Abstract
Background
The active arterial-to-arterial collaterals are a significant factor in the prevention of ischemia and extensive tissue necrosis in the case of arterial blockage of various tissues. The present study investigates the mucogingival vasculature in the maxillary esthetic zone mucosa in human cadavers and functionally evaluates the area, which is supplied by the terminal arterioles, on the individual level.
Methods
In the human cadaver study, macroscopic arterial analyses of the anterior maxillary vestibule in 7 specimens were scrutinized by latex milk injection. The tracks of the mucosal branches in relation to the mucogingival junction were investigated. In the functional study, individual gingival blood flow (GBF) changes were measured by laser speckle contrast imaging (LSCI) in 31 young subjects with healthy gingiva before and during 30-s compressions. This was conducted with a ball-shaped condenser. The data was analyzed by the linear mixed model.
Results
The vertically aligned branches of the superior labial artery (SLA) divided into small, slightly deviating sub-branches near the mucogingival junction. These arteries created collateral plexuses and supplied the attached gingiva. The compression of these branches resulted in ischemia coronally with significant individual variation. The ischemia was either apico-mesial, apico-distal, or straight apical to the compression. A significant correlation was found between the ischemic area and the magnitude of the decrease in GBF (r = 0.81, p < 0.001). In males, 77% of the subjects, and 50% of the female subjects had an ischemic response in either region. The horizontal extension of the ischemic area ranged between 0.26 mm and 8.76 mm. Males had significantly higher baseline GBF and larger ischemia than females. At the base of the papilla, significant restoration of GBF was observed during compression in males, but not in females.
Conclusion
The arcade anastomoses formed by the small arteries in the keratinized gingiva of the upper esthetic zone explain the consequences of vertical incisions. The considerable individual variations in ischemic responses might be the reason for unexpected surgical outcomes in some cases. Furthermore, there is increasing evidence that men have different vascular reactivity and/or regulation of collateral circulation than women, which may affect wound healing.
Similar content being viewed by others
Background
Clinical observation findings suggest that flap elevations without vertical incisions favor an accelerated blood supply and uneventful wound healing, consequently improving esthetic outcomes and minimizing the risk of scarring [1,2,3,4,5,6]. However, in some cases of ridge augmentation or surgical endodontics, the incision can be placed at the mesial border of the flap to avoid cutting through the vessels coming from posterior to anterior [7]. It is also suggested to plan the incision perpendicularly from the mesial- or distal marginal course of the gingiva toward the midline of the papilla and gradually turn parallel to the tooth axis [8]. Prolonged periodontal and implant surgery with a vertical incision on the marginal gingiva and additional excessive local anesthetics with epinephrine result in long-term ischemia in the mucogingival flap [9,10,11]. However, further data are required regarding the influence of the incision design or wounding on the blood flow or angiogenesis in the anterior maxillary vestibule in humans [12,13,14,15,16].
There is a considerable variation regarding a flap’s survival after surgery [5, 13, 16,17,18,19,20]. This phenomenon could be explained by individual variations in surgical skills, but it may be more rational to consider differences between patients. Previous data suggest [5, 18, 19] the existence of significant variations among subjects in blood flow changes after periodontal surgery. Active arterial-to-arterial or arteriole-to-arteriole collaterals are crucial in the prevention of extensive tissue necrosis in cases of arterial blockage extent of heart failure and stroke [21,22,23]. The abundance is controlled by genes and is relatively uniform across the tissue within a specific subject [23, 24]. Nevertheless, there is scarce evidence available regarding the abundance, functionality, and individual variations of arterial-to-arterial collaterals in human gingiva. The gingiva and the connective tissue of the maxillary esthetic zone are mainly furnished by tributaries of the superior labial artery (SLA) and the infraorbital artery (IOA) [6]. Based on a corrosion cast of dog’s gingiva [25, 26], the sub-branches of these arteries ramify near the mucogingival junction and subsequently split into the arterioles. The arterioles pass deeply through the connective tissue of the attached gingiva and subdivide into smaller branches, forming a vascular network in the keratinized gingiva [26, 27].
The aims of the present human study are i) to characterize the mucogingival vasculature in maxillary esthetic zone mucosa by utilizing latex milk injection in cadavers and ii) to functionally evaluate the terminal arterioles branching from the vascular network in the keratinized gingiva via laser speckle contrast imaging (LSCI) in healthy volunteers.
