Abstract
Background
Some head and neck cancer surgeons found that many patients with locally advanced head and neck squamous cell carcinoma (LA-HNSCC) without postoperative radiotherapy (PORT) also have a good prognosis. The purpose of this study was to determine the effect of PORT on survival in patients with LA-HNSCC.
Methods
A case-match cohort analysis was performed at two institutions on patients with LA-HNSCC. Patients who received surgery alone were case-matched 1: 1 with patients treated by surgery plus PORT based on pT, pN, tumor subsite etc.
Results
114 patients were matched into 57 pairs, with a median follow-up period of 40.2 months. No difference in overall survival (OS, HR 0.88; 95% CI 0.50–1.58; P = 0.79) or disease-specific survival (DFS, 0.86; 95% CI 0.50–1.50; P = 0.76) was observed with no PORT.
Conclusions
PORT isn’t necessary for patients with LA-HNSCC who are treated for the first time as long as the head and neck cancer surgeon adhere to appropriate surgical concepts. The indications of PORT for patients with LA-HNSCC need to be further discussed.
Similar content being viewed by others
Background
Head and neck cancer is one of the most common malignancies, and squamous cell carcinoma (SCC) accounts for approximately 90% of all head and neck cancers [1]. Head and neck squamous cell carcinoma (HNSCC) arises in the oral cavity, oropharynx, larynx or hypopharynx and is the sixth leading cancer by incidence worldwide, with more than 600,000 cases diagnosed annually. Only 40%–50% of patients with HNSCC survive for 5 years [2]. Surgery, radiotherapy and chemotherapy are the mainstays of primary treatment in patients with HNSCC [3, 4]. However, despite the progress of multiple treatments and multidisciplinary treatments, the effect of treatment on HNSCC is not good, especially for locally advanced HNSCC (LA-HNSCC) [5, 6]. LA-HNSCC is cancer that has grown outside the origin organ but has not yet spread to distant parts of the body (Stage III/IV disease, pT3-4N0 or pT1-4N+). Most patients present with locoregionally advanced disease, and more than 50% have recurrence within 3 years [7]. At the same time, comprehensive treatment brings more complications and social and economic burden to patients. However, many experienced head and neck cancer surgeons have found that not all patients with LA-HNSCC, especially those with oral squamous cell carcinoma (OSCC) need adjuvant radiotherapy to achieve an ideal prognosis [8, 9]. Our previous study [10] (including some unpublished data) found that patients with locally advanced OSCC did not necessarily need postoperative radiotherapy (PORT) to achieve a good prognosis under the premise of high-quality surgical resection of the tumour.
Based on the above results and the clinical data of our unit, we propose two questions: (1) Is the benefit of PORT for head and neck squamous cell carcinoma as important as we expected and (2) Should the indication of PORT be further discussed? To answer these questions, we performed a case-match cohort analysis on patients with HNSCC at two institutions.
Methods
Eligibility criteria
Patients with newly diagnosed HNSCC between February 2010 and August 2016 were identified from two institutions. The retrospective study is approved by the ethics committee, and all participants provided written informed consent. Experimental protocols were approved by the appropriate institutional review committee (2019–222) and meet the guidelines of their responsible governmental agency. Patients are informed of all surgery-related and post-operative procedures and prognoses, and all patients are given their own choice of treatment. The including criteria were as follow: (1) HNSCC treated by primary surgery, including neck dissection; (2) cT3-4N0 or cT1-4N+; (3) at least 3-year follow-up. The exclusion criteria were: (1) previously treated HNSCC; (2) positive margin of tumour resection on histological examination; (3) distant metastasis; (4) incomplete follow-up data; (5) death within 1 month after surgery; (6) receiving PORT but not completing the radiotherapy plan and (7) age < 18 years.
