Abstract
Objective
Many studies have investigated the impact of precocious puberty on cardiovascular disease (CVD) outcomes and the association between lipid profile levels and precocious puberty. However, the results have been inconsistent. The aim of this meta-analysis was to evaluate whether triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL)and low density lipoprotein (LDL) levels were altered in girls with precocious puberty compared with healthy controls.
Methods
References published before June 2022 in the EMBASE, Cochrane Library, PubMed and Web of Science databases were searched to identify eligible studies. A DerSimonian-Laird random-effects model was used to evaluate the overall standard mean difference (SMD) between precocious puberty and healthy controls. Subgroup analyses and sensitivity analyses were preformed, and publication bias was assessed.
Results
A total of 14 studies featuring 1023 girls with precocious puberty and 806 healthy girls were selected for analysis. The meta-analysis showed that TG (SMD: 0.28; 95% CI: 0.01 to 0.55; P = 0.04), TC (SMD: 0.30; 95% CI: 0.01 to 0.59; P = 0.04), LDL (SMD: 0.45; 95% CI: 0.07 to 0.84; P = 0.02)levels were significantly elevated in girls with precocious puberty. HDL levels did not change significantly (SMD: -0.06; 95% CI: -0.12 to 0.61; P = 0.62). Subgroup analyses revealed that the heterogeneity in the association between lipid profile and precocious puberty in this meta-analysis may arise from disease type, region, sample size, chronological age, body mass index difference and drug usage.
Conclusion
Lipid profile levels altered in girls with precocious puberty compared with healthy controls. In order to minimize the risk of CVD morbidity and mortality, early interventions were needed to prevent obesity in children and adolescents, especially those with precocious puberty.
Similar content being viewed by others
Introduction
Precocious puberty is defined as the development of secondary sexual characteristics in girls by the age of 8 years and in boys by the age of 9 years [1]. A growing percentage of girls are going through precocious puberty [2, 3]. According to whether the hypothalamic-pituitary-gonadal axis (HPGA) occurs or not, precocious puberty is classified as central precocious puberty (CPP) with the HPGA occurring driven by early increased gonadotropin-releasing hormone (GnRH) secretion, which accounts for roughly 80% [4, 5], and as peripheral precocious puberty (PPP) which is independent of GnRH secretion, as well as incomplete precocity, which is the variation of CPP, including premature pubarche (PP), premature adrenarche (PA), premature thelarche (PT) and premature menarche [6,7,8,9]. PT is caused by transient partial activation of HPGA and overproduction of follicle stimulating hormone (FSH) and is characterized by isolated breast development without any other signs of sexual maturation [10]. However, 13% of PT cases may develop into CPP [11]. PA is referred to as an increase of adrenal androgen level independent of the HPGA, it is identified as pubarche including the presence of pubic and axillary hair, apocrine body odor and acne by age 8 for girls and age 9 for boys [12].
In girls, early maturational timing is linked to increased all-cause, cancer, and cardiovascular disease (CVD) [13, 14]. Accordingly, an earlier age of menarche is linked to a higher risk of obesity [15,16,17,18], hypertension [13], type 2 diabetes [18], ischemic heart disease, and stroke occurrences [13], as well as other conditions in later life. According to a recent study, high childhood obesity may be to blame for the effects of premature menarche on cardiovascular risk in adults [19]. In accordance, high adiposity during adolescence predicts premature menarche [20, 21]. On the other hand, women who have a history of early menarche have greater increases in adiposity during adolescence and in the early years of adulthood [15, 20]. As a result, it does appear that early maturation plays a role in unfavorable metabolic programming.
There are conflicting reports about the effects of precocious puberty on lipid metabolism so far. Ibáñez et al. [22]. found the serum levels of triglyceride (TG), total cholesterol (TC) and low density lipoprotein (LDL) increased, and the level of high density lipoprotein (HDL) decreased in girls with premature pubarche.And similar findings were found in several cohort studies [23, 24]. In contrast, no relationship between early maturational timing and lipid profile has been found in other studies [25,26,27,28,29,30,31,32].
