout the procedure. Then, 3 sterile paper points (Gapadent Co. Ltd., Korea) were put in each canal for 60 s, withdrawn under laminar flow, and transferred into the tubes containing 1 ml of BHI. The tubes were vortexed for 60 s and the corollary solution was consecutively diluted tenfold in BHI broth. Aliquots of 100 μl of the suspension were smeared on BHI agar plates and incubated at 37 °C for 1 day. By using the CFU/ml counts of E. faecalis, bacterial growth was deliberated and validated with colony morphology and gram stain.
Intracanal medication
The root canals were re-prepared with 5 ml of sterile saline solution, followed by 17% EDTA, which lasted 3 min. The root canals were irrigated with sterile saline solution and dried with sterile paper points. The microplates containing the roots were randomly assigned to be medicated with one of the following intracanal medicaments as GO (n = 30), DAP (n = 30), GO-DAP (n = 30), positive control (sterile saline, n = 9), and negative control (no bacterial contamination, n = 9).
DAP was applied to the root canals by using size 30 spiral fillers (Dentsply; Mailer, Ballaigues, Switzerland) and condensed with hand pluggers (Dentsply India Pvt Ltd.; Mumbai, India). The graphene oxide was applied by a #30 K-file (Mani Inc.; Tochigi-Ken, Japan). Any excess medicament was eliminated and the access cavities were blocked by sterile cotton pellets. Each group was subdivided into three subgroups with respect to the exposure time of 24 h, 7 days, and 14 days. In other words, specimens were randomly allocated into 15 groups including 9 experimental (n = 10) and 3 control groups (n = 3).
The samples were incubated in a microaerophilic environment at 37 ℃ for the predetermined contact time defined for each subgroup. The medicaments were removed by using #30 K-file and rinsed with 5 ml of sterile saline. Except for the controls, the specimens were rinsed again with 2 ml of sterile saline. Microbiological harvests were conducted for the predetermined incubation duration with medicaments by using sterile Gates Glidden drills #5 (Mani Inc.; Tochigi-Ken, Japan). This sampling technique was adopted from previous studies [31, 33, 34]. To standardize dentinal shavings collection, roots with similar morphology and length were chosen. The sampling and preparation technique was identical in all experimental groups. The canals were drilled up to 10 mm of canal length 3 times, 10 s each. Dentin shavings were transferred into 1 ml of sterile BHI and vortexed for 60 s, followed by serial tenfold dilutions (up to 5 times) on BHI broth. Eventually, they were incubated in an anaerobic setting at 37 °C for 1 day. Aliquots of 100 μl from the suspensions were smeared on BHI agar plates, followed by incubation at 37 °C for a day. CFU/ml counts of E. faecalis were used for measuring bacterial growth.
Statistical analysis
Statistical analyses were done with SPSS software (version 22, SPSS Inc., IL, USA). Given the abnormal distribution of data, intergroup and intragroup CFU/ml percentage reduction over different contact times were compared through the Kruskal–Wallis and Friedman tests, respectively. P < 0.05 was regarded as statistically significant.
Results
Characterization
The morphology of GO, DAP, and GO-DAP was characterized through scanning electron microscopy (SEM) and TEM (Fig. 2). The loosely stacked and typical wrinkled structure of the GO and GO-DAP was observed in SEM micrographs. This wrinkled nature is essential to prevent collapse-back in a graphitic structure [35]. The wrinkled surface of GO and GO-DAP was also visible in TEM micrographs.
Figure 3 demonstrates the FTIR spectra of the GO for the wavelength range of 450–4000 cm−1. The main characteristic peaks of the GO group were in good agreement with what was previously reported [36,37,38,39]. The carbonyl, alkoxy, and epoxy functional groups were confirmed by the FTIR peaks at 1703 cm−1, 1055 cm−1, and 1229 cm−1, respectively. The peaks at 3408 cm−1 and 1634 cm−1 were assigned to OH stretching vibration and C=C benzenoid vibration. Figure 4 displays the FTIR spectra of the DAP and GO-DAP groups. At the FTIR spectra of DAP, the characteristic peaks respectively contributed to NH stretching vibration (3398 cm−1), (CH) aromatic vibration (3035 cm−1), (CH) aliphatic stretching (2924 cm−1), C=C vibration (1624 cm−1), N=O (1452 cm−1), and ring torsion band (826 cm−1), which were in line with previous reports [40, 41].