Surgical technique
Our team has been committed to clinical research of the surgical treatment of HNSCC. Through long-term clinical studies, a large number of clinical cases were summarised. A series of improvements have been made to the surgical resection of HNSCC, which we call the anatomic unit (subunit) resection of HNSCC [10, 11]. The primary lesion excision was performed with anatomy unit resection surgery. All patients were performed radical resection of the primary lesion and neck dissection (suprascapulohyoid or full) with appropriate reconstruction (pedicle or free flap). The standard treatment of the surgery procedure is performed unified by two professional surgeons. All patients received en bloc excision with primary tumour excision combined with neck dissection. During the surgery, frozen sections were performed to confirm adequate margins. Figure 1 shows a typical case.
Postoperative radiotherapy
Radiotherapy was started 4–6 weeks after surgery. A dose of 1.8–2 Gy per day, 5 days per week, for 6 weeks (54–60 Gy in total) was used as standard conformal or intensity-modulated radiotherapy. A total radiation dose of 66 Gy was recommended in patients with high-risk features. A small number of high-risk patients received concurrent chemotherapy with PORT.
The irradiation field includes the scope of the primary tumor and subclinical (including postpharyngeal lymph nodes) before surgery. All cases require postoperative CT/MRI scans for postoperative radiotherapy reference. Clinical target volume (CTV) and planning target volume (PTV) are defined as follows:
CTV: (1) Primary tumor before surgery; (2) In patients with positive lymph nodes, the area of the affected lymph nodes found on clinical and imaging studies (CT or MRI scans). (3) In the primary area and neck, potentially subclinical affected areas in the microscope. (4) CTV1 (High-risk): a area with visible lesions (i.e., tumor bed/operating bed) found by clinical or radiological methods before surgery, and/or pathological examination revealed positive borders/lymph node invasion + extracapsular spread/no extracapsular spread multiple lymph node invasion. (5) CTV2 (low risk): is considered a potential subclinical lesion but not in a high-risk area.
PTV: (1) PTV1 needs to cover CTV1; (2) PTV2 needs to cover CTV2.
Grouping and pairing
A case-match cohort analysis (1:1) was performed between patients who were treated with surgery plus PORT and patients treated with surgery alone, eliminating patients who received chemotherapy after surgery. The investigator was blinded to the outcome during the matching. The included criteria is based on cT category. However, to make the statistical analysis more accurate, we used pT category in patient grouping. The hierarchy of matching was as follows: (1) pT category; (2) pN category; (3) tumour subsite; (4) age of the patient; (5) sex of the patient and (6) tumour differentiation.
Follow-up and outcomes
After completion of operation (surgery-alone) or radiotherapy (surgery plus PORT), patients were monitored every month during the first year, every 3 months during the second year, every 6 months during the third year, and once per year thereafter until death or data censoring. At each follow-up visit, the patients underwent a standard postoperative assessment performed upon hospital admission. The assessment included the following: head and neck/abdomen ultrasound examination (every visit), chest X-rays (every 6 months), head and neck computed tomography (CT) (every 6 months), head and neck magnetic resonance imaging (MRI) (every 6 months) and positron emission tomography (PET)-CT (if required). If the patients did not return, we contacted the patient or his/her family to inquire about the patient’s condition. Overall survival (OS) was calculated from the date of operation to the date of death. Disease-free survival (DFS) were calculated from the date of operation to recurrence or death resulting from any cause.
Statistical considerations
The primary end point was OS and the secondary end point was DFS. For descriptive analysis, categorical data were expressed as number and percentage. The survival analysis was conducted using the Kaplan–Meier method and log-rank test. Hazard ratios (HRs) were calculated using the Cox proportional hazards model. All hypothesis-generating tests were two sided, at a significance level of.05. Statistical analysis was performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 6.