Hence, understanding the potential link between lipid profile and precocious puberty has public health implications for reducing the risk of cardiovascular morbidity and mortality in women. Precocious puberty may provide important information to unravel the effects of pubertal onset and age on lipid profile levels. Therefore, the aim of this systematic review and meta-analysis was to assess lipid profile levels in girls with precocious puberty and healthy controls.
Materials and methods
Reporting guidelines
This systematic review and meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [33], and was prospectively registered on PROSPERO (registration number: CRD42022357819). This study was conducted according to the guidelines for Meta-analysis of Observational Studies in Epidemiology (MOOSE) [34].
Search strategy
The population/intervention/comparison/outcome (PICO) components were as follows: P (girls before the age of 10), I (girls with precocious puberty), C (healthy girls), O (serum levels of TG, TC, HDL and LDL).To identify eligible studies, an exhaustive literature search was conducted in the Cochrane Library, PubMed, EMBASE and Web of Science databases (without restricting by language, location and journal) through June 2022 to identify published research using the following keywords: “precocious puberty” OR “sexual precocity” OR “premature puberty” OR “precocious sexual maturation” OR “early puberty” OR “earlier puberty” OR “early pubertal timing” OR “early maturation” OR “isolated premature thelarche” OR “premature thelarche” OR “premature pubarche” OR “premature adrenarche” OR “premature menarche” OR “early age of menarche” OR “PP” OR “CPP” OR “PPP” OR “IPT” OR “PT”AND “lipid profile” OR “total cholesterol” OR “triacylglycerol” OR “triglyceride” OR “triglycerides” OR “high-density lipoprotein” OR “low-density lipoprotein” OR “TC” OR “TG” OR “HDL” OR “LDL” OR “HDL-C” OR “LDL-C”.
Inclusion and exclusion criteria
The inclusion criteria for eligible studies were as follows:(1) original observational study of humans; (2) all patients involved in studies were diagnosed with precocious puberty, including CPP, PP, PA and PT before 8 years old; (3) all subjects involved in studies were younger than 10 years old; (4) studies focusing on the association between the serum TG, TC, HDL, LDL levels and precocious puberty; and (5) studies included data on the lipid profile levels for patients with precocious puberty and healthy age-matched prepubertal girls.
The exclusion criteria were as follows: (1) laboratory or animals studies; (2) reviews or case reports; (3) duplicate publications; (4) subjects involved in studies were diagnosed with sex hormone releasing tumors and the Cushing’s Syndrome; (5) subjects participating in studies were diagnosed with diabetes, thyroid dysfunction or hyperprolactinemia; (6) studies without healthy control groups; and (7) studies that did not provide definitive data on serum TG/TC/HDL/LDL levels.
Study selection and data extraction
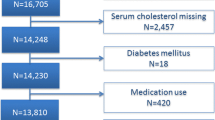
Literature screening and data extraction were done independently by two researchers (MJ and YG). Discrepancies between two reviewers were resolved in consultation with a third reviewer (LH). Firstly, citations were imported into EndNote to identify duplicate citations. Secondly, research titles and abstracts were screened, and those did not meet the inclusion criteria were excluded. Finally, the full literature was read, and the studies were further excluded based on inclusion and exclusion criteria. A flow chart of the PRISMA interpretation the selection process was shown in Fig. 1. TG, TC, HDL and LDL [mean ± standard deviation (SD)] levels were extracted from the literature, and all data were reviewed by LH.
Flow chart of the selection process. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi: https://doi.org/10.1371/journal.pmed1000097
When no continuous variables were reported, each author was contacted and asked to provide the raw data. In cases where contact failed, standard deviation values were calculated from the median, quartiles or ranges or [35, 36], if the data were displayed graphically only, values were estimated by digital ruler software (Getdata Graph Digitizer, version 2.25) [37, 38].
Quality assessment
The quality of the selected studies was evaluated according to the Newcastle-Ottawa Scale (NOS) star system [39].
