Abstract
Background
Type 2 diabetes mellitus (T2DM) is often linked to chronic inflammation, which can be influenced by both lifestyle and dietary choices. However, the relationship between the inflammatory potential of diet and lifestyle factors and the risk of developing T2DM remains unclear. The present study aimed to investigate the associations of the empirical dietary inflammatory index (EDII), dietary inflammatory score (DIS), and lifestyle inflammatory score (LIS) with the risk of T2DM among Iranian adults.
Methods
The current study was conducted on 5714 individuals from Yazd Health Study (YaHS) who were followed up for a mean period of six years. YaHS is a prospective cohort study which has been conducted since 2014. Dietary intakes were collected at baseline using the food frequency questionnaire. The relative risk (RR) of T2DM was calculated by Cox regression analysis across tertiles of EDII, DIS, and LIS, adjusted for potential confounders.
Results
The mean ± SD for the age and body mass index of the study population were 47.0 ± 9.2 years and 26.7 ± 5.1 Kg.m2, respectively. A significant association between LIS and the risk of T2DM was observed (RR: 4.05, 95% CI: 2.61–6.27 P-trend < 0.001). Individuals in the highest compared to the lowest tertile of EDII-LIS (RR: 3.07, 95%CI: 2.01–4.68; P for trend < 0.001) and DIS-LIS (RR: 2.42, 95%CI: 1.69–3.49; P for trend < 0.001) had a higher risk of T2DM. However, no significant association was found between EDII and DIS scores and the risk of T2DM.
Conclusion
Greater adherence to LIS, EDII-LIS, and DIS-LIS scores was associated with a higher risk of T2DM, while no significant association was found between EDII and DIS with T2DM risk.
Similar content being viewed by others
Introduction
Over the past few decades, the prevalence of type 2 diabetes mellitus (T2DM) has increased dramatically worldwide, emerging as a global public health crisis that demands urgent attention [1]. In 2020, an alarming 6.28% of the adult population, equivalent to approximately 462 million individuals, were affected by T2DM [2]. If current trends persist, projections indicate that this number could skyrocket to 592 million by 2035, underscoring the unchecked growth of the T2DM epidemic [3]. Among the countries grappling with a severe escalation of T2DM cases, Iran stands out as a particularly affected nation [4]. A recent study has revealed an alarmingly high prevalence of T2DM among the adult population in Iran, with the prevalence rate for Iranians over the age of 25 standing at a concerning 10.8% [5]. This growing disease burden in Iran has considerable adverse impacts, negatively affecting the quality of life and increasing healthcare costs, comorbidities, and mortality rates [6].
A growing body of research highlights chronic inflammation as a key factor underlying the development of T2DM [7, 8]. At the same time, certain modifiable health behaviors have been shown to heighten inflammatory processes. For example, poor diet quality, excess adiposity, physical inactivity, smoking, and heavy alcohol intake can all contribute to systemic inflammation [9, 10]. These observations have sparked increasing interest in the public health field to find ways to mitigate lifestyle-related inflammation as a strategy for preventing the onset of chronic diseases linked to inflammation, such as T2DM [11].
It is possible to estimate the contribution of dietary exposures to chronic disease development by a pre-defined score known as the dietary inflammatory index (DII) [12]. Accordingly, recent studies found a positive association between a higher DII and a higher risk of diabetes [13, 14]. However, a limitation of the DII is its heavy focus on certain nutrients, which may not account for a wide range of other known and unknown dietary components that could affect inflammation. To overcome such limitations, novel indices have been developed to provide a more comprehensive assessment of the inflammatory potential of both diet and lifestyle. The Empirical Dietary Inflammatory Index (EDII) is a novel index that evaluates the inflammatory potential of an individual’s overall diet based on food groups rather than specific nutrients [15]. Moreover, the Dietary Inflammatory Score (DIS) and Lifestyle Inflammatory Score (LIS) were introduced to assess the inflammatory potential of diet and lifestyle factors, respectively, taking into account various dietary components and lifestyle behaviors such as physical activity, smoking, and body mass index (BMI) [16]. These indices offer a more holistic approach to evaluating the inflammatory potential associated with chronic disease development, overcoming the narrow focus of traditional measures such as the DII.