Results
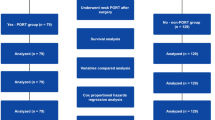
A total of 432 patients were eligible for this study. After performing the case-match-designed matching, only 57 pairs (114 patients) were included. The quality of the matching was excellent, as summarised in Table 1. P values for chi-square tests of homogeneity were greater than 0.4 for all matches and for all variables except groups. 57 matched pairs of patients (110 men and 4 women) with a mean age of 51 ± 9.8 years were identified. The median follow-up period was 40.2 months (range 3–101 months). All patients had LA-HNSCC. Of the 57 patients who received adjuvant treatment, surgery with radiotherapy were 44 patients, other 13 had surgery with chemoradiotherapy. In statistical analysis, some included patients turned from cT3-4N0 to pT1-2N0. In order to be more accurate, we eliminated pT1-2N0 patients. The characteristics were summarised in Table 2. 37 pairs of patients (71 men and 3 women) with a mean age of 48 ± 9.9 years were remained. Of the 37 patients who received adjuvant treatment, surgery with radiotherapy were 28 patients, other 9 had surgery with chemoradiotherapy. The study selection criteria, including the relevant reasons for exclusion, are illustrated in Fig. 2.
The 3-year estimated OS rate was 57.9% for those treated with surgery alone and 54.4% for those receiving surgery plus PORT. There was no significant difference in OS between the two arms (HR 0.88; 95% CI 0.50–1.58; P = 0.79; Fig. 3A). Similarly, there was no significant difference in DFS between the two groups (HR 0.86; 95% CI 0.50–1.50; P = 0.76; Fig. 3B). The 3-year DFS was 52.6% and 49.1% in the surgery-alone group and surgery plus PORT, respectively. Locoregional recurrence occurred in 24 cases in the surgery-alone group and 26 in surgery plus PORT group. There was no statistically significant difference between the two groups. The surgery-alone group had three patients with distant metastasis, and all three patients had pulmonary metastasis, one of whom had multiple metastasis, including adrenal gland and pelvis. The surgery plus PORT group had three distant metastasis: two to the lungs and one to the ribs. After the elimination, the result was the same. No differences in OS (HR 0.90; 95% CI 0.48–1.68; Fig. 4A) or DFS (HR 0.88; 95% CI 0.47–1.65; Fig. 4B) were observed.
Uni- and multi-variable analyses are shown in Table 3. In univariate analysis, only subsite and lymph node metastasis were associated with an increased risk of OS (RR = 1.331; 95% CI 1.063–1.667, 1.638; 95% CI 1.164–2.305, respectively), DFS (RR = 1.345; 95% CI 1.084–1.669, 1.726; 95% CI 1.241–2.400, respectively). Adjuvant chemotherapy resulted in a significant improvement in DFS (RR 0.483; 95% CI 0.248–0.943). In multivariable analysis, after control for age, smoking and alcohol status, no difference in OS (RR 1.184; 95% CI 0.649–2.162) or DFS (RR 1.169; 95%CI 0.659–2.073) was observed with the addition of PORT. Subsite was associated with an increased risk of OS (RR 1.457; 95% CI 1.122–1.890), and DFS (RR 1.423; 95% CI 1.104–1.834). Lymph node metastasis was associated with an increased risk of OS (RR 1.628; 95% CI 1.143–2.317), and DFS (RR 1.725; 95% CI 1.227–2.424). After the elimination, Subsite and lymph node metastasis were associated with an increased risk of OS and DFS in the remaining 37 pairs.
Based on the case-match cohort analysis of 57 pairs, 37 patients relapsed within 1 year after surgery, including 19 patients in the surgery-alone group and 18 patients in the surgery plus PORT group. A total of 46 patients (including the 37 patients who relapsed in 1 year) relapsed 2 years after surgery (22 in the surgery-alone group and 24 in the surgery plus PORT group). A total of 57 patients (27 in the surgery-alone group and 30 in the surgery plus PORT group) relapsed within 3 years after surgery. The mean recurrence time of patients was 33.7 months, the median recurrence time was 30 months, and the recurrence cases within 1 year accounted for 32.5% (37/114) of all the recurrence cases. After eliminating pT1-2N0 patients including 37 pairs, 27 patients relapsed within 1 year after surgery, including 14 patients in the surgery-alone group and 13 patients in the surgery plus PORT group. 33 patients (including the 27 patients who relapsed in 1 year) relapsed 2 years after surgery (16 in the surgery-alone group and 17 in the surgery plus PORT group). A total of 44 patients (21 in the surgery-alone group and 23 in the surgery plus PORT group) relapsed within 3 years after surgery. The mean recurrence time of patients was 33.2 months, the median recurrence time was 29 months, and the recurrence cases within 1 year accounted for 36.5% (27/74) of all the recurrence cases.