Statistical analysis
A meta-analysis was performed on the data extracted from the included studies. As the available literature was inconsistent in terms of units, the combined standardized mean difference (SMD) and 95% confidence intervals (CI) were used to determine the associations between serum TG, TC, HDL, LDL levels and precocious puberty. The Cochran’s Q two-sided homogeneitytest [40] was used to test for heterogeneity of the studies. A Mantel-Haenszel fixed-effect model was used if I-square (I2) < 50% and conversely, a DerSimonian-Laird random-effects model was used [41, 42]. Subgroup analyses were performed to determine the association between serum TG, TC, HDL, LDL levels and study characteristics to test whether this could explain heterogeneity.Sensitivity analyses were performed to test the robustness of the combined SMD by excluding each study. The funnel plot method (in the case of the number of inclusions was ≥ 10) was used to test for publication bias. All analyses were performed using RevMan software, and differences were considered statistically significant at P < 0.05.
Results
Literature search
After reading the titles and abstracts of the initial 405 articles, 303 were excluded because they did not meet the inclusion criteria. 102 articles were included for full-text assessment, of which 88 were excluded: 26 without healthy controls; 11 subjects aged 10 years or older; one review; and fifty research topics were inappropriate. A total of 14 eligible papers were included (Fig. 1), all studies were observational in design and included individual data from 1023 cases and 806 healthy controls [22,23,24,25,26,27,28,29,30,31,32, 43,44,45]. The baseline characteristics, such as author, year, geographical location, study design, sample size, chronological age (CA), bone age (BA), tanner stage, body mass index (BMI), medication usage, measurement method, lipid profile and key findings included in the studies were shown in Table 1.
Quality assessment
Quality assessment of literature were performed according to the NOS star system, and all selected studies were scored 7 or more (Table 2).
Meta-analysis
Fourteen studies (n = 1829 participants) compared serum TG levels between girls with precocious puberty and healthy controls (Fig. 2A), and there was significant heterogeneity between studies (I2 = 84%; P < 0.00001). Precocious puberty was significantly associated with elevated serum TG levels (SMD: 0.28; 95% CI: 0.01 to 0.55; P = 0.04).
Forest plot of the levels of serum lipid profile in cases and healthy controls. Weights are from random effects analysis. (A) Meta-analysis of triglyceride. (B) Meta-analysis of total cholesterol. (C) Meta-analysis of high density lipoprotein. (D) Meta-analysis of low density lipoprotein. CI, confidence interval; SD, standard difference
Twelve studies (n = 1707 participants) compared serum TC levels between girls with precocious puberty and healthy controls (Fig. 2B), and there was significant heterogeneity between studies (I2 = 85%; P < 0.00001). Precocious puberty was significantly associated with elevated serum TC levels (SMD: 0.30; 95% CI: 0.01 to 0.59; P = 0.04).
Thirteen studies (n = 1361 participants) compared serum HDL levels between girls with precocious puberty and healthy controls (Fig. 2C), and there was significant heterogeneity between studies (I2 = 72%; P < 0.0001). There was no significant correlation between precocious puberty and serum HDL levels (SMD: -0.06; 95% CI: -0.12 to 0.61; P = 0.62).
Thirteen studies (n = 1361 participants) compared serum LDL levels between girls with precocious puberty and healthy controls (Fig. 2D), and there was significant heterogeneity between studies (I2 = 90%; P < 0.00001). Precocious puberty was significantly associated with elevated serum LDL levels (SMD: 0.45; 95% CI: 0.07 to 0.84; P = 0.02).
Results of subgroup analysis
There was significant heterogeneity between studies including meta-analysis of lipid profile levels in precocious puberty. In order to find the sources of heterogeneity and to more accurately assess the differences between girls with precocious puberty and healthy controls, subgroup analyses were conducted by disease type, region, sample size, case and control BMI differences.