Previous research has linked higher scores of EDII, DIS, or LIS to various chronic diseases [17,18,19,20,21]. In a study by Farhadnejad et al., it was found that a higher score of DIS and LIS is associated with an increased risk of metabolic syndrome (MetS) [20]. According to Lee et al., individuals in the highest quintile of the Empirical Dietary Inflammatory Pattern (EDIP) had a 3.11-fold higher risk of developing T2DM compared to those in the lowest quintile [19]. However, to our knowledge, no study has yet comprehensively examined the relationship between all three inflammation measures, namely EDII, DIS, and LIS, and the incidence of T2DM. Only a single study conducted by Teymoori et al. [22] specifically investigated the associations of these inflammation measures with T2DM incidence. Their findings demonstrated that higher inflammatory potential, as assessed by EDII and LIS, was associated with an increased risk of T2DM, while the DIS did not show a significant relationship. This highlights the need for further research to evaluate the combined impact of these inflammation measures on T2DM risk. Therefore, we aimed to address the limited scope of previous research in this area, as there is currently only one study that has explored the relationship between the inflammatory potential of diet and lifestyle, as assessed by indices including EDIP, DIS, LIS, and T2DM incidence. Furthermore, this existing study did not investigate the combined effects of these indices on T2DM risk. By considering combinational indices that incorporate multiple inflammation measures simultaneously, our study aims to fill this crucial research gap and provide a more comprehensive understanding of the potential links between the inflammatory potential of diet and lifestyle and the risk of T2DM. Through this approach, we aimed to address the limitations of previous research, such as potential variations in dietary habits and lifestyle factors across different regions, and a lack of investigation into combinational indices that consider multiple inflammation measures simultaneously. We hypothesized that higher inflammatory potential, as assessed by higher scores of EDII, DIS, LIS, EDII-LIS, and DIS-LIS would be associated with an increased risk of developing T2DM among Iranian adult participants in a prospective cohort study.
Materials and methods
Study design and population
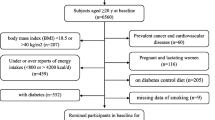
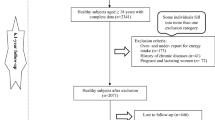
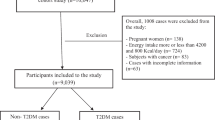
The data used in this study were gathered from the Yazd Health Study (YaHS), a prospective cohort study of adults aged 20–70 living in Yazd, Iran, that has been previously described in detail [23]. Briefly, the study employed a two-phase data collection approach. In the initial phase, conducted in November 2014, trained interviewers obtained comprehensive information from participants through structured interviews and assessments. The data collected included personal and dietary habits, physical activity levels, medical history, mental health status, social well-being, anthropometric measurements, and biochemical data. The second phase, carried out in November 2015, involved the establishment of the Yazd biobank (Zist Bank-e-Yazd-ZIBA) and a nutrition-focused sub-study named the TAMYZ study. This phase facilitated the collection of nutritional data from the participants [24]. Participants had to complete the relevant questionnaires during two implementation phases in order to be included in the present study. Following the completion of the two initial data collection phases in 2014 and 2015, where participants completed the relevant questionnaires, they were then prospectively followed up annually for a period of 6 years to assess various outcomes or changes over time. Participants were excluded from this study if they had a history or diagnosis of diabetes, cardiovascular diseases (CVDs), cancer, or were pregnant, as well as those with under- or over-reported dietary energy intakes (< 500 or > 5000 kcal/d), or those who failed to respond to at least 20% of the items on the dietary questionnaire.
In the present study, of 8965 individuals with complete dietary and anthropometric data, participants with chronic diseases including CVD (n = 752), different cancers (n = 103), diabetes (n = 1279), individuals with calorie intake less than 500 kcal/d or higher than 5000 kcal/d (n = 1572), pregnant women (n = 116), and those with missing data of diabetes (n = 51) were excluded. Some individuals fell into more than one exclusion category. Finally, for the analysis related to the EDII and DIS, the final sample size was 5714 individuals who were followed up. However, for the analysis specifically focusing on the association between LIS and T2DM, we had to exclude participants with missing data on LIS components, namely BMI (n = 40), physical activity (n = 853), and smoking (n = 159). As a result, the sample size for this particular analysis was reduced to 4714 participants. In addition, for the combination indices of EDII-LIS and DIS-LIS analyses, 4714 individuals remained for entry into the final analysis (Fig. 1).
YaHS was approved by the ethics committee of Shahid Sadoughi University of Medical Sciences and the current study was approved by the ethics committee of Iran University of Medical Sciences (IR.IUMS.REC.1399.1415). The experimental procedures adhered to the principles outlined in the Declaration of Helsinki guidelines and all subjects provided written informed consent prior to participating in the study.
Anthropometric measurements
Body weight of each participant was measured to the nearest 0.1 kg using a digital Omron BF-511 scale with participants standing in minimal clothing and without shoes. Participants’ height was measured in a standing position using a tape measure on a straight wall to the nearest centimeter barefoot while their heads, shoulder blades, buttocks, and heels were rested against the wall. Then, BMI was calculated as weight (kg) divided by height in meters squared (m2) [25]. These anthropometric measurement and calculation methods align with established guidelines [24].
Dietary assessment
Dietary intake was assessed using a comprehensive 178-item food frequency questionnaire (FFQ), which was a modified version of a previously validated 168-item questionnaire [26] with 10 additional questions related to food items commonly consumed in the Yazd region added by trained interviewers [24, 26, 27]. Participants reported two key aspects for each food item: 1) the frequency of consumption over the past year, ranging from ’10 or more times per day’ to ‘never or less than once a month’, and 2) the portion size or amount consumed per intake. To enhance accuracy, participants referred to a photo book illustrating standard portion sizes for different foods. The questionnaire assessed both the frequency and amount of intake through multiple-choice questions with predefined response categories tailored to each food item. The reported frequencies and portion sizes for each food over the past year were then converted to gram amounts using guidelines provided by household measurement scales [28]. To calculate energy and nutrient intakes per gram of each food item, we used the Iranian food composition table (FCT) as well as the U.S. Department of Agriculture FCT, which were used for any foods that were not included in the Iranian FCT [29].