Methods
The investigation is divided into two sections: i) human cadaver study of anterior vestibule arterial analyses in seven maxillae (five dentate, two partially dentate). Seven bodies (four males, three females; 43–69 years of age) donated to science were investigated at the Department of Macroscopic and Clinical Anatomy of the Medical University of Graz, Austria. The bodies were donated in accordance with the Department’s Donation Program and Styrian burial law. Donations were provided exclusively by the person’s own last will and written consent. ii) gingival blood flow (GBF) study involved 31 volunteers (13 males, 18 females) with healthy periodontal statuses. Their ages ranged from 21 to 29 years (mean age: 24). Exclusion criteria were pregnancy, smoking, clinically relevant systemic diseases, and any medication except for contraceptives. The participants granted their written informed consent before they underwent any procedure. The research was performed according to the Declaration of Helsinki (amended in 2013). Ethical permission was granted by the Hungarian authority, called the Committee of the Health Registration and Training Center (approval number: 092642/2015/OTIG).
-
(i)
Latex milk injection in human cadavers
The protocol ensued from the previous study [28]. The common carotid arteries (CCAs) were dissected and canulated. Then, intravascular Thiel solution (to flush the vessels) was introduced to the CCAs [28]. Cautiously, a red-colored agent (Pintasol red E-L3 mix paste, Mixol-products Diebold GmbH, Kirchheim, Germany) was combined with latex milk (Creato Latexmilch, Zitzmann Zentrale, Baden, Germany), and the mixture was injected via the CCAs [6, 28]. The cadavers were embalmed within Thiel solution for about 6–8 months [6, 28]. After that, the path of the arteries was examined.
-
(ii)
Blood flow measurements in healthy volunteers
Subjects were asked to avoid brushing, gargling, rinsing, eating or drinking 60 min before the session. Fifteen minutes before and during blood flow measurements, the patients were comfortably seated and relaxed in a standard supine position on a dental chair in a quiet room with a constant temperature of 24 °C. The patient’s head was fixed by a vacuum pillow. The lips were retracted carefully with a lip retractor (Spandex®, Hager & Werken, Germany). At the beginning, during and end of the intervention, each participant’s blood pressure was measured with an automatic blood pressure meter (Omron M2, Omron Healthcare Inc., Kyoto, Japan) on the left arm. The GBF was evaluated by LSCI (PeriCam PSI HR System, Perimed AB, Stockholm, Sweden). The LSCI illuminates the object with infrared red laser light. The laser light reflected from static objects forms a speckle pattern. However, moving cells blur this pattern proportionally to their velocity [29, 30]. By calculating the contrast of the captured image, LSCI can assess the microcirculation in a non-contact and non-invasive manner with high spatial and temporal resolution. It has an adequate reproducibility in human gingiva [31].
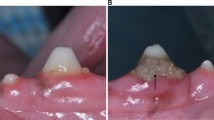
The region of interest measured approximately 2 × 3 cm; five images/sec were recorded, and they were averaged, thus registering one value per second. The resolution was set to 0.02 mm/pixel. The GBF was expressed in an arbitrary laser speckle perfusion unit (LSPU). After stabilization of GBF, the baseline value was recorded. The compression was made by a ball-headed metal instrument with a diameter of 1.5 mm (Fig. 1c). This procedure was followed by a single investigator that applied a constant force of approximately 20 g for 30 s. If a branch of the vessel was identified above the papilla, then it was selectively compressed. If no distinct vessel was seen, then the middle the most reddish area was compressed.
The upper oral vestibule esthetic zone with regions of interest. a The superior labial artery (SLA) gives a vertically orientated sub-branches that ramify (near the mucogingival junction) to slightly deviated tributaries moving toward the gingival papillae. The regions analogus to the LSCI’s ROIs are indicated by red rectangules. b The vertical branch of the SLA above the right upper incisors, bisects to small arteries and supply the mucosa. c Selection of the primary ROIs during the occlusion on the native LSCI image. d The mean and the standard error of the blood flow values, expressed in LSPU, in various ROIs in males (red line) and females (blue line). Significant differences compared to the baseline (TOI 1) are indicated by an asterisk, * in males and + in females, p < 0.05 after Bonferroni adjustment. Green arrows indicate a significant rebound in males during the second half of the compression
Primarily, seven regions of interest (ROI) were selected on the native speckle image (Fig. 1c) to obtain an overview of the affected area. ROI 1 and ROI 2 were selected on the papillary area by dividing the height of the papilla into half. ROI 3 and ROI 4 were just above ROI 2 and below the compression tool. ROI 5 and ROI 6 were the same size as ROI 3 and ROI 4 which were placed in the mid-buccal line of teeth 11 and 12. ROI 7 was selected on the papilla between teeth 12 and 13. This had the most significant distance from the compression; therefore, it was expected to be suitable for the reference region. Time of interests (TOI) were selected spanning 10-s intervals. The elapsed time between TOI 1 and TOI 2 (in the baseline period) and between TOI 3 and TOI 4 (in the compression period) was 10 s. Based on the findings of the primary ROIs, the selection of ROI 8 was constructed horizontally, connecting at the mid-buccal point of the teeth 11 and 12 (Fig. 2a).