Antibacterial evaluation
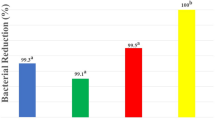
Contamination of all specimens with E. faecalis was confirmed at the very first step of the experiment. The CFU/ml counts of the positive control samples revealed no statistically significant difference among the three studied contact times. Bacterial growth was denied in the negative control samples at the three studied time points. Table 1 summarizes the results of the descriptive analysis. The GO-DAP was the most efficient intracanal medicament against E. faecalis which completely eradicated this microorganism within 1 day. The results of the Kruskal–Wallis test revealed a significant difference in the CFU/ml counts among the experimental groups at all time points (P < 0.05). After 1 day and 7 days, GO-DAP remarkably eliminated higher bacterial count compared to GO and positive control (P < 0.05). Also, the reduction of bacterial count by DAP was significantly higher than positive control group after 1 day and 7 days (P = 0.027). After 14 days, GO-DAP and DAP resulted in a remarkable reduction of bacterial count compared to GO (P = 0.005 and P = 0.039, respectively).
The bacterial count remarkably decreased from the initial sampling after 1 day to the last sampling after 14 days. Statistically significant reduction of CFU/ml counts after 1 day was only seen in the GO-DAP group (P = 0.002). At all allocated contact times, the GO-DAP and DAP showed superior antibacterial efficacy against E. faecalis than GO per se (P < 0.05). Intragroup comparisons revealed a significant reduction of CFU/ml count in GO, GO-DAP, and DAP groups after 7 and 14 days (P < 0.05).
Discussion
For optimum use of biomaterials in endodontics, their antibacterial activity, biocompatibility, mechanical properties, sustainability, and shelf life should be considered [42, 43]. Given the excellent properties and favorable antibacterial activity of GO against different bacterial pathogens, the present study assessed the antibacterial efficacy of GO-DAP as a novel intracanal medicament against E. faecalis. The null hypothesis was rejected as a significant difference in the CFU/ml counts among the experimental groups was observed at all time points.
Three-week-old preformed biofilms of E. faecalis, facultative anaerobic gram-positive cocci, were chosen for the present study as they are the dominant pathogen in failed endodontic therapy [44]. Their unique features make them a highly resistant endopathogen that complicates endodontic therapy and necessitates the development of more efficient alternative irrigants and medicaments [45,46,47].
According to the present findings, the GO-DAP was the only agent that significantly reduced and entirely eradicated the E. faecalis bacterial load within 1 day (P = 0.002). Even the reduction of bacterial count with this agent was statistically higher than the DAP (P = 0.042). There is no exactly similar report in the literature the results of which can be directly compared with the present findings. However, some partly similar reports have stated that brief intracanal exposure to DAP was unable to completely disrupt E. faecalis [7, 48]. Unlike DAP, GO-DAP could completely eradicate E. faecalis within 24 h, which highlights the promising synergistic antibacterial efficacy of GO-DAP.
In the present study, the antibacterial efficacy of DAP against E. faecalis was higher than that of the GO at all the allocated contact times (P < 0.05). DAP reduced the initial bacterial count up to 98.22% in just 1 day and eradicated all the bacteria after 14 days. In accordance with the present findings, Sadek et al. [49] and Sabrah et al. [50] reported that DAP was a potent eradicator of viable bacteria counts (> 99%).
The antibacterial mechanism of GO is attributed to its particular two-dimensional structure that vigorously interacts with the bacterial lipid bilayer, separates the lipid molecules from the bacterial membrane, and, as a result, destroys it [51]. Nanda et al. [52] investigated the precise molecular mechanism of GO against E. faecalis by Raman spectroscopy. They concluded that GO degrades the inner and outer bacterial cell membrane of E. faecalis, thereby, Adenine and protein are released from the bacteria and it dies. Additionally, they reported that GO had an identical mechanism of antibacterial activity against both gram-positive and gram-negative microorganisms. It was also noted that increasing the GO concentration induced degradation of the inner and outer bacterial cell membranes of both gram-negative and gram-positive bacteria. In the current study, GO decreased the initial bacterial load by 42.78% within 24 h and 82.90% after one week. Complete eradication of E. faecalis was not achieved even after 2 weeks (97.54%).