Physical activity assessment
Physical activity was assessed using the validated Persian translation of the International Physical Activity Questionnaire Short Form (IPAQ-SF) [30]. Participants were asked to report the frequency and time spent on sedentary, moderate, and intense activities throughout the past week based on a list of common activities of daily life. The resulting physical activity scores were expressed as Metabolic Equivalent hours (MET-h) per week for analysis.
Blood sampling and laboratory assessments
Biochemical assessments were carried out by trained staff following standardized laboratory protocols. Blood specimens were obtained from participants following a 12-hour overnight fasting period. Serum levels of fasting blood glucose (FBG) and lipid profile (high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and total cholesterol) were measured enzymatically using kits from Pars Azmoon (Tehran, Iran). Analyses were performed using properly calibrated Ciba Corning auto-analyzers (Ciba Corp., Basle, Switzerland) [24].
Assessment of other covariates
A general information questionnaire was used to collect demographic and health details from all participants. The information gathered included age, marital status, education level, employment status, residence location, immigration status, insurance coverage, religious affiliation, and number of children. The prevalence of major chronic conditions, including CVD and cancer, was determined through existing electronic medical records accessed via Iran’s National Health Record System (SEPAS). This system consolidates data from all public and private hospitalizations nationwide, allowing reliable ascertainment of disease status [24].
For calculating socioeconomic status variable (SES) [31], a point system was used where participants received one point for each of the following: owned housing, employed, high school diploma or above, less than four household members. Otherwise, zero points were assigned if they did not meet those criteria. The total SES score was obtained by summing the points for the four components, yielding a range of 0 to 4. Based on their overall scores, participants were categorized into low SES (0–2 points), middle SES (3 points) or high SES (4 points).
Calculation of indices
The EDII score was calculated for each participant based on their reported dietary intake, following the approach of Tabung et al. [15]. However, three food groups involving alcoholic beverages were excluded due to the lack of consumption data and cultural differences. Therefore, we calculated EDII score with 15 instead of 18 food groups based on dietary intakes of tea, coffee, dark yellow vegetables, leafy green vegetables, snacks, fruit juice, pizza, processed meat, red meat, organ meat, other fish (canned tuna or fish), other vegetables (vegetables other than leafy green vegetables and dark yellow vegetables), refined grains, high-energy beverages, and tomatoes. The inflammatory weighting coefficient proposed for each food group was multiplied by the mean daily intake reported. These weighted values were summed to create an overall EDII score [15], which was then rescaled by dividing by 1000 to improve interpretability. We additionally calculated dietary and lifestyle inflammation scores for participants as proposed by Byrd et al. [16]. The DIS comprised 18 food groups, excluding supplement intake, which was not assessed. Food groups included were leafy greens and cruciferous vegetables, tomatoes, apples, berries, yellow or orange vegetables and fruits, other fruits, fruit juices, legumes, fish, poultry, red/organ meats, processed meats, added sugars, high-fat dairy, low-fat dairy, tea, nuts, refined grains, starchy vegetables, and other fats. Each participant’s standardized intake (to a mean of zero and SD of 1) for each food group was summed to create the overall DIS. The LIS incorporated BMI, physical activity, and smoking status. Alcohol intake was excluded given the lack of data. Dummy variables were first generated for each component, weighted by regression coefficients, and summed to obtain the LIS as follows: [16] Participants were classified into weight categories based on their BMI: those with a BMI < 25 were classified as normal weight, those with a 25 ≤ BMI < 30 were classified as overweight, and those with a BMI ≥ 30 were classified as obese. The respective scores assigned to these weight categories were 0.0, 0.89, and 1.57. Physical activity levels were categorized into tertiles: participants in the first (0.28–8.66 MET-h/week), second (8.67–18.38 MET-h/week), and third (18.39–76.5 MET-h/week) tertiles were assigned scores of 0.0, -0.18, and − 0.41, respectively. Smokers and non-smokers were assigned regression coefficients of 0.50 and 0.0, respectively.
To integrate the dietary and lifestyle inflammation measures, we derived two novel indices: the EDII-LIS and DIS-LIS. First, the EDII and LIS as well as the DIS and LIS were converted to z-scores to equalize their weight in the new index. The EDII-LIS was then calculated by summing the z-scores of the energy-adjusted EDII and LIS for each participant. Similarly, the DIS-LIS summed the z-scores for the DIS and LIS. For data analysis and population stratification, the EDII and DIS scores were computed for each participant per 1000 kcal of energy intake.
T2DM definition and ascertainment
T2DM diagnosis was based on American Diabetes Association criteria: FBG ≥ 126 mg/dL (7.0 mmol/L) or 2-hour post-glucose load ≥ 200 mg/dL (11.1 mmol/L) [32, 33]. Diabetes status was ascertained using both baseline lab measurements and self-reported data collected through validated questionnaires [24]. However, T2DM incidence was verified through the national Electronic Health Record System (SEPAS) which compiles hospital data across Yazd province [24].