Measurements of the horizontal extension of the ischemia. a Selection of ROI 8 for the assessment of horizontal dimension of ischemia. b The method of analysis of the raw data. The rectangle indicates the area between the crossing points of the blood flow curves measured at the baseline and under occlusion. The dashed line indicates the midline of the papilla
Data processing
The means of each primary ROI within each TOI were exported to a Microsoft Excel file. The data were analyzed in multiple steps. First, the LSPUs of all primary ROIs were statistically tested to ensure the stability of the reference ROI 7 and to calculate the mean change within a ROI during vascular occlusion. Then, the reference ROI 7 was selected to calculate the variance component between the TOIs, indicating the variance in time due to the random, within-subject noise. The repeatability coefficient (r) was utilized to determine the smallest difference that indicated a real change (with a 95% confidence interval) at the individual level [20, 31,32,33]. If the change in any of the 31 cases during occlusion was less then ‘r’ value, then the case was classified as ischemic due to the occlusion.
The ROI 8 was analyzed by exporting every single pixel that contained perfusion values (several thousand each) within the ROI into Excel from the TOI 2 (baseline) and TOI 3 (occlusion). The mean LSPUs for each column (vertical lines) of the horizontal ROI were calculated and graphically depicted (Fig. 2b). In the case of ischemia, the curves of the TOI 2 and TOI 3 formed two crossing-points. The number of pixels between the crossing points were utilized to calculate the horizontal dimension of the ischemic area; the dimension was calculated in mm by multiplying the number of pixels by 0.02 mm. The area under the curve of the TOI 2 was deducted from the area under the curve of TOI 3. This value was divided by number of pixels to calculate the mean magnitude of the ischemia for each case.
Statistical analysis
Data are presented as mean ± standard error of the mean. Blood flow alterations were investigated with a linear mixed model. The ROIs and TOIs were the main factors, and their interactions were integrated into the model; the respective baseline values were covariates. Pairwise comparisons were made with the least significant difference post hoc test. The p values were adjusted utilizing the sequential Bonferroni method for multiple pairwise comparisons, which was set at 0.05. The relationship between the horizontal dimension of the ischemic area and the mean magnitude of the ischemia was calculated with the Pearson correlation coefficient. The proportion between genders was tested via the Chi-square test. Statistical assessment was made by SPSS 25 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp).
Results
Vascular distribution in the maxillary esthetic zone of gingiva
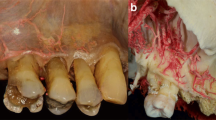
At least three vertically positioned arteries originating from the SLA supplied the mucosa of the maxillary esthetic zone (Fig. 1). Following the border of the movable and attached mucosae, these arteries ramified into small, slightly deviating branches (Figs. 1, 3). As the vessels neared the gingival margin and the papilla, the number of arterial mesh increased, and their diameters decreased. According to our observations, the midpoint of the gingiva did not receive an individual branch. Additionally, the pattern of arcade collateral anastomoses above and below the mucogingival junction was observed (Figs. 1, 3). Furthermore, the aligning of the arteries in the posterior and anterior aspects of the maxillary gingiva were compared (Fig. 3a, d). Due to the track of the posterior superior alveolar artery (PSAA), the orientation of the mucosal branches at the premolar zone was more mesialized than the esthetic zone (Fig. 3a, d).
Distribution of the arterial network before and after the maxillary canines in cadavers. a The small arteries in the upper right premolar area are mesialized compared to the esthetic zone. b The meshwork of anastomoses at the level of the upper right canine, supplies several parts of the gingiva. c Magnified small arterial network in the esthetic zone gingiva. d Envisioning the tributaries of the posterior superior alveolar artery (PSAA) and superior labial artery (SLA) under the mucosa
Effect of compression on the microcirculation of GBF
The vascular pattern of the compressed area was categorized into two groups. In 8 cases (26%), the pattern appeared diffuse (Fig. 4c, i), and in 23 cases (74%), a single vessel could be identified for compression (Fig. 4a, e, g). The center of the compression varied in localization between 1.58 mm distal or 2.20 mm mesial to the midline of the papilla. However, it was always located within the dimension of the papilla base. The change in blood flow after compression was not dependent on the baseline patterns (Fig. 4).