The antibacterial efficacy of GO was significantly inferior to that of GO-DAP and DAP per se. To date, no study has evaluated the efficacy of GO as an intracanal medicament; although, few investigations have assessed the antibacterial efficacy of graphene or its derivatives against E. faecalis [26, 27, 53,54,55]. In accordance with the present findings, Ioannidis et al. [26] reported that Ag-GO reduced the bacterial load by 57%, being less efficient than NaOCl, the gold standard of endodontic irrigants. On the contrary, Sharma et al. [27] observed that the total microbial biovolume of Ag-GO nanoparticles was 86.85%, which was superior to NaOCl (80.40%), although it was statistically insignificant. However, two other studies declared that the incorporation of GO was promising in photodynamic therapy against E. faecalis and significantly decreased the bacteria count (up to 99.4%) [53, 54].
Another study stated that GO significantly decreased the bacterial load after 72 h of incubation [55]. Likewise, Mahmoud et al. [56] fabricated graphene quantum dots@gemifloxacin@hybrid double-layered Fe/Al, which effectively acted against E. faecalis. Furthermore, the minimum inhibitory concentration of cystamine-conjugated GO against E. faecalis revealed its strong antibacterial activity and great reactive oxygen species effects with low cytotoxicity [57]. Niranjan et al. [58] assessed the antibacterial activity of reduced graphene oxide (rGO) loaded with sulfur and sulfur-selenium nanoparticles. Their findings elucidated that, unlike rGO-S/Se NPs, the antibacterial efficacy of rGO and rGO-S was not favorable. Since the GO fight against E. faecalis is concentration- and time-dependent, the contrasting findings of the aforementioned studies could be attributed to the different concentrations of GO or its time of contact with the root canal system [59]. Additionally, medium culture conditions and the purity of GO may affect its antibacterial activity [60]. Another influencing factor of the antibacterial activity of nanoparticles is their size, which may lead to differences between studies [58].
Conclusion
Within the limitations of the present study, GO-DAP revealed antibacterial efficacy against E. faecalis even after 24 h and represented a promising candidate as an intracanal medicament in root canal therapy.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- E. faecalis :
-
Enterococcus faecalis
- TAP:
-
Triple antibiotic paste
- DAP:
-
Double antibiotic paste
- GO:
-
Graphene oxide
- CFU:
-
Colony forming unit
- TEM:
-
Transmission electron microscopy
- EDS:
-
Energy-dispersive X-ray spectroscopy
- FTIR:
-
Fourier transform infrared
- EDTA:
-
Ethylenediaminetetraacetic acid
- rGO:
-
Reduced graphene oxide
- H3PO4 :
-
Phosphoric acid
- H2SO4 :
-
Sulfuric acid
- KMnO4 :
-
Potassium permanganate
- H2O2 :
-
Hydrogen peroxide
References
Abouelenien SS, Ibrahim SM, Shaker OG, Ahmed GM. Evaluation of postoperative pain in infected root canals after using double antibiotic paste versus calcium hydroxide as intra-canal medication: a randomized controlled trial. F1000Research. 2018;7:1768.
Mofidi H, Khosravani SR, Garjani S, Mirghotbi T. Evaluate the efficacy of laser therapy in root canal disinfection with different irrigants protocols: a systematic review and meta-analysis. Eurasia J Biosci. 2020;14:4697–701.
Alfadda S, Alquria T, Karaismailoglu E, Aksel H, Azim AA. Antibacterial effect and bioactivity of innovative and currently used intracanal medicaments in regenerative endodontics. J Endod. 2021;47(8):1294–300.
Sahebi S, Asheghi B, Samadi Y, Eskandari F. Effect of calcium hydroxide and nano calcium hydroxide on push-out bond strength of epoxy resin sealer to root canal dentin. Iran Endod J. 2022;17(1):13–39.
Adl A, Razavian A, Eskandari F. The efficacy of EndoActivator, passive ultrasonic irrigation, and Ultra X in removing calcium hydroxide from root canals: an in-vitro study. BMC Oral Health. 2022;22(1):1–7.
Akcay M, Arslan H, Topcuoglu HS, Tuncay O. Effect of calcium hydroxide and double and triple antibiotic pastes on the bond strength of epoxy resin–based sealer to root canal dentin. J Endod. 2014;40(10):1663–7.
Devaraj S, Jagannathan N, Neelakantan P. Antibiofilm efficacy of photoactivated curcumin, triple and double antibiotic paste, 2% chlorhexidine and calcium hydroxide against Enterococcus fecalis in vitro. Sci Rep. 2016;6(1):1–6.