Statistical analysis
Statistical analysis of data was done using SPSS 20 software (version 20.0; SPSS Inc, Chicago IL). The normal distribution of the data was evaluated using histogram charts and the Kolmogorov-Smirnov test. EDII, DIS, and LIS were categorized into tertiles and then, the characteristics of the participants were represented accordingly. Data are presented as the mean and standard variation (mean ± SD) or median (25–75) or interquartile range (IQR) for continuous variables and percentages for categorical variables across tertiles of EDII, DIS, and LIS. To test the trend of quantitative and qualitative variables across tertiles of these indices (as median value in each tertile), linear regression and Chi-square were used, respectively. Cox proportional hazard regression was used to estimate the relative risk and 95% confidence intervals (RRs and 95% CIs) of diabetes risk across tertiles of the EDII, DIS, and LIS. To determine the association between EDII and DIS and diabetes risk, in addition to the crude model, age and sex were adjusted in model 1. Model 2 was further adjusted for smoking, physical activity, family history of diabetes, marital status, SES, menopausal status, and dietary intake of energy. P-value < 0.05 was considered statistically significant. P-trend was also determined using Cox regression analysis between the inflammatory indices (as median value in each tertile) as the independent variable and the incidence of diabetes as the dependent variable in various regression models.
Results
Baseline characteristics and dietary intakes of participants
The mean age and BMI in all study population were 47.0 ± 9.2 years and 26.7 ± 5.1 kg/m2, respectively. The baseline characteristics and dietary intakes of the participants based on the tertiles of EDII, DIS, and LIS indices are presented in Tables 1 and 2, and 3, respectively. Table 1 shows that compared with those in the lowest tertiles of EDII, significant differences were observed for the variables of level of education, menopausal status, and smoking status of the participants. Those in the third tertile of EDII were fewer smokers, had a lower percentage of menopausal women, and a higher percentage of people with a higher educational level compared to the first tertile. In addition, dietary intake did differ significantly across tertiles of EDII except for protein (P for trend = 0.07). In terms of EDII components, individuals in the highest tertile had lower intakes of anti-inflammatory food groups. However, dietary intakes of the pro-inflammatory food group significantly increased across EDII score tertiles.
Table 2 shows that participants in different tertiles of DIS exhibited variations in age, BMI, and physical activity levels, while no other significant differences were observed. Individuals in the third tertile of DIS had a significantly higher energy, carbohydrate, and fat intake than those in the first tertile. In contrast, protein, PUFA, and fiber intake were significantly lower in the third tertile compared to the first tertile. Moreover, participants in the highest quartile of the DIS had lower intakes of leafy greens and cruciferous vegetables, tomatoes, apples and berries, deep yellow or orange vegetables and fruit, other fruits and real fruit juices, other vegetables, legumes, poultry, low-fat dairy, coffee and tea, nuts and higher intakes of added sugars, high-fat dairy, other fats, and refined grains and starchy vegetables.
Table 3 shows significant differences in age, sex, BMI, physical activity, family history of diabetes, level of education, menopausal, marital, socioeconomic, and smoking status among participants in the highest tertile of LIS compared to those in the lowest tertile. Specifically, those in the highest tertiles were older, had a higher proportion of men, higher BMI, lower physical activity levels, more smokers, had a higher proportion of married individuals, more menopausal women, a higher percentage of employed individuals, had a lower educational level, a lower SES, and a higher prevalence of family history of diabetes compared to those in the lowest tertiles. The intakes of energy and macronutrients did not differ significantly across tertiles of LIS except for fiber (P for trend = 0.022).
EDII, DIS, and LIS with the risk of diabetes
The association of EDII, DIS, and LIS with the risk of diabetes is shown in Table 4. In the crude model, there was an increased risk of T2DM for subjects at the highest compared to the lowest tertile of the LIS (RR: 6.34, 95% CI: 4.12–9.76, P for trend < 0.001). The same trend was observed in the age and sex-adjusted model (RR: 4.41, 95% CI: 2.85–6.82, P for trend < 0.001). Also after further adjustment for energy intake, marital status, SES, menopausal status, and family history of diabetes in the multivariable-adjusted model, the direct association of LIS and diabetes risk remained significant (RR: 4.05; 95% CI: 2.61–6.27, P for trend < 0.001). There was no association between EDII and T2DM either in the crude or in the fully adjusted models. In the crude model, there was a significant inverse association between the DIS and risk of T2DM (RR: 0.72, 95% CI: 0.54–0.95, P-trend = 0.028). However, no significant association was observed between a higher score of DIS and the risk of T2DM after adjustments in models 1 and 2.