Five cases demonstrate considerable individual variability. a In Subject 6, a single vessel can be seen, and b ischemia developed in the attached gingiva, distal to the compression with a minor effect on the papilla. c In Subject 11, no single vessel can be seen, and d ischemia developed in the attached gingiva mesial to the compression with a minor effect on papilla. e In Subject 8, a single small vessel was compressed, and f only the papilla appears ischemic. g In Subject 7, a single large vessel was seen, and h a wide, symmetrical ischemic area with complete papillary involvement was developed. i In Subject 25, a diffuse reddish area can be seen, and j a wide, symmetrical ischemic area with papillary involvement was developed
No significant change in GBF was observed between two baseline TOIs (TOI 1 and TOI 2) in any regions (Fig. 1d). Men had significantly higher GBF than women in all regions and all TOIs (Fig. 1d). In females, the GBF of ROI 1, 2, 3, and 4 significantly (p < 0.001–0.05) dropped by -25 to -31 LSPU with similar magnitudes in TOI 3 and TOI 4. In males, the GBF of ROI 1, 2, 3, and 4 significantly (p < 0.001) dropped in TOI 3 by -42 to -64 LSPU. However, the GBF of TOI 4 was significantly (p < 0.001) higher than that of TOI 3 in ROI 3 and ROI 4. The GBF was restored to the baseline in these ROIs during the occlusion. The significant interaction between time and gender was observed during occlusion (TOI 3, p < 0.05), indicating that ischemia was higher in males than in females. In ROI 5 and 6, no ischemic response was observed, but in the ROI 5 at TOI 4, the blood flow increased significantly by 25 LSPU (p < 0.01). In ROI 7, no significant change was observed in any TOIs (+ 1–5 LSPU).
The horizontal extension of the ischemia in the attached gingiva was assessed by analyzing of ROI 8. The mean position of the ischemic area was shifted mesially by 0.27 mm relative to the compression. The shifting varied significantly between subjects; it ranged from 1.51 mm distal to 2.59 mm mesial. The mean width of the ischemic area was 3.8 ± 0.38 mm and ranged between 0.26 mm and 8.76 mm between subjects. The width was not significantly different between genders (3.29 ± 0.46 mm in females, 4.51 ± 0.62 in males, p = 0.116).
The calculated ‘r’ of the reference ROI 7 was 33.3 LSPU. Every change less than -33 LSPU or greater than 33 LSPU was considered a real difference within a specific subject with 95% confidence. In males, 10 subjects of 13 (77%) had an ischemic response in either the papillary or attached gingiva. In females, only 9 of the 18 subjects (50%) indicated an ischemic response, but the difference in proportion between genders was not statistically significant (p = 0.129). The vascular rebound during the 30-s ischemic period (blood flow was elevated by at least 33 LSPU from the TOI 3 to 4) was seen in 8 (38%) of the ischemic cases. The incidence of the rebound was not significantly different between genders (5 males and 3 females). The distribution of biotypes between genders was not different (thin-scalloped: 6 females and 5 males, thick-flat: 7 females and 3 males, thick-scalloped: 5 females and 5 males; p = 0.635). No differences in blood flow were observed between biotypes.
A moderate correlation in the magnitude of ischemia was observed between the attached gingiva (ROI 8) and the papilla (r = 0.56, p < 0.01 for ROI 1 and r = 0.68, p < 0.001 for ROI 2; Fig. 5). There was a strong correlation between the magnitude of the horizontal extent of the ischemia in the attached gingiva (ROI 8) and the mean drop in blood flow (r = 0.81, p < 0.001).