Varshini R, Subha A, Prabhakar V, Mathini P, Narayanan S, Minu K. Antimicrobial efficacy of Aloe vera, lemon, Ricinus communis, and calcium hydroxide as intracanal medicament against Enterococcus faecalis: a confocal microscopic study. J Pharm Bioallied Sci. 2019;11(Suppl 2):S256.
Goswami M, Baveja C, Bhushan U, Sharma S. Comparative evaluation of two antibiotic pastes for root canal disinfection. Int J Clin Pediatric Dent. 2022;15(Suppl 1):S12.
Rahimi S, Ghasemi N, Jabbari G, Zaheri Z, Torabi ZS, Darehchi NR. Effect of different intracanal medicaments on the fracture resistance of the human root. Dent Res J. 2022;19:9.
Madhukumar M, Geetha P, Nair KR, Unnikrishnan M. The effects of double antibiotic paste and amoxicillin-clavulanate paste used in endodontic regeneration on microhardness of radicular dentine: an in vitro study. J Pharm Bioallied Sci. 2021;13(Suppl 1):S510.
Chouhan A, Mungse HP, Khatri OP. Surface chemistry of graphene and graphene oxide: a versatile route for their dispersion and tribological applications. Adv Coll Interface Sci. 2020;283:102215.
Sun X, Huang C, Wang L, Liang L, Cheng Y, Fei W, et al. Recent progress in graphene/polymer nanocomposites. Adv Mater. 2021;33(6):2001105.
Valentini F, Calcaterra A, Ruggiero V, Pichichero E, Martino A, Iosi F, et al. Functionalized graphene derivatives: antibacterial properties and cytotoxicity. J Nanomater. 2019;2019.
Ji H, Sun H, Qu X. Antibacterial applications of graphene-based nanomaterials: recent achievements and challenges. Adv Drug Deliv Rev. 2016;105:176–89.
Mohammed H, Kumar A, Bekyarova E, Al-Hadeethi Y, Zhang X, Chen M, et al. Antimicrobial mechanisms and effectiveness of graphene and graphene-functionalized biomaterials. A scope review. Front Bioeng Biotechnol. 2020;8:465.
Ge Z, Yang L, Xiao F, Wu Y, Yu T, Chen J, et al. Graphene family nanomaterials: properties and potential applications in dentistry. Int J Biomater. 2018;2018.
Palmieri V, Papi M, Conti C, Ciasca G, Maulucci G, De Spirito M. The future development of bacteria fighting medical devices: the role of graphene oxide. Expert Rev Med Devices. 2016;13(11):1013–9.
Liu C, Tan D, Chen X, Liao J, Wu L. Research on graphene and its derivatives in oral disease treatment. Int J Mol Sci. 2022;23(9):4737.
Croitoru A, Oprea O, Nicoara A, Trusca R, Radu M, Neacsu I, et al. Multifunctional platforms based on graphene oxide and natural products. Medicina. 2019;55(6):230.
Singh AA, Makade CS, Krupadam RJ. Graphene nanoplatelets embedded polymer: an efficient endodontic material for root canal therapy. Mater Sci Eng C. 2021;121:111864.
Yousefi M, Dadashpour M, Hejazi M, Hasanzadeh M, Behnam B, de la Guardia M, et al. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater Sci Eng C. 2017;74:568–81.
Ouyang L, Deng Y, Yang L, Shi X, Dong T, Tai Y, et al. Graphene-oxide-decorated microporous polyetheretherketone with superior antibacterial capability and in vitro osteogenesis for orthopedic implant. Macromol Biosci. 2018;18(6):1800036.
Guo C, Lu R, Wang X, Chen S. Antibacterial activity, bio-compatibility and osteogenic differentiation of graphene oxide coating on 3D-network poly-ether-ether-ketone for orthopaedic implants. J Mater Sci Mater Med. 2021;32(11):1–14.
Rodrigues CT, De Andrade F, De Vasconcelos L, Midena R, Pereira T, Kuga M, et al. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int Endod J. 2018;51(8):901–11.
Ioannidis K, Niazi S, Mylonas P, Mannocci F, Deb S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent Mater. 2019;35(11):1614–29.
Sharma D, Bhat M, Kumar V, Mazumder D, Singh S, Bansal M. Evaluation of antimicrobial efficacy of graphene silver composite nanoparticles against E. faecalis as root canal irrigant: an ex-vivo study. Int J Pharm Med Res. 2015;3(5):267–72.