EDII-LIS and DIS-LIS with the risk of diabetes
Table 5 presents the results of the combined role of the inflammatory potential of diet and lifestyle, as determined by EDII-LIS and DIS-LIS, in predicting the risk of diabetes. In the crude model, the higher scores of EDII-LIS and DIS-LIS were associated with increased risk of diabetes (RR: 4.52, 95% CI: 3.00-6.81, P for trend < 0.001 and RR: 3.42, 95% CI: 2.39–4.89, P for trend = 0.001, respectively). This positive association remained significant after adjustment for potential confounders and in the final adjusted model, the RR (95%CI) of EDII-LIS and DIS-LIS was 3.07 (2.01–4.68), P for trend < 0.001 and 2.42 (1.69–3.49), P for trend < 0.001, respectively.
Discussion
In the present study, we evaluated the association of EDII, DIS, LIS, and their combinations with the risk of T2DM in the framework of a longitudinal population-based study after 6 years of follow-up. Our findings suggest that higher scores of LIS, EDII-LIS, and DIS-LIS are associated with a higher risk of T2DM, while no significant association was found between the individual EDII and DIS scores and the risk of T2DM. To our knowledge, only one previous study has investigated the association between EDIP, DIS, and LIS indices and the risk of T2DM. However, as that study was conducted in a different population and region [22], it is important to examine these associations in other populations to evaluate their generalizability.
Our finding of a positive association between LIS and T2DM aligns with previous research linking inflammatory lifestyles to adverse health outcomes. Studies have related higher LIS to increased colorectal adenoma risk [34], all-cause, cancer- and cardiovascular disease-specific mortality [18], and MetS incidence [20, 35]. Most relevantly, a recent cohort study by Teymoori et al. [22] reported similar associations between elevated LIS and increased T2DM incidence. Together, these studies provide consistent evidence that lifestyle components such as smoking, obesity, and inactivity, which heighten systemic inflammation, may contribute to T2DM pathogenesis. Our study replicates this relationship between lifestyle inflammation and T2DM in a new population while utilizing an adapted LIS tailored to available data. Our findings further establish lifestyle-induced inflammation as a potentially modifiable risk factor for T2DM that warrants attention alongside dietary and metabolic factors. Targeted lifestyle interventions may help reduce T2DM risk by promoting positive changes in obesity, exercise, smoking, and overall inflammatory lifestyle patterns.
The positive association between LIS and T2DM risk may be partly attributable to unhealthy lifestyle patterns among those with higher scores, which can promote systemic inflammation and consequent insulin resistance through multiple interrelated pathways. Our baseline data showed that participants in the top LIS tertile had a higher prevalence of obesity, smoking, and lower levels of physical activity, factors associated with increased risk for T2DM [36,37,38]. Excess adiposity leads to increased secretion of pro-inflammatory adipokines and cytokines (e.g. leptin, TNF-α, IL-6) from dysfunctional adipose tissue, which can impair insulin signaling and action in skeletal muscle, liver, and pancreatic beta cells [9, 39]. Simultaneously, components of tobacco smoke induce oxidative stress and an inflammatory state by activating redox-sensitive transcription factors such as NF-κB, upregulating inflammatory mediators [40]. This heightened oxidative and inflammatory milieu directly promotes insulin resistance and beta cell dysfunction, independently increasing T2DM risk [41]. Notably, inflammation may interact synergistically with other adverse lifestyle factors such as physical inactivity, further exacerbating insulin resistance through shared molecular mechanisms [42]. For instance, physical inactivity can induce beta cell insufficiency and reduce pancreatic islet mass [43], which can compromise the ability to maintain normal glucose regulation [44]. Physical inactivity can also promote the onset and progression of a low-grade inflammatory response [45], which, in turn, induces insulin resistance. Therefore, the combined pro-inflammatory effects of coexisting unhealthy lifestyle factors likely promoted a persistent low-grade inflammatory state that impaired glycemic control, contributing to the elevated T2DM risk observed with higher LIS scores.
We did not observe significant associations between EDII or DIS and T2DM risk. However, a recent study [22] reported a positive relationship between EDIP and T2DM risk, which is inconsistent with our findings. This discrepancy could be attributed to differences in population characteristics, such as demographic factors or genetic backgrounds that may influence the relationship between inflammatory diets and T2DM risk. Moreover, our study was conducted in a different geographic region, where regional variations in dietary patterns, food sources, and preparation methods could impact the inflammatory potential of specific food components. Furthermore, coffee and pizza, which are part of the anti-inflammatory group of the EDII, were reported to have low consumption in the mentioned study [22]. This lower consumption in their study, compared to our study, may have influenced the overall inflammatory balance of the index and could explain why they observed a higher EDII score associated with increased T2DM risk, while we did not find a significant relationship. On the other hand, the same study [22] found no significant association between DIS and T2DM risk, which is in line with our study’s findings. Dietary inflammatory indices, such as EDII and DIS, aim to provide a comprehensive assessment of an individual’s usual dietary intake by evaluating a wide range of foods. However, their ability to estimate disease risk may be attenuated by complex interactions between anti- and pro-inflammatory dietary components that are not fully captured by the scores. It is also important to note that the EDII and DIS indices were developed and validated for US populations, so their applicability to other populations requires further study. Although these scores have shown reliability and validity for measuring dietary inflammation, and prior research has linked inflammatory diets to diabetes development [19, 22, 46], our study did not find significant associations. This may be due to limitations in capturing all relevant dietary and lifestyle inflammation factors and there may be other confounding variables that we were unable to control for. For example, other potential influencing variables that we could not account for include medications, supplements, genetics, and long-term diet patterns exceeding the period assessed. In addition, the scores are based on population averages that likely do not reflect individual variations in food-related inflammatory impacts. Differences in associations across populations and in calculation methods may also contribute to inconsistent findings. Further research is needed to determine specific dietary drivers of inflammation in our population and refine the utility of these scores for T2DM risk.