In terms of disease type (Table 3), TG levels were higher in the PT group than in healthy controls (p = 0.01), while there was no significant difference between the CPP (p = 0.66), PP (p = 0.05) and PA (p = 0.58) groups, and reduced heterogeneity in CPP subgroup (I2 = 0%). TC levels were higher in the PT group than in healthy controls (p = 0.03), while there was no significant difference between the CPP (p = 0.66), PP (p = 0.22), and PA (p = 0.09) groups, and there was no heterogeneity in CPP subgroup (I2 = 0%).HDL levels were lower in PP girls compared with healthy controls(p = 0.03), but not significantly different in CPP (p = 0.98), PA (p = 0.32) and PT (p = 0.87)cases, and there was reduced heterogeneity in CPP subgroup (I2 = 0%). LDL levels were higher in girls with PT compared with healthy controls (p < 0.0001), but no significant differences in CPP (p = 0.86), PP (p = 0.05) and PA (p = 0.32)cases, and no heterogeneity in CPP subgroup (I2 = 0%).
In terms of geographical location (Table 4),TG levels were higher in European girls with precocious puberty than in healthy controls(p = 0.02), but not significantly different in the American(p = 0.18), Central Asian (p = 0.58) and Asian cases (p = 0.39), with reduced heterogeneity in the Americas (I2 = 0%) and Asian (I2 = 57%) subgroups. TC levels did not correlate with precocious puberty in all subgroups, but there was reduced heterogeneity in the American (I2 = 0%) and Asian (I2 = 53%) subgroups. HDL levels did not correlate with precocious puberty in all subgroups, but heterogeneity was reduced in the American (I2 = 0%) and Asian (I2 = 0%) subgroups. There was no correlation between LDL level and precocious puberty in all subgroups, but the heterogeneity of Americas subgroup decreased (I2 = 8%).
In terms of sample size (Table 5), the sample size < 50 group had higher TG levels than the healthy control group (p = 0.03), while the sample size ≥ 50 group did not differ significantly (p = 0.91). TC levels were higher in the sample size < 50 group than in the healthy controls (p = 0.03), whereas they were not higher in the sample size ≥ 50 group (p = 0.52), with reduced heterogeneity (I2 = 2%).HDL levels were not statistically different between cases and healthy controls in the sample size < 50 (p = 0.14) and sample size ≥ 50 (p = 0.38) subgroups. LDL levels were higher in girls with precocious puberty than in healthy controls (p = 0.03)with sample size < 50.However, serum LDL was not associated with precocious puberty in the subgroup with a sample size ≥ 50 (p = 0.77).
As for case and control BMI differences (Table 5), in the subgroup with difference, the TG levels in the cases were higher than that in healthy controls (p = 0.03), but not in the subgroup without difference (p = 0.25), the heterogeneity decreased in this subgroup (I2 = 19%).In the subgroup with difference, girls with precocious puberty had a higher TC level than the healthy controls (p = 0.03), but not in the subgroup with without difference (p = 0.20),while the heterogeneity was reduced in this subgroup (I2 = 9%). Serum HDL was not associated with precocious puberty in the subgroup with difference (p = 0.34) and without difference (p = 0.60), while there was no heterogeneity in the subgroup without difference (I2 = 0%). LDL level were not associated with precocious puberty in the subgroup with difference (p = 0.05) and without difference (p = 0.38).
Sensitivity analysis
For TG, HDL and LDL levels, there was no qualitative change in the total effect size after study-by-study exclusion, indicating that this meta-analysis results were stable and reliable. For TC level, the results of this meta-analysis were weakly stable and sensitive to Guven’s study [44]. Heterogeneity was reduced after excluding this study (I2 from 85 to 55%), and TC levels were not statistically different between cases and healthy controls (P from 0.04 to 0.23).
Publication bias
Any publishing bias was found using the funnel plot method. Systematic reviewers may be at fault for publication bias if they draw final statistical findings that do not match the data by omitting previously unpublished literature. The shapes of the funnel plots were asymmetric for TG, TC, HDL and LDL, and some studies fell outside the 95% confidence interval, indicating that the asymmetry of funnel plot might be caused by the heterogeneity among the studies. This suggested that there was heterogeneity among the studies (Fig. 3).
