Abstract
Background
The changes in bone homeostasis observed during pregnancy and lactation could result in alterations in the rate of orthodontic tooth movement, but research in human subjects presents significant ethical and practical limitations. Our aim was to compare the amount of orthodontic tooth movement between pregnant/lactating or not animals.
Methods
We searched without restrictions 8 databases and performed hand searching until July 2019 (PubMed, Central, Cochrane Database of Systematic Reviews, SCOPUS, Web of Science, Arab World Research Source, ClinicalTrials.gov, ProQuest Dissertations and Theses Global). We searched for studies comparing quantitatively the amount of orthodontic tooth movement between pregnant/lactating or not animals. Following retrieval and selection of studies, the collection of related data was performed and the risk of bias was assessed using the SYRCLE’s Risk of Bias Tool. Exploratory synthesis was carried out using the random effects model.
Results
Four studies were finally identified raising no specific concerns regarding bias. One study showed that lactation increased the rate of tooth movement by 50 % [p < 0.05]. Although an overall increase was noted in the pregnancy group as well, it did not reach statistical significance [3 studies, Weighted Mean Difference: 0.10; 95% Confidence Interval: − 0.04 - 0.24; p = 0.165].
Conclusions
The metabolic changes occurring during pregnancy and lactation may have an impact on the rate of tooth movement in animals. Although these animal experimental results should be approached cautiously, it could be safe practice to consider the impact of these physiological changes in the clinical setting.
Registration
PROSPERO (CRD42018118003).
Similar content being viewed by others
Background
During pregnancy females experience physiological changes associated with increases in oestrogen and progesterone levels, which lead to functional and tissue metabolism alterations critical to ensure a healthy gestation [1]. Regarding the skeletal system, the needs for maternal minerals increase in order to fulfil the mineralization of the developing foetal skeleton [2]. As a response, the calcium content of the maternal skeleton augments during the initial stages of pregnancy; later small reductions in bone mineral density might also be observed [3].
It is well recognised that oestrogen levels exert a critical role regarding bone mass preservation during gestation [4]. Oestrogen receptors have been observed in human cells [5] and several lines of evidence support that inhibition of bone remodelling by oestrogen is a result of osteoclastogenesis prevention from marrow precursors, as well as by induction of the Fas/FasL system that leads to osteoclast apoptosis [6, 7]. Oestrogen exerts a further inhibitory role on bone resorption through effects on the receptor activator of nuclear factor-Kappa B (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) system and the production of some pro-resorptive cytokines (e.g. IL-1, IL-6, IL-7, TNF) [8,9,10,11,12,13,14]. However, oestrogen also affects directly the cells of the osteoblastic lineage contributing to bone preservation [15, 16].
Progesterone has also been shown to exert bone protective effects [17]. These results seem to be moderated directly via progesterone receptors in osteoblasts [18], as well as indirectly by acting as a ligand to the glucocorticoid receptor [17, 19]. Furthermore, progesterone may participate in the regulation of bone matrix, through its inhibitory action on metalloproteinases [20, 21].
Following pregnancy, lactation constitutes an important part of mammalian reproduction by ensuring the continuation of the supply of nutrients to the offspring [22]. The preparation of the female body begins already from pregnancy with increasing prolactin levels [23]. During lactation, prolactin that plays the principal role in stimulating the proliferation and differentiation of mammary cells [24], acts also as a key regulator of bone resorption by modulating sex hormone level [25, 26]. In general, lactation is characterized by a phase of oestrogen deficiency and attenuation of its bone protective effects [27]. Also, increases in osteoclasts are observed and overall bone remodelling alters in the direction of bone mass reduction [28]. In addition to oestrogen deficiency, other mechanisms including fluctuations in the levels of androgens and direct effects of prolactin on bone metabolism have been implicated with bone loss in women during lactation [29, 30]. At the same time, the requirements from the maternal system continue to be increased as the new-born gains minerals from the mother. If the dietary sources are insufficient, then a greater amount will be drawn from maternal skeletal sources, an event that could further affect negatively the maternal skeletal structure and lead to additional loss of bone mass [2].