Previous studies have primarily concentrated on only one inflammation-related index and have not taken into account the synergistic effects of different inflammation-related factors or their relative contributions to inflammatory status and chronic diseases. In our study, we used two newly-developed innovative indices that incorporate all inflammation-related diet and lifestyle components and evaluate their combined contribution to low-grade systemic inflammation.
Our findings highlight the significant positive association between higher combined diet and lifestyle inflammation scores (EDII-LIS and DIS-LIS) and increased risk of T2DM. These novel indices provide a comprehensive assessment of an individual’s inflammatory potential by accounting for both dietary and lifestyle factors, aligning with a recent study that showed a direct association between the diet and lifestyle inflammation score (DLIS) and the risk of insulin resistance [47]. From a public health perspective, these findings highlight the importance of promoting lifestyle modifications as a preventive strategy against T2DM. Interventions targeting multiple aspects of an individual’s lifestyle, including physical activity levels and other health-related behaviors such as smoking cessation, may be more effective in reducing chronic low-grade inflammation and mitigating T2DM risk. Furthermore, the use of comprehensive inflammation indices such as EDII-LIS and DIS-LIS could be used as screening tools to identify individuals at higher risk of developing T2DM, allowing for targeted interventions and personalized lifestyle counseling.
Overall, our findings suggest that individuals with a more pro-inflammatory lifestyle may be at a higher risk of developing T2DM. However, it is important to note that other factors, such as genetics and underlying health conditions, may also play a role in the observed association [48, 49]. Further research is needed to fully understand the mechanisms behind this relationship and to determine whether interventions targeting pro-inflammatory factors can lower the risk of T2DM.
The current study had several strengths, including its population-based prospective design with a large sample size for both genders in a Middle Eastern country, and its adjustment for various potential confounding factors. A valid and reliable FFQ was also used to accurately calculate scores, and a validated physical activity questionnaire was used to assess energy expenditure for physical activity by trained interviewers. This study also had some limitations that need to be acknowledged. Firstly, 15 out of 18 food groups were utilized to compute the EDII score, and certain items were omitted in calculating the DIS and LIS scores. Consequently, the final scores were calculated based on 18 food groups for DIS and 3 components for LIS, instead of the usual 19 and 4, respectively. Secondly, as with all self-reported dietary assessments, potential measurement errors and recall bias cannot be entirely ruled out, despite using culturally-adapted, validated questionnaires to minimize such issues. While the present study has adjusted for many confounding variables, potential residual confounding factors that were not controlled for in the analysis, such as circulating inflammatory markers, medications, supplement intake, genetic data, and long-term dietary patterns, could still influence our results and limit our ability to perform additional analyses and comprehensively interpret the findings. Furthermore, there may be residual confounding due to unknown or unmeasured factors. In addition, it should be noted that these indices were validated for the US population, and using the given weights may not be suitable for other populations.
Conclusion
This longitudinal analysis of a Middle Eastern cohort found that heightened inflammation from lifestyle factors, captured by the LIS, EDII-LIS, and DIS-LIS scores, was associated with increased 6-year T2DM incidence. These findings suggest that lifestyle modifications to mitigate inflammation may have protective effects against T2DM development in at-risk individuals. However, the isolated EDII and DIS scores did not show significant relationships, highlighting the need for further research to clarify the role of dietary versus lifestyle inflammation in T2DM pathogenesis across diverse populations. Future studies should investigate the potential behavioral and biological mechanisms linking pro-inflammatory lifestyle components to T2DM risk, which could inform targeted public health interventions for T2DM prevention.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- EDII:
-
Empirical dietary inflammatory index
- DIS:
-
Dietary inflammatory score
- LIS:
-
Lifestyle inflammatory score
- FFQ:
-
Food frequency questionnaire
- FBG:
-
Fasting blood glucose
- RR:
-
Relative risk
- T2DM:
-
Type 2 diabetes mellitus
- YaHS:
-
Yazd Health Study
References
Li M-z, Su L, Liang B-y, Tan J-j, Chen Q, Long J-x et al. Trends in prevalence, awareness, treatment, and control of diabetes mellitus in mainland China from 1979 to 2012. International journal of endocrinology. 2013;2013.
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of Disease and Forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–11.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Javanbakht M, Baradaran HR, Mashayekhi A, Haghdoost AA, Khamseh ME, Kharazmi E, et al. Cost-of-illness analysis of type 2 diabetes mellitus in Iran. PLoS ONE. 2011;6(10):e26864.