Discussion
A total of 14 studies were selected for this meta-analysis, with a total of 1829 girls participating. Subgroup analyses were conducted according to disease type, region, sample size, case and control BMI differences. This meta-analysis revealed significant changes in lipid levels in girls with precocious puberty: serum TG, TC and LDL levels were significantly higher in girls with precocious puberty than in healthy controls, while serum HDL levels in girls with precocious puberty were not significantly different from those in healthy controls.
Accelerated growth rates in children, especially increased fat mass, may lead to an earlier onset of puberty [46] and an increased risk of cardiometabolic disease [47], in addition, increased cardiovascular morbidity and mortality in adulthood are associated with early menarcheal age [13, 18]. Furthermore, the earlier the onset of menstruation, the greater the risk of hypertension and cardiovascular disease in adulthood [13]. Recent data, however, indicates that this relation might be mostly attributed to higher childhood obesity [19]. Early puberty causes girls to develop more quickly than girls who will experience later puberty, and as their epiphyses fuse, they seem to maintain this growth by laying down fat. Although greater adiposity may be principally responsible for the association between early maturation and higher cardiovascular risk, this does not fully explain the association [13, 14], early maturity timing per se appears to also be involved in this harmful metabolic programming [15, 20]. Girls who experience adrenarche (the rise in androgen secretion from the adrenal cortex right before puberty) earlier in life are more likely to develop insulin resistance, dyslipidemia, and obesity later in life [48]. Studies show that even in the absence of obesity, girls with PA are more likely to develop polycystic ovarian syndrome (PCOS) and have a higher future risk of developing metabolic alterations (hyperlipidemia, diabetes, and hypertension) [49]. PP, the primary clinical symptom of PA, is also associated to dyslipidemia and insulin resistance [43]. In both animal and human models, androgens are associated with changes in the distribution of body fat in females; however, gonadotropin releasing hormone agonist (GnRHa) medication does not affect this mechanism [50]. Furthermore, the prevalence of obesity and metabolic syndrome usually occurrs between the third and 50th years of life and appears to be the same in treated and untreated women with a history of CPP in childhood [51].
How dyslipidaemia causes CVD is well established. The risk of CVD is increased by hyperlipidemia, which is frequently diagnosed by elevated serum LDL levels. Furthermore, due to oxidative modification of LDL, the prevalence of atherosclerosis rises with LDL levels. As oxidised LDL accumulates in the arterial walls of the cardio-vascular region, atherosclerotic plaques gradually form. The corresponding blood vessels are blocked by the atherosclerotic plaques, which also cause atherosclerosis and raise the risk of CVD [52]. Studies have demonstrated that even if the amount of blood LDL is normal [53], partial oxidation leading toatherosclerosis cannot be excluded. Additionally, increased blood TC and TG levels are responsible for inadequate antioxidant mechanisms, which contribute to preclinical atherosclerosis [54]. These changes are therefore partly responsible for the increased incidence of CVD.
Interventions to prevent or reverse higher adiposity trajectories in girls with precocious puberty at a very young age may have significant effects on cardiovascular health in adulthood. Firstly, lipid profile should be closely monitored to detect and prevent PCOS and metabolic syndrome. Secondly, intervention to modify the lifestyle (e.g., additional diet and physical activity counseling) of the girls could prevent the long-term complications of dyslipidemia. Finally, it appears that GnRHa therapy cannot undo the undesirable body compositional alterations brought on by early maturation, inhibition of pubertal progression by GnRHa treatment is instead associated with a continual increase in obesity [55, 56]. Hence, it is worth initiating treatment with insulin sensitizers, such as metformin, which has been demonstrated to be effective in girls with precocious puberty [57, 58]. Treatment with metformin resulted in reversible reductions in TG, TC, and LDL levels as well as a significant rise in HDL level [59]. Additionally, metformin appears to have a direct impact on ovarian steroidogenesis, specifically by lowering the production of both androgen and estradiol [60, 61]. Metformin also reduces levels of plasminogen activator inhibitor type 1, known to be animportant inhibitor of fibrinolysis [62, 63]. Therefore, in order to offer the greatest promise to decrease morbidity and mortality, girls with risk factors, especially precocious puberty, should be investigated and intervened for atherosclerosis.