Correlation between the change in blood flow in the attached gingiva (ROI 8) and papilla (ROI 1 and 2). ROI 1 (blue points) is localized at the papilla tip; ROI 2 (orange points) is localized at the base of the papilla. The blue and orange dots are usually close together. Dashed lines indicate the linear regression between attached gingiva and, ROI 1 (blue) and ROI 2 (orange). A green circle indicates a subject with significant ischemia in the attached gingiva, but lack of significant ischemia in the papilla. The purple circle indicates papillary ischemia. Red circles indicate two subjects with extremely high ischemia in all three ROIs
Discussion
During incision and flap design, acknowledgement of vascular track and decent circulation results in proper angiogenesis, vasculogenesis, and primary wound healing [7, 34, 35]. The maxillary vestibule presents a complex vascular circulation [6], which might be the cause of intra- and postoperative complications. Specifically, the movable mucosa of the maxillary esthetic zone receives at least three vertical branches originating from the SLA. According to our observations, the arteries were approximately localized above the upper front teeth. The high-resolution image captured by the LSCI indicated that there is a zone above the papilla which has higher microcirculation, and it is in accordance with previous findings [20, 36, 37]. In cadavers, no sign of a rich arterial network was discerned in the mid-papillary area compared to the mid-buccal area. Therefore, the differences could be explained by the level of the blood flow regulation and imply more intense microcirculation of the interdental area.
Previously [15, 16, 20], it has been emphasized that the arterioles in the attached gingiva are positioned primarily vertically. Similarly, Shahbazi et al. [6] have proposed that the sub-branches of the SLA (mainly supplying the mucosa/gingiva) and IOA (mainly supplying the periosteum/gingiva) in the upper esthetic zone have vertical orientations. Latex milk injection in both earlier [6] and current experiments reveal that following the junction of the movable and attached mucosae, the vertical arteries bisect to smaller branches. These diminutive sub-branches became slightly deviated and moved toward different parts of the attached gingiva. Similarly, the direction of the ischemic (mesial, distal oblique, or straight vertical) pattern after compression of the terminal branch varied among patients. Cadaver specimens also indicated a collateral anastomoses arcade network above and below the mucogingival junction in the upper esthetic zone. In periodontal and implant-related surgeries, predominantly, the arcade anastomoses in the attached mucosa are significant in the circulation of the gingiva [6]. During secondary wound healing of the exposed periosteum, the revascularization primarily begins laterally [5], which emphasizes the significance of horizontal branches. The study [5] also suggested limiting the horizontal extension of the surgical wound. Therefore, to maintain proper circulation and prevent ischemia in the keratinized gingiva, it is suggested to avoid vertical incisions in the marginal gingiva [5, 38]. The scar tissue formation directly correlates with the tissue hypoxia and delayed revascularization [5, 38, 39]. This coincides with the root coverage study [3] stating vertical incision can let in keloid formation.
In the cadaver investigation, number and information regarding the clinical condition of the gingivae were limited. Furthermore, previous studies indicated that vascular resistance, microcirculation pulse curve, and vascular morphology tend to change with the age [40,41,42]. Therefore, we aimed to functionally confirm the heterogeneity among patients by studing only young subjects with healthy gingivae. A previous study [20] applied a 10 mm wide horizontal compression mid-buccally. During the compression, no blood flow was distinguished in any of the cases, indicating a complete closure of the blood supply within the affected area. Some individuals in the present study experienced little or no ischemia after 1.5 mm wide compression. Therefore, the size of the area without effective compensation can be confined between 1.5 and 10 mm. This is in the range of the average distance of the vessels that supplied the attached gingiva observed in cadavers; however, it does not explain the individual variations. Generally, the arteriole-to-arteriole anastomoses interconnect a small part (generally < 0.05%) of arterioles in the crowns of neighboring arterial trees [43]. Their size on average is less than 100 μm in diameter in the majority of healthy species (including humans), and can be detected in most of the tissues [39]. The arteriole-to-arteriole anastomosis was rarely observed in the attached gingiva in a recent study [27]. This could explain the reason that some of our patients had extensive ischemia horizontally and vertically, although only a 1.5 mm wide area was blocked. Furthermore, these subjects also had the highest ischemia in magnitude. The subjects may have a single large vessel supplying a large area, up to 8 mm, without any existing collaterals. The size of vessels in the attached gingiva are less than 100 µm, according to the previous literature [25, 44], which indicates that the whole attached gingiva belongs to the microcirculation network. Conversely, large arteries (> 200 µm) in the deep layer of the attached gingiva have recently been revealed [27]. We suppose that in some instances the large artery may move directly toward the papilla without branching to attached gingiva, explaining the outcome in which only the papilla was ischemic. In this study, only 61% of the volunteers presented ischemia in different directions. The lack of response could be explained by the small sub-branches may run parallel (not visible in cadavers), and they could bypass the occluded branch. By acknowledging individual variations, surgical complications and failure might be prevented.