Hazar N, Jokar M, Namavari N, Hosseini S, Rahmanian V. An updated systematic review and Meta-analysis of the prevalence of type 2 diabetes in Iran, 1996–2023. Front Public Health. 2024;12:1322072.
Werfalli M, Raubenheimer P, Engel M, Peer N, Kalula S, Kengne AP, et al. Effectiveness of community-based peer-led diabetes self-management programmes (COMP-DSMP) for improving clinical outcomes and quality of life of adults with diabetes in primary care settings in low and middle-income countries (LMIC): a systematic review and meta-analysis. BMJ Open. 2015;5(7):e007635.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
Brahimaj A, Ligthart S, Ghanbari M, Ikram MA, Hofman A, Franco OH, et al. Novel inflammatory markers for incident pre-diabetes and type 2 diabetes: the Rotterdam Study. Eur J Epidemiol. 2017;32(3):217–26.
Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–78.
Schulze MB, Hu FB. Primary prevention of diabetes: what can be done and how much can be prevented? Annu Rev Public Health. 2005;26:445–67.
Gaesser G, Angadi S, Ryan D, Johnston C. Lifestyle measures to reduce inflammation. Am J Lifestyle Med. 2012;6:4–13.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96.
Denova-Gutiérrez E, Muñoz-Aguirre P, Shivappa N, Hébert JR, Tolentino-Mayo L, Batis C et al. Dietary inflammatory index and type 2 diabetes Mellitus in adults: the diabetes Mellitus Survey of Mexico City. Nutrients. 2018;10(4).
King DE, Xiang J. The Dietary Inflammatory Index is Associated with Diabetes Severity. J Am Board Fam Med. 2019;32(6):801–6.
Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and validation of an empirical Dietary Inflammatory Index. J Nutr. 2016;146(8):1560–70.
Byrd DA, Judd SE, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Development and Validation of Novel Dietary and Lifestyle inflammation scores. J Nutr. 2019;149(12):2206–18.
Li Z, Gao Y, Byrd DA, Gibbs DC, Prizment AE, Lazovich D, et al. Novel dietary and lifestyle inflammation scores directly Associated with All-Cause, All-Cancer, and all-Cardiovascular Disease Mortality Risks among women. J Nutr. 2021;151(4):930–9.
Byrd D, Holmes A, Judd S, Flanders WD, Bostick RM. Abstract 3287: associations of whole food and lifestyle-based inflammation scores with all-cause, cancer- and cardiovascular disease-specific mortality. Cancer Res. 2017;77(13Supplement):3287.
Lee DH, Li J, Li Y, Liu G, Wu K, Bhupathiraju S, et al. Dietary inflammatory and insulinemic potential and risk of type 2 diabetes: results from three prospective U.S. Cohort studies. Diabetes Care. 2020;43(11):2675–83.
Farhadnejad H, Parastouei K, Rostami H, Mirmiran P, Azizi F. Dietary and lifestyle inflammatory scores are associated with increased risk of metabolic syndrome in Iranian adults. Diabetol Metab Syndr. 2021;13(1):30.
Saber N, Hosseinzadeh M, Shab-Bidar S, Mirzaei M, Najarzadeh A, Rahideh ST. Empirical dietary inflammatory index and lifestyle inflammation score relationship with obesity: a population-based cross-sectional study. Food Sci Nutr. 2023;11(11):7341–51.
Teymoori F, Farhadnejad H, Mokhtari E, Sohouli MH, Moslehi N, Mirmiran P, et al. Dietary and lifestyle inflammatory scores and risk of incident diabetes: a prospective cohort among participants of Tehran lipid and glucose study. BMC Public Health. 2021;21(1):1293.
Mirzaei M, Sharifnia G, Khazaei Z, Sadeghi E, Fallahzadeh H, Namayandeh SM. Prevalence of general obesity and central adiposity and its related factors in adult population of Yazd. J Shahid Sadoughi Univ Med Sci. 2017;25(9):736–47.
Mirzaei M, Salehi-Abargouei A, Mirzaei M, Mohsenpour MA. Cohort Profile: the Yazd Health Study (YaHS): a population-based study of adults aged 20–70 years (study design and baseline population data). Int J Epidemiol. 2018;47(3):697–h8.
Weir CB, Jan A, BMI Classification Percentile And Cut Off Points. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Arif Jan declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2023. StatPearls Publishing LLC.; 2023.
Zimorovat A, Moghtaderi F, Amiri M, Raeisi-Dehkordi H, Mohyadini M, Mohammadi M, et al. Validity and reproducibility of a semiquantitative multiple-choice food frequency questionnaire in Iranian adults. Food Nutr Bull. 2022;43(2):171–88.
Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol. 2010;20(2):150–8.
Ghafarpour M, Houshiar-Rad A, Kianfar H. The Manual for Household measures. Cooking Yields Factors and Edible Portion of Food; 1999.
Azar M, Sarkisian E. Food composition table of Iran. Tehran: National Nutrition and Food Research Institute, Shaheed Beheshti University. 1980;65.
Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18(8):1073–80.