Subgroup analyses were conducted to further explore the sources of heterogeneity and to more accurately evaluate the association between lipid profile and precocious puberty. Heterogeneity in this meta-analysis of the correlation between TG levels and precocious puberty might be derived from disease type, region and BMI differences. The correlation between TC levels and precocious puberty might be related to disease type, region, sample size and BMI differences. The correlation between HDL levels and precocious puberty might be derived from disease type, region and BMI differences. And the correlation between LDL levels and precocious puberty might be related to disease type and region. (1) Disease types including PP, PA, PT and CPP were focused in this meta-analysis. PP, PA and PT were the variations of CPP and therefore have different degrees of impact on lipid profile levels; (2) people in different regions have different dietary habits, lifestyles and economic circumstances, which have different effects on serum lipid profile; (3) all literature included in this meta-analysis were case-control studies. If there were more cases in the case-control studies, the findings would have been influenced by more confounding factors; (4) being overweight has a significant effect on lipid profiles and therefore BMI,which is often used as a measure of total body fat,might be one of the sources of heterogeneity.
Subgroup analyses revealed partial sources of heterogeneity, but heterogeneity was still evident, so sensitivity analyses were performed. In this meta-analysis,the combined results of TC levelsassociated with precocious puberty were more sensitive to Guven’s study [44]. After excluding this reference, the heterogeneity decreased (I2 decreased from 85 to 55%), therefore, Guven’s study [44] had a significant impact on the heterogeneity of the combined results. After a careful reading of the literature, TC levels in the study was found to be estimated by enzyme assay method, unlike other studies with colourimetric methods or fully automated analyzers, and that other confounding factors may have contributed to the heterogeneity. The results of the sensitivity analysis showed that the combined correlation between TG, HDL, LDL levels and precocious puberty turned out to stable and reliable. In addition, there are possible signs of publication bias in this study. There is a thing called publication bias in the medical literature, it implies a higher probability of favorable results being reported. Therefore, additional thought is still required before possibly implementing the findings in clinical practice.
To our knowledge, this is the first meta-analysis to explore the relationship between lipid profile and precocious puberty. Clear eligibility criteria was developed, comprehensive search was conducted, eligibility and bias risks were both assessed, key outcomes were addressed, sensitivity and subgroup analyses were conducted, and quality assessment of literature were performed using the NOS star system. However, some limitations of this meta-analysis should be acknowledged. (1) there was significant heterogeneity between the original studies due to differences in sample size, background of study participants and methods of detecting lipid levels.The shapes of the funnel plots were asymmetric for TG, TC, HDL and LDL, and some studies fell outside the 95% confidence interval, also indicating the heterogeneity among the studies; (2) subgroup analyses of bone age and duration of precocious puberty could not be performed due to incomplete data. Instead, the heterogeneity may be related to the duration of precocious puberty or bone age, rather than physiological age per se. Because of these factors, HDL levels in girls with precocious puberty may be similar to those of healthy controls; (3) the main confounding factors affecting the lipid profiles of patients in the original study (e.g. diet, ethnicity, physical activity, etc.) were not adjusted, which might affect the conclusion of this study. So more cohort studies adjusting for these confounders are needed to justify the results of this meta-analysis; (4) the studies included in this study were all case-control studies, which would limit causal inferences–whether dyslipidemia causes precocious puberty or vice versa. Therefore, more cohort studies are needed to predict how the lipid profile develop over time in girls with precocious puberty; (5) the diagnostic criteria for identifying precocious puberty and methods for detecting lipid profile levels used in various studies varied slightly, which might also increase heterogeneity. For these reasons, we suggest that our conclusions should be taken with a grain of salt.