The blood vessels of the interdental papilla arise from interdental septa, periodontal ligament, and the gingiva [45]. The significant contribution of the gingiva in maintaining the papillary blood flow has recently been demonstrated [20]. The complete compression of the papilla base resulted in a considerable drop in the blood flow of the papilla. In the present study, although only a 1.5 mm area at the mucogingival line was occluded, in many cases, we found significant ischemia in the papilla. These results suggest that in physiological conditions, the main blood supply of the papilla originate from gingival vessels. During incision/flap designs, such as papilla preservation flap [10] or horizontal incision [5], the previously described alveolar and periodontal supplements may become activated by a mechanism called collateralization [43].
In addition to the suggested cross-connection between branches in the attached gingiva, collaterals between supraperiosteal and periodontal plexuses were elucidated in histological studies in dogs and cats [25, 44]. Conversely, the wide horizontal compression of the attached gingiva in humans demonstrateed no collateral circulation from the periodontal ligament [20]. Wounding the attached gingiva by 1.5 mm diameter punching either mid-buccal or mid-papillary [15] revealed seven days of ischemia coronal to the wound, which confirmed the significance of the supply from the vestibule. The study [15] measured 2.06 mm of the horizontal width of the ischemic area six hours after wounding. Notably, in our investigation, we applied a similar localization and dimension for a reversible blockade. We found that the mean horizontal width of the ischemic area was double in size (3.8 mm) than in the wounding study. The difference in extent might be explained by vasodilation and by collateralization within the six hours of healing. The relative standard deviation (i.e., coefficient of variation) of the whole ischemic area in the earlier wound study [15] resembles our findings (70%), similarly indicating significant individual variations. They [15] also observed the quicker revascularization of the mid-papillary area than the mid-buccal and confirmed the higher vascular perfusion of the mid-papillary zone.
The arteriovenous bundles run together when they enter keratinized gingiva; they branch and form a microcirculation mesh [25, 26]. The simultaneous blockade of venular outflow could prevent the refill of the vessels from the collaterals. However, in some cases, a significant rebound could be observed with a considerable variation in anastomosis mesh between individuals. In this study, males were found to be more prone to compensate the ischemia by opening collaterals. It has been previously found that males experience higher hyperemia responses after a complete closure of vessels in the attached gingiva [20]. Furthermore, the circulation of the coronally advanced flap was restored much earlier in males than in females [18], and there is evidence that males have better wound healing than females after a third molar surgery [46,47,48,49] as well as after palatal wounding [50]. Better blood flow recovery may facilitate wound healing in males. In previous investigations [5, 51, 52], blood flow changes induced either by nitric oxide, epinephrine, or surgery were not correlated with the gingival thickness measured by the ultrasonic instrument. Therefore, gender differences cannot be explained by biotype. A higher magnitude of ischemia in males indicates a higher vascular reactivity; however, the similar extent of the ischemic area in males and females suggests a similar distribution of the vessels.
Conclusion
Generally, the main arteries originating in the vestibule supply the attached gingiva by providing small, slightly deviated branches. This even distribution does not explain the considerable heterogeneity of the resting blood flow of the attached gingiva; thus, the regulation of the microcirculation may vary between sites. Furthermore, the localization and responsiveness of the collaterals considerably depend on the individual and the genders.
Availability of data and materials
The datasets utilized and analyzed during the study.
Abbreviations
- LSCI:
-
Laser Speckle Contrast Imaging
- SLA:
-
Superior labial artery
- IOA:
-
Infraorbital artery
- PSAA:
-
Posterior superior alveolar artery
- GBF:
-
Gingival blood flow
- CCAs:
-
Common carotid arteries
- LSPU:
-
Laser Speckle Perfusion Units
- ROI:
-
Regions of interest
- TOI:
-
Time of interests
References
Zuhr, O., et al., Surgery without papilla incision: tunneling flap procedures in plastic periodontal and implant surgery. Periodontol 2000, 2018;77(1):123–149.
de Sanctis M, Clementini M. Flap approaches in plastic periodontal and implant surgery: critical elements in design and execution. J Clin Periodontol. 2014;41(Suppl 15):S108–22.
Zucchelli G, et al. Coronally advanced flap with and without vertical releasing incisions for the treatment of multiple gingival recessions: a comparative controlled randomized clinical trial. J Periodontol. 2009;80(7):1083–94.
Zucchelli G, De Sanctis M. The coronally advanced flap for the treatment of multiple recession defects: a modified surgical approach for the upper anterior teeth. J Int Acad Periodontol. 2007;9(3):96–103.
Fazekas R, et al. Blood flow kinetics of a xenogeneic collagen matrix following a vestibuloplasty procedure in the human gingiva-An explorative study. Oral Dis. 2019;25(7):1780–8.