Garmaroudi GR, Moradi A. Socio-economic status in Iran: a study of measurement index. Payesh (Health Monitor). 2010;9(2):137–44.
Association AD. 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes—2021. Diabetes Care. 2020;44(Supplement1):S15–33.
Mirzaei M, Rahmaninan M, Mirzaei M, Nadjarzadeh A, Dehghani tafti AA. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes in Central Iran: results from Yazd health study. BMC Public Health. 2020;20(1):166.
Byrd DA, Judd S, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Associations of Novel Dietary and Lifestyle inflammation scores with Incident, sporadic colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2300–8.
Dehghani Firouzabadi F, Jayedi A, Asgari E, Akbarzadeh Z, Janbozorgi N, Djafarian K et al. Association of Dietary and Lifestyle inflammation score with metabolic syndrome in a sample of Iranian adults. Front Nutr. 2021;8.
Will JC, Galuska DA, Ford ES, Mokdad A, Calle EE. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30(3):540–6.
Aras M, Tchang BG, Pape J. Obesity and diabetes. Nurs Clin North Am. 2021;56(4):527–41.
Joseph JJ, Echouffo-Tcheugui JB, Golden SH, Chen H, Jenny NS, Carnethon MR, et al. Physical activity, sedentary behaviors and the incidence of type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis (MESA). BMJ Open Diabetes Res Care. 2016;4(1):e000185.
Okura T, Nakamura R, Fujioka Y, Kawamoto-Kitao S, Ito Y, Matsumoto K, et al. Body mass index ≥ 23 is a risk factor for insulin resistance and diabetes in Japanese people: a brief report. PLoS ONE. 2018;13(7):e0201052.
Barr J, Sharma CS, Sarkar S, Wise K, Dong L, Periyakaruppan A, et al. Nicotine induces oxidative stress and activates nuclear transcription factor kappa B in rat mesencephalic cells. Mol Cell Biochem. 2007;297(1–2):93–9.
Stadler M, Tomann L, Storka A, Wolzt M, Peric S, Bieglmayer C, et al. Effects of smoking cessation on β-cell function, insulin sensitivity, body weight, and appetite. Eur J Endocrinol. 2014;170(2):219–7.
Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Pathophysiology of physical inactivity-dependent insulin resistance: a theoretical mechanistic review emphasizing clinical evidence. J Diabetes Res. 2021;2021:7796727.
Hudish LI, Reusch JE, Sussel L. β cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129(10):4001–8.
Bauer S, Wennberg Huldt C, Kanebratt KP, Durieux I, Gunne D, Andersson S, et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: towards a novel human ex vivo type 2 diabetes model. Sci Rep. 2017;7(1):14620.
Højbjerre L, Sonne MP, Alibegovic AC, Nielsen NB, Dela F, Vaag A, et al. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34(10):2265–72.
Jin Q, Shi N, Aroke D, Lee DH, Joseph JJ, Donneyong M, et al. Insulinemic and inflammatory dietary patterns show enhanced predictive potential for type 2 diabetes risk in Postmenopausal Women. Diabetes Care. 2021;44(3):707–14.
Farhadnejad H, Teymoori F, Mokhtari E, Mirmiran P, Azizi F. Higher scores of dietary and lifestyle inflammatory indices are associated with increased risk of insulin-related disorders in Iranian adults. Eur J Clin Nutr. 2022;76(11):1566–75.
Hadjadj S, Fumeron F, Roussel R, Saulnier PJ, Gallois Y, Ankotche A, et al. Prognostic value of the insertion/deletion polymorphism of the ACE gene in type 2 diabetic subjects: results from the non-insulin-dependent diabetes, hypertension, Microalbuminuria or Proteinuria, Cardiovascular events, and Ramipril (DIABHYCAR), Diabete De type 2, Nephropathie et genetique (DIAB2NEPHROGENE), and Survie, Diabete De type 2 et genetique (SURDIAGENE) studies. Diabetes Care. 2008;31(9):1847–52.
Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291(16):1978–86.
Acknowledgements
We thank the people of Yazd and investigators of YaHS for their time and efforts.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
N.S contributed to the original draft preparation, review and editing of the manuscript. M.M founded YaHS and approved its methodology as its chief investigator. M.H and A.N were involved in the methodology and proof read the manuscript draft. S.S, F.T, and S.H contributed in statistical analysis. ST. R supervised the project from conception to manuscript preparation. The final version of the paper was reviewed and approved by all authors. Mahdieh Hosseinzadeh and Niloufar Saber equally contributed to this work (equally first author).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods of this study were carried out under the Declaration of Helsinki’s ethical principle for medical research involving human subjects. YaHS was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences. Additionally, the Ethics Committee of Iran University of Medical Sciences approved the protocol of this study (IR.IUMS.REC.1399.1415). All participants in the study provided written informed consent.
Accordance statement
The authors declare that all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hosseinzadeh, M., Saber, N., Bidar, S.S. et al. Association of dietary and lifestyle inflammatory indices with type 2 diabetes risk in Iranian adults. BMC Endocr Disord 24, 131 (2024). https://doi.org/10.1186/s12902-024-01673-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01673-7