Conclusion
In summary, this systematic review and meta-analysis showed that serum TG, TC and LDL levels were significantly higher in precocious puberty subjects. This implies that girls who reach sexual maturity too early can lead to some changes in lipid profile and increase the risk of developing CVD in adulthood. Thus, keeping an eye out for CVD risk markers in early-maturing girls may be a useful and effective disease prevention strategy during adolescence. We recommend that lipid profile should be assessed and comprehensively studied to ensure that early lifestyle (e.g. diet and exercise) and/or medical intervention (e.g., metformin) and to minimize the morbidity and mortality of CVD associated with dyslipidemia in girls with precocious puberty at follow-up. In addition, the relationship between serum HDL levels and precocious puberty needs to be further investigated.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22(1):111–51. https://doi.org/10.1210/edrv.22.1.0418
Mogensen SS, Aksglaede L, Mouritsen A, Sørensen K, Main KM, Gideon P, et al. Diagnostic work-up of 449 consecutive girls who were referred to be evaluated for precocious puberty. J Clin Endocrinol Metab. 2011;96(5):1393–401. https://doi.org/10.1210/jc.2010-2745
Chen M, Eugster EA. Central precocious puberty: update on diagnosis and treatment. Paediatr Drugs. 2015;17(4):273–81. https://doi.org/10.1007/s40272-015-0130-8
Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–93. https://doi.org/10.1210/er.2002-0019
Niwa K, Mori S, Kuwabara K, Nagata K, Takenaka M, Shiga T, et al. Primary ovarian carcinosarcoma: Cytological, pathological, immunocytochemical, and immunohistochemical features. Open J Pathol. 2021;11:11.
Brito VN, Spinola-Castro AM, Kochi C, Kopacek C, Silva PC, Guerra-Júnior G. Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch Endocrinol Metab. 2016;60(2):163–72. https://doi.org/10.1590/2359-3997000000144
Sun T, Tian L, Guo Y, Zheng Y, Ouyang L, Zhang X, et al. Anaplastic carcinoma showing rhabdoid features combined with ovarian mucinous borderline cystadenoma: a case report and literature review. J Int Med Res. 2021;49(5):3000605211013159. https://doi.org/10.1177/03000605211013159
Terzic M, Aimagambetova G, Norton M, Della Corte L, Marín-Buck A, Lisón JF, et al. Scoring systems for the evaluation of adnexal masses nature: current knowledge and clinical applications. J Obstet Gynaecol. 2021;41(3):340–7. https://doi.org/10.1080/01443615.2020.1732892
Lidaka L, Grasmane A, Lazdane G, Dzivite-Krisane I, Gailite L, Viberga I. Can a mother’s polycystic ovary syndrome (PCOS)-related symptoms be used to predict the future clinical profile of PCOS in her adolescent daughter? A pilot study. Eur J Contracept Reprod Health Care. 2021;26(1):17–22. https://doi.org/10.1080/13625187.2020.1795118
Berberoğlu M. Precocious puberty and normal variant puberty: definition, etiology, diagnosis and current management. J Clin Res Pediatr Endocrinol. 2009;1(4):164–74. https://doi.org/10.4274/jcrpe.v1i4.3
Bereket A. A critical Appraisal of the Effect of Gonadotropin-Releasing Hormon Analog treatment on adult height of girls with central precocious puberty. J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):33–48. https://doi.org/10.4274/jcrpe.2017.S004
Novello L, Speiser PW. Premature Adrenarche. Pediatr Ann. 2018;47(1):e7–e11. https://doi.org/10.3928/19382359-20171214-04
Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–60. https://doi.org/10.1210/jc.2009-1789
Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976-88. Int J Epidemiol. 2009;38(1):245–52. https://doi.org/10.1093/ije/dyn251
Garn SM, LaVelle M, Rosenberg KR, Hawthorne VM. Maturational timing as a factor in female fatness and obesity. Am J Clin Nutr. 1986;43(6):879–83. https://doi.org/10.1093/ajcn/43.6.879
Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 2003;3:3. https://doi.org/10.1186/1471-2431-3-3