Trusek A, Kijak E. Drug carriers based on graphene oxide and hydrogel: opportunities and challenges in infection control tested by amoxicillin release. Materials. 2021;14(12):3182.
Zancan RF, Cavenago BC, Oda DF, Bramante CM, Andrade FBD, Duarte MAH. Antimicrobial activity and physicochemical properties of antibiotic pastes used in regenerative endodontics. Braz Dent J. 2019;30:536–41.
Mousavi SM, Hashemi SA, Ghahramani Y, Azhdari R, Yousefi K, Gholami A, et al. Antiproliferative and apoptotic effects of graphene oxide@ AlFu MOF based saponin natural product on OSCC line. Pharmaceuticals. 2022;15(9):1137.
Abbaszadegan A, Dadolahi S, Gholami A, Moein MR, Hamedani S, Ghasemi Y, et al. Antimicrobial and cytotoxic activity of Cinnamomum zeylanicum, calcium hydroxide, and triple antibiotic paste as root canal dressing materials. J Contemp Dent Pract. 2016;17(2):105–13.
Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care. 2002;6(4):1–7.
Jacques Rezende Delgado R, Helena Gasparoto T, Renata Sipert C, Ramos Pinheiro C, Gomes de Moraes I, Brandão Garcia R, et al. Antimicrobial activity of calcium hydroxide and chlorhexidine on intratubular Candida albicans. Int J Oral Sci. 2013;5(1):32–6.
Javidi M, Afkhami F, Zarei M, Ghazvini K, Rajabi O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust Endod J. 2014;40(2):61–5.
Gholami A, Emadi F, Nazem M, Aghayi R, Khalvati B, Amini A, et al. Expression of key apoptotic genes in hepatocellular carcinoma cell line treated with etoposide-loaded graphene oxide. J Drug Deliv Sci Technol. 2020;57:101725.
Abu-Nada A, Abdala A, McKay G. Isotherm and kinetic modeling of strontium adsorption on graphene oxide. Nanomaterials. 2021;11(11):2780.
Ahmad W, Ahmad Q, Yaseen M, Ahmad I, Hussain F, Mohamed Jan B, et al. Development of waste polystyrene-based copper oxide/reduced graphene oxide composites and their mechanical, electrical and thermal properties. Nanomaterials. 2021;11(9):2372.
Croitoru A-M, Moroșan A, Tihăuan B, Oprea O, Motelică L, Trușcă R, et al. Novel graphene oxide/quercetin and graphene oxide/juglone nanostructured platforms as effective drug delivery systems with biomedical applications. Nanomaterials. 2022;12(11):1943.
Mahmoodi H, Fattahi M, Motevassel M. Graphene oxide–chitosan hydrogel for adsorptive removal of diclofenac from aqueous solution: preparation, characterization, kinetic and thermodynamic modelling. RSC Adv. 2021;11(57):36289–304.
Ali HRH, Ali R, Batakoushy HA, Derayea SM. Solid-state FTIR spectroscopic study of two binary mixtures: cefepime-metronidazole and cefoperazone-sulbactam. J Spectrosc. 2017;2017.
Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S. Spectroscopic characterization of biofield treated metronidazole and tinidazole. Med Chem. 2015;5(7):340–4.
Eskandari F, Razavian A, Hamidi R, Yousefi K, Borzou S. An updated review on properties and indications of calcium silicate-based cements in endodontic therapy. Int J Dent. 2022;2022:6858088.
Ramburrun P, Pringle NA, Dube A, Adam RZ, D’Souza S, Aucamp M. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials. 2021;14(12):3167.
Ismail IH, Al-Bayaty FH, Yusof EM, Khan HBSG, Hamka FA, Azmi NA. Evaluation of antimicrobial effect of Malaysian geopropolis with Aloe vera against Enterococcus faecalis to be used as an intracanal medicament in endodontics. J Conserv Dent JCD. 2020;23(5):489.
Alghamdi F, Shakir M. The influence of Enterococcus faecalis as a dental root canal pathogen on endodontic treatment: a systematic review. Cureus. 2020;12(3):e7257.
El-Telbany M, El-Didamony G, Askora A, Ariny E, Abdallah D, Connerton IF, et al. Bacteriophages to control multi-drug resistant Enterococcus faecalis infection of dental root canals. Microorganisms. 2021;9(3):517.
Wu S, Liu Y, Zhang H, Lei L. Nano-graphene oxide with antisense walR RNA inhibits the pathogenicity of Enterococcus faecalis in periapical periodontitis. J Dent Sci. 2020;15(1):65–74.