Discussion
This paper is a multi-institutional matched study of surgery-alone compared with surgery plus PORT in patients with LA-HNSCC. Our results showed that PORT did not improve OS or DFS in patients with LA-HNSCC when compared with surgery alone. The results are strengthened by the strict matching criteria. Two large head and neck cancer centres were utilised to recruit patients. Two institutions have adopted very similar treatment paradigms in their approaches to LA-HNSCC with a preference for withholding PORT wherever possible.
PORT has been included in the treatment guidelines for LA-HNSCC for about 50 years [12]. The results of our previous clinical study showed that patients with LA-HNSCC could obtain a good prognosis without PORT [10, 11, 13]. We designed this multicentre case-matching study to validate the conclusions of our previous study. The advantage of the case-matching study is that the factors and conditions affecting the experiment can be controlled in advance to make it as balanced as possible, reduce errors, and at the same time reduce the individual differences of the experimental subjects (patients). In order to eliminate the prescription bias associated with cohort studies comparing outcomes for patients receiving surgery alone with those treated by surgery plus PORT, we applied rigid matching criteria. Although minimised, the prescription bias cannot be completely eliminated. Medical comorbidity seriously affects the survival of patients, and because of the limited amount of data in our database, we did not use this factor as a match. That is unavoidable in all types of clinical studies, including RCTs. Meanwhile, the surgical techniques used in this study are similar to those of some scholars [14, 15]. The basic concept for the anatomic unit (subunit) resection is removal of the entire anatomical subunit in which tumour is contained rather than removing tumour with a 1–2 cm histopathological margin. The results of this clinical study showed that PORT did not significantly improve the OS rate or tumour-free survival rate of patients with LA-HNSCC. Multivariate analysis showed that only lymph node metastasis and tumour site were independent factors affecting survival.
In contrast with our study, many other trials [16,17,18] show that PORT shows an improvement in the outcome of patients with LA-HNSCC. The study by Yanamoto et al. [16] showed that after the propensity score analysis, PORT/concurrent chemoradiotherapy significantly improved OS (HR 0.554; 95% CI 0.38–0.80; P = 0.001) and DSS (HR 0.641; 95% CI 0.43–0.96; P = 0.030) compared to surgery only. The finding of Wang et al. [17] suggested that patients undergoing surgery had a 5-year OS of 27% compared with 66% for patients undergoing surgery plus adjuvant radiotherapy (P = 0.003); patients undergoing surgery had a 5-year DFS of 34% compared with 74% for patients undergoing surgery plus adjuvant radiotherapy (P = 0.001). However, compared with our study, these studies had some differences. Firstly, by the prescription bias associated with an unmatched comparative study of this nature, these above data are confounded; case-matching study can minimise the error in this aspect. Secondly, the concept of surgery in our study is different from that in the above studies. Another two case-matching studies [19, 20] came to the similar conclusion as our study: with the addition of adjuvant radiation (or radiation and chemotherapy), no difference in OS or DSS was observed. Another possible reason is that only five of the 114 patients in this study were oropharyngeal SCC and the rest were OSCC.
About the local toxicity associated with PORT on health-related quality of life, the negative impact is well documented [21, 22]. During and after treatment, overall health-related quality of life declines, and it will be at least 1 year before the baseline levels are recovered. However, physical function related to saliva, swallowing and chewing remain persistently low [23]. For conventional radiotherapy compared to intensity-modulated radiotherapy, this effect is worse [24].
Conclusions
Based on the results of our clinical trial, we conclude that PORT does not improve the OS of patients with LA-HNSCC, but may benefit the DFS of patients. Therefore, we believe that PORT is not necessary for patients with LA-HNSCC who are treated for the first time as long as the head and neck cancer surgeon adheres to appropriate surgical concepts (specifically the anatomic unit (subunit) resection). The evidence for this conclusion is not strong enough. Furthermore, the prognoses of the PORT for different surgical approaches need to be confirmed by other high-quality clinical studies.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to the requests of hospitals but are available from the corresponding author on reasonable request.