Shahbazi A, et al. Vascular survey of the maxillary vestibule and gingiva-clinical impact on incision and flap design in periodontal and implant surgeries. Clin Oral Investig. 2021;25(2):539–46.
Kleinheinz J, et al. Incision design in implant dentistry based on vascularization of the mucosa. Clin Oral Implants Res. 2005;16(5):518–23.
Velvart P, Peters CI. Soft tissue management in endodontic surgery. J Endod. 2005;31(1):4–16.
Ambrosini P, et al. A laser Doppler study of gingival blood flow variations following periosteal stimulation. J Clin Periodontol. 2002;29(2):103–7.
Retzepi M, Tonetti M, Donos N. Comparison of gingival blood flow during healing of simplified papilla preservation and modified Widman flap surgery: a clinical trial using laser Doppler flowmetry. J Clin Periodontol. 2007;34(10):903–11.
Sheikh R, et al. Hypoperfusion in response to epinephrine in local anaesthetics: Investigation of dependence on epinephrine concentration, spread of hypoperfusion and time to maximal cutaneous vasoconstriction. J Plast Reconstr Aesthet Surg. 2017;70(3):322–9.
Tatarakis N, et al. Blood flow changes using a 3D xenogeneic collagen matrix or a subepithelial connective tissue graft for root coverage procedures: a pilot study. Clin Oral Investig. 2018;22(4):1697–705.
Kaner, D., et al., Early Healing Events after Periodontal Surgery: Observations on Soft Tissue Healing, Microcirculation, and Wound Fluid Cytokine Levels. Int J Mol Sci. 2017;18(2).
Alssum L, et al. Gingival Perfusion and Tissue Biomarkers During Early Healing of Postextraction Regenerative Procedures: A Prospective Case Series. J Periodontol. 2017;88(11):1163–72.
Mormann W, Meier C, Firestone A. Gingival blood circulation after experimental wounds in man. J Clin Periodontol. 1979;6(6):417–24.
Mormann W, Ciancio SG. Blood supply of human gingiva following periodontal surgery. A fluorescein angiographic study. J Periodontol. 1977;48(11):681–92.
Logue T. Managing patients with gingival graft failure or loss. J Can Dent Assoc. 2014;80:e17.
Molnar E, et al. Evaluation of laser speckle contrast imaging for the assessment of oral mucosal blood flow following periodontal plastic surgery: an exploratory study. Biomed Res Int. 2017;2017:4042902.
Molnar B, et al. Assessment of palatal mucosal wound healing following connective-tissue harvesting by laser speckle contrast imaging: an observational case series study. Int J Periodontics Restorative Dent. 2019;39(2):e64–70.
Fazekas R, et al. Functional characterization of collaterals in the human gingiva by laser speckle contrast imaging. Microcirculation. 2018;25(3):e12446.
Meier P, et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116(9):975–83.
Zhang H, et al. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30(5):923–34.
Zhang H, Faber JE. Transient versus permanent MCA occlusion in mice genetically modified to have good versus poor collaterals. Med One. 2019;4.
Chalothorn D, et al. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30(2):179–91.
Nuki K, Hock J. The organisation of the gingival vasculature. J Periodontal Res. 1974;9(5):305–13.
Nobuto T, et al. Periosteal microvasculature in the dog alveolar process. J Periodontol. 1989;60(12):709–15.
Le NM, et al. A noninvasive imaging and measurement using optical coherence tomography angiography for the assessment of gingiva: An in vivo study. J Biophotonics. 2018;11(12):e201800242.
Shahbazi A, et al. Detection of vascular pathways of oral mucosa influencing soft- and hard tissue surgeries by latex milk injection. J Vis Exp. 2020(159).
Briers JD, Webster S. Laser speckle contrast analysis (LASCA): a nonscanning, full-field technique for monitoring capillary blood flow. J Biomed Opt. 1996;1(2):174–9.
Briers D, et al. Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt. 2013;18(6):066018.
Molnar E., et al. Assessment of the test-retest reliability of human gingival blood flow measurements by Laser Speckle Contrast Imaging in a healthy cohort. Microcirculation, 2018;25:2.
Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313(7049):106.
Gaverth J, et al. Test-retest and inter-rater reliability of a method to measure wrist and finger spasticity. J Rehabil Med. 2013;45(7):630–6.
Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther. 1991;52(3):407–22.
Shahbazi A, et al. Analysis of blood supply in the hard palate and maxillary tuberosity-clinical implications for flap design and soft tissue graft harvesting (a human cadaver study). Clin Oral Investig. 2019;23(3):1153–60.