As orthodontic tooth movement can be modulated by any condition that is implicated in the associated molecular pathways [31], the adaptive changes in bone homeostasis and the alterations in the balance between osteoclastic bone resorption and osteoblastic bone deposition observed during pregnancy and lactation could result in alterations in terms of the rate of tooth movement. However, to the best of our knowledge, this information has yet to be summarized in an evidence-based manner. As research in human subjects during these periods presents significant ethical and practical limitations, the use of animal models may provide a mean to improved understanding.
Objective
The objective of the present review was to systematically investigate and appraise the quality of the most up to date available evidence regarding the differences in terms of the rate of orthodontic tooth movement between pregnant/lactating or not animals.
Methods
Protocol and registration
Initially a special protocol was developed (registration in PROSPERO: CRD42018118003) [32]. Regarding conduct and reporting we adhered to relevant methodological guidelines [33,34,35]. As the present study was a systematic review, ethical approval was not required.
Eligibility criteria
The eligibility criteria were defined according the Participants, Intervention, Comparison, Outcomes and Study design domains (Table 1). We aimed to include prospective studies that compared quantitatively the amount of orthodontic tooth movement between pregnant/lactating or not animals of any kind [36]. We excluded the following types of studies: investigation on humans; studies involving animals subjected to additional clinical interventions such as tooth extraction, animals under medication, animals with pathological conditions or dietary deficiencies, like calcium deficiency that leads to additional decrease in bone density [37]. Also, we excluded ex vivo, in vitro, in silico studies; case studies; cross-over studies and studies without a separate control group; reviews (traditional reviews, systematic reviews and meta-analyses) and studies with less than 5 subjects per group analysed, based on relevant methodological suggestions [36].
Information sources and search strategy
Following the development of detailed search strategies, the two authors searched the whole content in 8 electronic databases until July 2019 (PubMed, Central, Cochrane Database of Systematic Reviews, SCOPUS, Web of Science, Arab World Research Source, ClinicalTrials.gov, ProQuest Dissertations and Theses Global) (Supplementary Table 1). The searches were conducted without placing restrictions on language and were supplemented by reviewing the bibliography in any relevant paper retrieved. Moreover, we had planned to contact the responsible author in the event we needed some clarifications on the content of a potentially eligible paper.
Study selection, data collection and data items
The two investigators assessed the retrieved records for inclusion separately without being blinded about the identity of the authors and kept a record on all decisions. Kappa statistics were not computed following relevant recommendations [34]. Subsequently, data extraction was carried out by filling in special forms the following items: bibliographic data; information on study design; animal and orthodontic mechanics characteristics; tooth movement measurement methodology and results.
Risk of bias in individual studies
The risk of bias was assessed by the authors using the SYRCLE’s risk of bias tool [38]. In all the processes described above any disagreements were resolved by discussion.
Summary measures, synthesis of results, risk of bias across studies and additional analyses
Data on the amount of tooth movement are continuous; thus, they were expressed as Weighted Mean Difference (WMD) accompanied by the 95% Confidence Intervals (CI). Exploratory synthesis for the effect of pregnancy on the amount of tooth movement at the point of the longest follow-up was carried out using the random effects model [39, 40]. The overlap of the 95% CI was inspected graphically and the I2 statistic was calculated [34]. Analyses were performed with Comprehensive Meta-analysis software 3.3.070 (©2014 Biostat Inc., Tampa, Florida, USA).
Based on the research protocol, subgroup analyses as well as analyses for “small-study effects” and publication bias were planned, but were not performed finally due to the lack of an adequate amount of data [34]. Despite the lack of extensive information, the quality of evidence was assessed following Guyatt et al. [41] in order to adopt a structured and transparent approach in formulating an interpretation of the evidence.
Results
Study selection
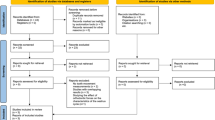
Database search rendered 452 records and 1 record was located through hand searching. Later, we excluded 80 records as duplicates and 368 based on their title and abstract. After the exclusion of one more paper because it involved animals with dietary calcium deficiency [42], four papers were considered eligible (Fig. 1) [43,44,45,46].