Jenks DB, Ehrlich Y, Spolnik K, Gregory RL, Yassen GH. Residual antibiofilm effects of various concentrations of double antibiotic paste used during regenerative endodontics after different application times. Arch Oral Biol. 2016;70:88–93.
Sadek RW, Moussa SM, El Backly RM, Hammouda AF. Evaluation of the efficacy of three antimicrobial agents used for regenerative endodontics: an in vitro study. Microb Drug Resist. 2019;25(5):761–71.
Sabrah AH, Yassen GH, Liu W-C, Goebel WS, Gregory RL, Platt JA. The effect of diluted triple and double antibiotic pastes on dental pulp stem cells and established Enterococcus faecalis biofilm. Clin Oral Invest. 2015;19(8):2059–66.
Zhong L, Yun K. Graphene oxide-modified ZnO particles: synthesis, characterization, and antibacterial properties. Int J Nanomed. 2015;10(Spec Iss):79.
Nanda SS, Yi DK, Kim K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci Rep. 2016;6(1):1–12.
Akbari T, Pourhajibagher M, Hosseini F, Chiniforush N, Gholibegloo E, Khoobi M, et al. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagn Photodyn Ther. 2017;20:148–53.
Ghorbanzadeh R, Assadian H, Chiniforush N, Parker S, Pourakbari B, Ehsani B, et al. Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: an ex vivo biofilm model. Photodiagn Photodyn Ther. 2020;29:101643.
Martini C, Longo F, Castagnola R, Marigo L, Grande NM, Cordaro M, et al. Antimicrobial and antibiofilm properties of graphene oxide on Enterococcus faecalis. Antibiotics. 2020;9(10):692.
Mahmoud ME, Fekry NA, Mohamed SM. Effective removal of Pb (II)/4-nitroaniline/E. faecalis and E. coli pollutants from water by a novel unique graphene quantum dots@ gemifloxacin@ double-layered Fe/Al nanocomposite. J Water Process Eng. 2022;46:102562.
Nanda SS, An SSA, Yi DK. Oxidative stress and antibacterial properties of a graphene oxide-cystamine nanohybrid. Int J Nanomed. 2015;10:549.
Niranjan R, Zafar S, Lochab B, Priyadarshini R. Synthesis and characterization of sulfur and sulfur-selenium nanoparticles loaded on reduced graphene oxide and their antibacterial activity against gram-positive pathogens. Nanomaterials. 2022;12(2):191.
Pulingam T, Thong KL, Ali ME, Appaturi JN, Dinshaw IJ, Ong ZY, et al. Graphene oxide exhibits differential mechanistic action towards gram-positive and gram-negative bacteria. Colloids Surf B. 2019;181:6–15.
Vi TTT, Kumar SR, Pang J-HS, Liu Y-K, Chen DW, Lue SJ. Synergistic antibacterial activity of silver-loaded graphene oxide towards Staphylococcus aureus and Escherichia coli. Nanomaterials. 2020;10(2):366.
Acknowledgements
The authors thank Dr. Mehrdad Vossoughi from the Dental Research Development Center, for helping with the statistical analyses. Appreciation is expressed to Ms. Farzaneh Rasooli for copyediting and improving the English structure of this manuscript.
Funding
This study was founded by the Vice-Chancellery of Research of Shiraz University of Medical Sciences (Grant#23061). This fund was used for the collection of samples, providing materials and tests, and statistical analysis of data.
Author information
Authors and Affiliations
Contributions
FE collected data,interpreted the results and was a major contributor in writing the manuscript. AA conceptualized the study and aided in research design. FE and AG performed all the laboratory works and tests. YG designed the study, supervised the research, and reviewed the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study design was approved by the Ethics in Human Research Committee of Shiraz University of Medical Sciences (IR.SUMS.DENTAL.REC.1400.021). All human teeth used for this study were collected from the Oral and Maxillofacial Surgery Department of Shiraz Faculty of Dentistry. The patients were informed that their teeth will be used for research purposes and signed a written consent form before the teeth extraction.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eskandari, F., Abbaszadegan, A., Gholami, A. et al. The antimicrobial efficacy of graphene oxide, double antibiotic paste, and their combination against Enterococcus faecalis in the root canal treatment. BMC Oral Health 23, 20 (2023). https://doi.org/10.1186/s12903-023-02718-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-02718-4