Abbreviations
- LA-HNSCC:
-
Locally advanced head and neck squamous cell carcinoma
- HNSCC:
-
Head and neck squamous cell carcinoma
- PORT:
-
Postoperative radiotherapy
- OS:
-
Overall survival
- DFS:
-
Disease-specific survival
- RCTs:
-
Randomized controlled trials
- SCC:
-
Squamous cell carcinoma
- OSCC:
-
Oral squamous cell carcinoma
References
Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA Cancer J Clin. 2015;65(5):401–21. https://doi.org/10.3322/caac.21293.
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. https://doi.org/10.1038/nrc2982.
Kaidar-Person O, Gil Z, Billan S. Precision medicine in head and neck cancer. Drug Resist Updates. 2018;40:13–6. https://doi.org/10.1016/j.drup.2018.09.001.
Koyfman SA, Ismaila N, Crook D, D’Cruz A, Rodriguez CP, Sher DJ, et al. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.18.01921.
Mesia R, Henke M, Fortin A, Minn H, Yunes Ancona AC, Cmelak A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):208–20. https://doi.org/10.1016/S1470-2045(14)71198-2.
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–8. https://doi.org/10.1016/S1470-2045(09)70311-0.
Tao Y, Auperin A, Sire C, Martin L, Khoury C, Maingon P, et al. Improved outcome by adding concurrent chemotherapy to cetuximab and radiotherapy for locally advanced head and neck carcinomas: results of the GORTEC 2007-01 phase III randomized trial. J Clin Oncol. 2018. https://doi.org/10.1200/jco.2017.76.2518.
Lee A, Givi B, Roden DF, Tam MM, Wu SP, Gerber NK, et al. Utilization and survival of postoperative radiation or chemoradiation for pT1-2N1M0 head and neck cancer. Otolaryngol Head Neck Surg. 2018;158(4):677–84. https://doi.org/10.1177/0194599817746391.
Brown JS, Blackburn TK, Woolgar JA, Lowe D, Errington RD, Vaughan ED, et al. A comparison of outcomes for patients with oral squamous cell carcinoma at intermediate risk of recurrence treated by surgery alone or with post-operative radiotherapy. Oral Oncol. 2007;43(8):764–73. https://doi.org/10.1016/j.oraloncology.2006.09.010.
Ren ZH, Gong ZJ, Wu HJ. Unit resection of buccal squamous cell carcinoma: description of a new surgical technique. Oncotarget. 2017;8(32):52420–31. https://doi.org/10.18632/oncotarget.14191.
Ren ZH, Wu HJ, Zhang S, Wang K, Gong ZJ, He ZJ, et al. A new surgical strategy for treatment of tongue squamous cell carcinoma based on anatomic study with preliminary clinical evaluation. J Craniomaxillofac Surg. 2015;43(8):1577–82. https://doi.org/10.1016/j.jcms.2015.07.034.
Maccomb WS, Fletcher GH. Planned combination of surgery and radiation in treatment of advanced primary head and neck cancers. Am J Roentgenol Radium Ther Nucl Med. 1957;77(3):397–414.
Onbasi Y, Lettmaier S, Hecht M, Semrau S, Iro H, Kesting M, et al. Is there a patient population with squamous cell carcinoma of the head and neck region who might benefit from de-intensification of postoperative radiotherapy? Strahlenther Onkol. 2019;195(6):482–95. https://doi.org/10.1007/s00066-018-1415-y.
Trivedi NP, Kekatpure V, Kuriakose MA. Radical (compartment) resection for advanced buccal cancer involving masticator space (T4b): our experience in thirty patients. Clin Otolaryngol. 2012;37(6):477–83. https://doi.org/10.1111/j.1749-4486.2012.02529.x.