Study characteristics
The retrieved studies were published between 1991 and 2018 and investigated the influence of pregnancy [43,44,45] and lactation [46] on the amount of orthodontic tooth movement in rats and mice. Orthodontic tooth movement was induced by placing coil springs between maxillary incisors and molars or between incisors, as well as by using expansion arches on the molars, for periods of maximum 3 weeks. The rate of orthodontic tooth movement was assessed either clinically or radiographically from occlusal or lateral cephalometric radiographs, as well as micro-CT (Table 2). We tried to contact the corresponding authors of two studies for further information, but we are unable to get in touch with them [44, 46].
Risk of bias within studies
Table 3 presents the summary of findings regarding risk of bias assessment. For many domains there was insufficient information to permit judgements of low or high risk, but no important concerns were raised overall.
Results of individual studies and synthesis of results
Two studies showed more movement in the pregnant animals [44, 45] while no difference was observed in the third [43] (Fig. 2). Exploratory data synthesis showed an overall increase in tooth movement in the pregnancy group that did not reach statistical significance [WMD: 0.10; 95% CI: − 0.04 - 0.24; p = 0.165; I2 = 72%]. Regarding lactation, Macari et al. [46] reported a significantly greater amount of tooth movement in lactating animals compared to the control group by 50% [p < 0.05].
Additional analyses and risk of bias across studies
It was not possible to conduct analyses for “small-study effects” and publication bias, nor for subgroup analyses. Regarding the effect of pregnancy and lactation on the amount of orthodontic tooth movement the quality of available evidence was considered as moderate (Supplementary Table 2).
Discussion
Summary of available evidence
The alterations in bone homeostasis occurring during pregnancy and lactation could possibly have an effect on the amount of orthodontic tooth movement. Based on the animal studies retrieved, lactation increased the amount of tooth movement. Exploratory synthesis showed an overall increase in the pregnancy group as well. However, this tendency did not reach statistical significance. Although these animal experiment results should be approached with some caution until more information becomes available, the clinician should not ignore the possibility that orthodontic patients during pregnancy or breastfeeding may exhibit changes in physiological bone remodelling, as well as the possible implications for clinical practice. Especially patients in lactation, might present increased needs for anchorage preparation during space closure. Furthermore, appointments might need to be more frequent in order to check and control the progress of treatment.
Quantitative synthesis of the information on pregnant and control animals revealed a tendency for increase in the rate of tooth movement. On the histological level, Hellsing and Hammarström [44] did not show a significant difference in the number of osteoclasts. Ghajar et al. [43] observed that the number of osteoclasts was significantly reduced in the pregnant rats, but on the clinical level the difference was not significant. The fact that paraffin histological analyses can only be performed in two dimensions might account for these differences in findings. Regarding osteoblasts, higher percentages have been observed in pregnant animals [47]. Kim and Lee [45] measured alkaline phosphatase and tartrate-resistant acid phosphatase activities in extracts of paradental alveolar bone, as a way to assess bone metabolism. Their results showed high activity in the pregnant group only at the early stages of the experiment. This information could suggest that, in the context of rat pregnancy that lasts 21–23 days [48] tooth movement could be promoted during pregnancy because the action of resorption is faster than deposition.
During pregnancy, the physiological maternal adaptations in the osseous metabolism result from the involvement of various regulators [49]. Oestrogens are known down-regulators of bone resorption and act to maintain bone mass [50]. In the context of orthodontic treatment, the administration of oestrogen reduced the rate of tooth movement in osteoporotic rats [51]. Progesterone also has been reported to lead to the same results directly through action on the osteoblasts, or indirectly by influencing the glucocorticoid receptors or the metalloproteinases [17] and has been linked with reduction in the rate of tooth movement [52]. On the contrary prolactin, which is present with increased levels during pregnancy, exhibits pro-resorptive action leading to reductions in bone mass [28]. A multitude of other hormones and biological factors have been implicated in the regulation of the processes associated with bone remodelling during pregnancy as well [49, 53, 54], which could potentially modify the rate of clinical movement under the influence of orthodontic forces.
Apart from the overall regulation of bone remodelling, local alterations in the periodontal tissues could account for the observed clinical changes. As periodontal ligament cells exhibit oestrogen receptors, the hormonal changes taking place in pregnancy might lead to water retention [55]. Thus, the periodontal ligament might become easily compressible in pregnant individuals when a mechanical force is applied. It is also expected that slight extrusion of the teeth will happen simultaneously which will facilitate the greater amount of tipping movement [44].