Kerdvongbundit V, et al. Microcirculation of the healthy human gingiva. Odontology. 2002;90(1):48–51.
Gleissner C, et al. Local gingival blood flow at healthy and inflamed sites measured by laser Doppler flowmetry. J Periodontol. 2006;77(10):1762–71.
Liu Q, et al. Increased blood flow in keloids and adjacent skin revealed by laser speckle contrast imaging. Lasers Surg Med. 2016;48(4):360–4.
DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100(5):979–84.
Ohsugi Y, et al. Age-related changes in gingival blood flow parameters measured using laser speckle flowmetry. Microvasc Res. 2019;122:6–12.
Matheny JL, Johnson DT, Roth GI. Aging and microcirculatory dynamics in human gingiva. J Clin Periodontol. 1993;20(7):471–5.
Wada-Takahashi S, et al. Effect of physical stimulation (gingival massage) on age-related changes in gingival microcirculation. PLoS ONE. 2020;15(5):e0233288.
Faber JE, et al. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34(9):1854–9.
Nobuto T, et al. The relationship between connective tissue and its microvasculature in the healthy dog gingiva. J Periodontal Res. 1989;24(1):45–52.
Carranza N, Zogbi C. Reconstruction of the interdental papilla with an underlying subepithelial connective tissue graft: technical considerations and case reports. Int J Periodontics Restorative Dent. 2011;31(5):e45-50.
Conrad SM, et al. Patients' perception of recovery after third molar surgery. J Oral Maxillofac Surg, 1999;57(11): 1288–94; discussion 1295–6.
Phillips C, et al. Risk factors associated with prolonged recovery and delayed healing after third molar surgery. J Oral Maxillofac Surg. 2003;61(12):1436–48.
Benediktsdottir IS, et al. Mandibular third molar removal: risk indicators for extended operation time, postoperative pain, and complications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(4):438–46.
Adeyemo WL, Ladeinde AL, Ogunlewe MO. Clinical evaluation of post-extraction site wound healing. J Contemp Dent Pract. 2006;7(3):40–9.
Engeland CG, et al. Mucosal wound healing: the roles of age and sex. Arch Surg, 2006;141(12):1193–7; discussion 1198.
Ganti B, et al. Evidence of spreading vasodilation in the human gingiva evoked by nitric oxide. J Periodontal Res. 2019;54(5):499–505.
Vag J, et al. Epinephrine penetrates through gingival sulcus unlike keratinized gingiva and evokes remote vasoconstriction in human. BMC Oral Health. 2020;20(1):305.
Acknowledgements
The authors thank Tamás László Nagy, a dental student, who participated in the evaluation of the data. Additionally, the authors thank the Department of Conservative Dentistry; the Department of Anatomy, Histology, and Embryology; the Department of Periodontology of Semmelweis University, and the Department of Macroscopic and Clinical Anatomy of the Medical University of Graz for their support.
Funding
The authors gratefully acknowledge the following supports. The Laser Speckle Contrast Analyzer's operation cost was covered by the Hungarian Human Resources Development Operational Program (EFOP-3.6.2-16-2017-00006). The purchasing of the chemicals and manufacturing of the compression tools was supported by the Thematic Excellence Programme (2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapy thematic programme of the Semmelweis University.
Author information
Authors and Affiliations
Contributions
BMikecs, JV, and AS designed the study and drafted the manuscript. BMikecs performed a functional investigation. Morphological evaluation of the data was performed by AS. GG, BMolnár and GF contributed to the interpretation of findings and provided a critical review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In the cadaver study, the bodies were donated to science at the Department of Macroscopic and Clinical Anatomy of the Medical University of Graz, Austria, according to the Department’s Donation Program and Styrian burial law. Donations were provided exclusively by the person’s own last will and written consent. Since this is the highest ethical donation, no ethical commission vote was necessary. As documents were sent to the persons before donations, all were informed about the scientific and teaching purposes. The gingival blood flow study was conducted in accordance with the Declaration of Helsinki (amended in 2013). The participants granted their written informed consent before they underwent any procedure. Ethical approval was given by the Hungarian authority called Committee of the Health Registration and Training Center (approval number: 092642/2015/OTIG).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mikecs, B., Vág, J., Gerber, G. et al. Revisiting the vascularity of the keratinized gingiva in the maxillary esthetic zone. BMC Oral Health 21, 160 (2021). https://doi.org/10.1186/s12903-021-01445-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-021-01445-y