Calabrese L, Bruschini R, Giugliano G, Ostuni A, Maffini F, Massaro MA, et al. Compartmental tongue surgery: long term oncologic results in the treatment of tongue cancer. Oral Oncol. 2011;47(3):174–9. https://doi.org/10.1016/j.oraloncology.2010.12.006.
Yanamoto S, Otsuru M, Ota Y, Okura M, Aikawa T, Kurita H, et al. Multicenter retrospective study of adjuvant therapy for patients with pathologically lymph node-positive oral squamous cell carcinoma: analysis of covariance using propensity score. Ann Surg Oncol. 2015;22(3):992–9. https://doi.org/10.1245/s10434-015-4824-5.
Wang JT, Palme CE, Morgan GJ, Gebski V, Wang AY, Veness MJ. Predictors of outcome in patients with metastatic cutaneous head and neck squamous cell carcinoma involving cervical lymph nodes: improved survival with the addition of adjuvant radiotherapy. Head Neck. 2012;34(11):1524–8. https://doi.org/10.1002/hed.21965.
Graboyes EM, Zhan KY, Garrett-Mayer E, Lentsch EJ, Sharma AK, Day TA. Effect of postoperative radiotherapy on survival for surgically managed pT3N0 and pT4aN0 laryngeal cancer: analysis of the national cancer data base. Cancer. 2017;123(12):2248–57. https://doi.org/10.1002/cncr.30586.
Barry CP, Wong D, Clark JR, Shaw RJ, Gupta R, Magennis P, et al. Postoperative radiotherapy for patients with oral squamous cell carcinoma with intermediate risk of recurrence: a case match study. Head Neck. 2017;39(7):1399–404. https://doi.org/10.1002/hed.24780.
Jackson RS, Sinha P, Zenga J, Kallogjeri D, Suko J, Martin E, et al. Transoral resection of human papillomavirus (HPV)-positive squamous cell carcinoma of the oropharynx: outcomes with and without adjuvant therapy. Ann Surg Oncol. 2017;24(12):3494–501. https://doi.org/10.1245/s10434-017-6041-x.
Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. https://doi.org/10.1093/jnci/djv403.
Lacas B, Bourhis J, Overgaard J, Zhang Q, Gregoire V, Nankivell M, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221–37. https://doi.org/10.1016/S1470-2045(17)30458-8.
Inhestern J, Schmalenberg H, Dietz A, Rotter N, Maschmeyer G, Jungehulsing M, et al. A two-arm multicenter phase II trial of one cycle chemoselection split-dose docetaxel, cisplatin and 5-fluorouracil (TPF) induction chemotherapy before two cycles of split TPF followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC-1). Ann Oncol. 2017;28(8):1917–22. https://doi.org/10.1093/annonc/mdx202.
Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. https://doi.org/10.1016/S1470-2045(10)70290-4.
Acknowledgements
Not applicable.
Funding
This work was supported by Shanghai Anticancer Association EYAS PROJECT (SACA-CY1B06); and The Interdisciplinary Program of Shanghai Jiao Tong University (ZH2018QNA08).
Author information
Authors and Affiliations
Contributions
ZHR drafted the work and prepared all figures and tablets. ZHR, JSL and ZMY have made substantial contributions to the acquisition, analysis and interpretation of data. SZ, JJY and HJW made contributions to the conception and design of the work, and have substantively revised it. All authors have contributed significantly, and all authors are in agreement with the content of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
A statement to confirm that all methods were carried out in accordance with relevant guidelines and regulations. A statement to confirm that all experimental protocols were approved by the ethics committee of the second Xiangya Hospital of CSU (2019-222; NO: JBWKQA001). The informed consent was obtained from all subjects or their legal guardians.
Consent for publication
Consent was obtained from patients to publish there images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ren, ZH., Lei, JS., Yang, ZM. et al. Postoperative radiotherapy may not be necessary for locally advanced head and neck squamous cell carcinoma: a case-match multicentre study. BMC Oral Health 22, 253 (2022). https://doi.org/10.1186/s12903-022-02288-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-022-02288-x