According to Macari et al. [46], lactation resulted in a significantly increased rate of tooth movement compared to the non-lactating group. Lactating animals exhibited elevated rates of bone turnover resulting in bone loss in the maxilla, femur and vertebra. These changes are consistent with those reported previously in long bones and the mandible of lactating calcium deficient mice [26, 29, 56,57,58] and can be associated with the bone mass reducing effect of prolactin [28]. On the contrary Shoji et al. [37] observed no effect of lactation on the density of the alveolar bone when calcium content of the diet is normal, while other researchers observed even increases in the height of alveolar bone [59]. Such discrepancies could be a result of the different methodologies employed.
Macari et al. [46] also observed that the osteopenic phenotype was associated with an increased expression of the RANK/RANKL/OPG signalling pathway in the alveolar bone. These findings were consistent with previous findings of increased expression of these factors in the calvaria of lactating mice [60] as well as prolactin treated osteoblast-like cells [61]. Increased bone turnover could also be attributed to the prolactin induced differentiation of osteoclasts [60]. Therefore, lactation associated alterations in the alveolar bone led to reductions in bone mineral density and to diminished trabecular bone architecture.
Strengths and limitations
For this review we followed well-established guidelines in an attempt to reduce methodological bias and we focused our unrestricted and comprehensive searches on controlled trials. We also performed an exploratory quantitative synthesis that albeit indicative until additional research becomes available, it is more transparent and potentially more valid than alternative summaries [62]. It has been suggested that if meaningful, even data from two studies can be combined [34, 63].
Furthermore, it has to be acknowledged that the data retrieved in the present systematic review relate mostly to rodents and cannot be directly extrapolated to humans. Investigations based on rats and mice have given important physiological information. However, significant differences between rodents and humans exist, not only in terms of bone physiology, but also of pregnancy/lactation endocrinology [23, 64]. Also, one should not forget that the biomechanical conditions were various and not analogous to clinical scenarios in humans [65]. Finally, as power sample calculations were not included in the methodology, the precision of the retrieved results could be potentially questioned. Consequently, it cannot be determined with certainty what would be the effect in everyday clinical practice. However, analogous human studies present significant ethical and practical limitations.
Recommendations for future research
Since, the number of adult female patients seeking orthodontic treatment appears to be on the rise, further well- designed experimental studies on the effects of pregnancy and lactation on orthodontic tooth movement would be useful for the clinician. It is highly desirable that study designs become standardized [66] and possible sources of risk of bias receive the appropriate attention [38]. Moreover, study designs should come closer to everyday clinical scenarios.
Conclusions
The metabolic changes occurring during pregnancy and lactation in animals may have an impact on the rate of tooth movement. Although these animal experiment results should be approached cautiously, it could be safe practice to consider the possible impact of these physiological changes in the clinical setting.
Availability of data and materials
All data and materials are available upon request.
Abbreviations
- CI:
-
Confidence Interval
- CT:
-
Computed Tomography
- IL:
-
Interleukin
- OPG:
-
Osteoprotegerin
- PICOS:
-
Participants, Interventions, Comparisons, Study design
- PROSPERO:
-
International prospective register of systematic reviews
- RANK:
-
Receptor activator of nuclear factor-Kappa B
- RANKL:
-
Receptor activator of nuclear factor-Kappa B ligand
- SYRCLE:
-
SYstematic Review Center for Laboratory animal Experimentation
- TNF:
-
Tumor Necrosis Factor
- WMD:
-
Weighted Mean Difference
References
Cornwall BC. Treatment of dental disease. In: Skouteris CA, editor. Dental Management of the Pregnant Patient. New York: John Wiley & Sons; 2018. p. 75–85.
Skouteris CA. Physiologic changes and their Sequelae in pregnancy. In: Skouteris CA, editor. Dental Management of the Pregnant Patient. New York: John Wiley & Sons; 2018. p. 5–24.
Tojo Y, Kurabayashi T, Honda A, Yamamoto Y, Yahata T, Takakuwa K, Tanaka K. Bone structural and metabolic changes at the end of pregnancy and lactation in rats. Am J Obstet Gynecol. 1998;178:180–5.
Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab. 2002;13:195–200.
Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, Riggs BL. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–6.
Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–23.
Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-kappa B ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276:8836–40.
Xu Y, Chu N, Qiu X, Gober HJ, Li D, Wang L. The interconnected role of chemokines and estrogen in bone metabolism. Biosci Trends. 2017;10:433–44.
Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–94.
Drake MT, Clarke BL, Lewiecki EM. The pathophysiology and treatment of osteoporosis. Clin Ther. 2015;37:1837–50.
Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–96.
Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92.
Turner RT, Colvard DS, Spelsberg TC. Estrogen inhibition of periosteal bone formation in rat long bones: down-regulation of gene expression for bone matrix proteins. Endocrinology. 1990;127:1346–51.
Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252:68–80.
Kondoh S, Inoue K, Igarashi K, Sugizaki H, Shirode-Fukuda Y, Inoue E, Yu T, Takeuchi JK, Kanno J, Bonewald LF, Imai Y. Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone. 2014;60:68–77.
Määttä JA, Büki KG, Gu G, Alanne MH, Vääräniemi J, Liljenbäck H, Poutanen M, Härkönen P, Väänänen K. Inactivation of estrogen receptor α in bone-forming cells induces bone loss in female mice. FASEB J. 2013;27:478–88.
Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–19.
Wei LL, Leach MW, Miner RS, Demers LM. Evidence for progesterone receptors in human osteoblast-like cells. Biochem Biophys Res Commun. 1993;195:525–32.
Prior JC. Progesterone as a bone-trophic hormone. Endocr Rev. 1990;11:386–98.
Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Altered circulating levels of matrix metalloproteinases 2 and 9 and their inhibitors and effect of progesterone supplementation in women with endometriosis undergoing in vitro fertilization. Fertil Steril. 2013;100:127–34.e1.
Allen TK, Feng L, Grotegut CA, Murtha AP. Progesterone receptor membrane component 1 as the mediator of the inhibitory effect of progestins on cytokine-induced matrix metalloproteinase 9 activity in vitro. Reprod Sci. 2014;21:260–8.
Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7:49–66.
Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41.
Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol. 2002;64:7–67.
Bernard V, Young J, Binart N. Prolactin - a pleiotropic factor in health and disease. Nat Rev Endocrinol. 2019;15:356–65.
VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144:5521–9.
Prior JC, Vigna YM, Wark JD, Eyre DR, Lentle BC, Li DK, Ebeling PR, Atley L. Premenopausal ovariectomy-related bone loss: a randomized, double-blind, one-year trial of conjugated estrogen or medroxyprogesterone acetate. J Bone Miner Res. 1997;12:1851–63.
Kasper D, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine. 20th ed. New York: McGraw Hill Education Medical; 2018.
Clément-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, Bouchard B, Amling M, Gaillard-Kelly M, Binart N, Baron R, Kelly PA. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999;140:96–105.
Abraham G, Halbreich U, Friedman RH, Josiassen RC. Bone mineral density and prolactin associations in patients with chronic schizophrenia. Schizophr Res. 2003;59:17–8.
Jiang N, Guo W, Chen M, Zheng Y, Zhou J, Kim SG, Embree MC, Songhee Song K, Marao HF, Mao JJ. Periodontal ligament and alveolar bone in health and adaptation: tooth movement. Front Oral Biol. 2016;18:1–8.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P. Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, Gøtzsche PC, Lasserson T, Tovey D. PRISMA for Abstracts Group PRISMA for Abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:e1001419.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. 2nd ed. UK: The Cochrane Collaboration and John Wiley & Sons Ltd; 2019.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Mead R, Gilmour SG, Mead A. Statistical principles for the Design of Experiments. Cambridge: Cambridge University Press; 2012.
Shoji K, Ohtsuka-Isoya M, Horiuchi H, Shinoda H. Bone mineral density of alveolar bone in rats during pregnancy and lactation. J Periodontol. 2000;71:1073–8.
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol. 2011;64:380–2.
Goldie RS, King GJ. Root resorption and tooth movement in orthodontically treated, calcium-deficient, and lactating rats. Am J Orthod. 1984;85:424–30.
Ghajar K, Olyaee P, Mirzakouchaki B, Ghahremani L, Garjani A, Dadgar E, Marjani S. The effect of pregnancy on orthodontic tooth movement in rats. Med Oral Patol Oral Cir Bucal. 2013;18:e351–5.
Hellsing E, Hammarström L. The effects of pregnancy and fluoride on orthodontic tooth movements in rats. Eur J Orthod. 1991;13:223–30.
Kim YS, Lee KS. Effects of pregnancy on alveolar bone turnover during experimental tooth movement in rats. Kor J Orthod. 2000;3:413–21.
Macari S, Sharma LA, Wyatt A, da Silva JM, Dias GJ, Silva TA, Szawka RE, Grattan DR. Lactation induces increases in the RANK/RANKL/OPG system in maxillary bone. Bone. 2018;110:160–9.
He Z, Chen Y, Luo S. Effects of pregnancy on orthodontic tooth movements: effects of progesterone on orthodontic tooth movements in pregnant rats. Hua Xi Kou Qiang Yi Xue Za Zhi. 1998;16:124–6.
Suckow M, Hankenson FC, Wilson R, Foley P. The laboratory rat. 3rd ed. London: Academic Press; 2020.
Sanz-Salvador L, García-Pérez MÁ, Tarín JJ, Cano A. Bone metabolic changes during pregnancy: a period of vulnerability to osteoporosis and fracture. Eur J Endocrinol. 2015;172:R53–5.
Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23:576–81.
Jin Z, Ding Y, Li X. Effects of estrogen on experimental tooth movement in osteoporosis rats. Zhonghua Kou Qiang Yi Xue Za Zhi. 2000;35:55–7.
Poosti M, Basafa M, Eslami N. Progesterone effects on experimental tooth movement in rabbits. J Calif Dent Assoc. 2009;37:483–6.
Gulson B, Taylor A, Eisman J. Bone remodeling during pregnancy and post-partum assessed by metal lead levels and isotopic concentrations. Bone. 2016;89:40–51.
Salles JP. Bone metabolism during pregnancy. Ann Endocrinol (Paris). 2016;77:163–8.
Lewko WM, Anderson A. Estrogen receptors and growth response in cultured human periodontal ligament cells. Life Sci. 1986;39:1201–6.
Messer HH, Goebel NK, Wilcox L. A comparison of bone loss from different skeletal sites during acute calcium deficiency in mice. Arch Oral Biol. 1981;26:1001–4.
Onal M, Galli C, Fu Q, Xiong J, Weinstein RS, Manolagas SC, O'Brien CA. The RANKL distal control region is required for the increase in RANKL expression, but not the bone loss, associated with hyperparathyroidism or lactation in adult mice. Mol Endocrinol. 2012;26:341–8.
Suntornsaratoon P, Wongdee K, Goswami S, Krishnamra N, Charoenphandhu N. Bone modeling in bromocriptine-treated pregnant and lactating rats: possible osteoregulatory role of prolactin in lactation. Am J Physiol Endocrinol Metab. 2010;299:E426.
Petrikowski CG, Overton TR. Quantitative radiographic changes in the mandible, femur and vertebra in lactating rats fed a low-calcium diet. Dentomaxillofac Radiol. 1996;25:136–45.
Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, Wysolmerski JJ. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology. 2007;148:3875–86.
Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, Suthiphongchai T, Krishnamra N. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone. 2008;42:535–6.
Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35:215–47.
Ryan, R. and Cochrane Consumers and Communication Review Group. 2016. Cochrane Consumers and communication review group: metaanalysis. http://cccrgcochraneorg Accessed January 31, 2020.
Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97:135–87.
Ren Y, Maltha JC, Kuijpers-Jagtman AM. The rat as a model for orthodontic tooth movement--a critical review and a proposed solution. Eur J Orthod. 2004;26:483–90.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010;1:94–9.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
EGK conceived the study and initiated the study design. MO and EGK contributed to data collection, data analysis, data interpretation and manuscript draft preparation. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Strategy for database search (up to July 18th 2019). Table S2. Quality of available evidence.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Omar, M., Kaklamanos, E.G. Does the rate of orthodontic tooth movement change during pregnancy and lactation? A systematic review of the evidence from animal studies. BMC Oral Health 20, 237 (2020). https://doi.org/10.1186/s12903-020-01223-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-020-01223-2