van Lenthe FJ, Kemper CG, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr. 1996;64(1):18–24. https://doi.org/10.1093/ajcn/64.1.18
Lakshman R, Forouhi N, Luben R, Bingham S, Khaw K, Wareham N, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia. 2008;51(5):781–6. https://doi.org/10.1007/s00125-008-0948-5
Kivimäki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Järvinen L, et al. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns study. Am J Clin Nutr. 2008;87(6):1876–82. https://doi.org/10.1093/ajcn/87.6.1876
Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27(11):1398–404. https://doi.org/10.1038/sj.ijo.0802422
Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90(5):2718–24. https://doi.org/10.1210/jc.2004-1991
Ibanez L, Valls C, Marcos MV, Ong K, Dunger DB, de Zegher F. Insulin sensitization for girls with precocious pubarche and with risk for polycystic ovary syndrome: Effects of prepubertal initiation and postpubertal discontinuation of metformin treatment. J Clin Endocrinol Metabolism. 2004;89(9):4331–7. https://doi.org/10.1210/jc.2004-0463
Ucar A, Saka N, Bas F, Hatipoglu N, Bundak R, Darendeliler F. Reduced atherogenic indices in prepubertal girls with precocious adrenarche born appropriate for gestational age in relation to the conundrum of DHEAS. Endocr Connections. 2013;2(1):2–11. https://doi.org/10.1530/ec-12-0059
Xu YQ, Li Y, Liang S, Li GM. Differential analysis of nutrient intake, insulin resistance and lipid profiles between healthy and premature thelarche Chinese girls. Ital J Pediatr. 2019;45(1). https://doi.org/10.1186/s13052-019-0758-z
Teixeira RJ, Ginzbarg D, Rodrigues Freitas J, Fucks G, Silva CM, Bordallo MA. Serum leptin levels in premature pubarche and prepubertal girls with and without obesity. J Pediatr Endocrinol Metabolism. 2004;17(10):1393–8. https://doi.org/10.1515/jpem.2004.17.10.1393
Larqué E, Gil-Campos M, Villada I, Ramírez-Tortosa MC, Cañete R, Gil A. Postprandial plasma adiponectin response is reduced in prepubertal premature pubarche girls. Metabolism. 2010;59(9):1319–26. https://doi.org/10.1016/j.metabol.2009.12.009
Sopher AB, Gerken AT, Lee EJ, Blaner WE, Deeds S, Gallagher D, et al. Retinol-binding protein 4 correlates with triglycerides but not insulin resistance in prepubertal children with and without premature adrenarche. J Pediatr Endocrinol Metabolism. 2011;24(9–10):683–7. https://doi.org/10.1515/jpem.2011.322
Su PH, Yang SF, Yu JS, Chen SJ, Chen JY. Study of leptin levels and gene polymorphisms in patients with central precocious puberty. Pediatr Res. 2012;71(4):361–7. https://doi.org/10.1038/pr.2011.69
Hur JH, Park S, Jung MK, Kang SJ, Kwon A, Chae HW, et al. Insulin resistance and bone age advancement in girls with central precocious puberty. Annals of Pediatric Endocrinology and Metabolism. 2017;22(3):176–82. https://doi.org/10.6065/apem.2017.22.3.176
Zurita-Cruz JN, Medina-Bravo P, Manuel-Apolinar L, Damasio-Santana L, Wakida-Kusunoki G, Padilla-Rojas M, et al. Resistin levels are not associated with obesity in central precocious puberty. Peptides. 2018;109:9–13. https://doi.org/10.1016/j.peptides.2018.09.009
Aydin BK, Kadioglu A, Kaya GA, Devecioglu E, Bas F, Poyrazoglu S, et al. Pelvic and breast ultrasound abnormalities and associated metabolic disturbances in girls with premature pubarche due to adrenarche. Clin Endocrinol. 2022;96(3):339–45. https://doi.org/10.1111/cen.14662
Bezen D, Tutunculer Kokenli F, Dilek E, Ag Seleci D, Erbas H. An evaluation of glucose metabolism and Cardiovascular Risk factors in Prepubertal girls with premature Pubarche. J Clin Res Pediatr Endocrinol. 2022. https://doi.org/10.4274/jcrpe.galenos.2022.2022-1-1
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Chatenoud L, Vecchia C, Comments. Meta-analysis of observational studies in epidemiology. Revue d Épidémiologie et de Santé Publique. 2000;48(4):411–2.
Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11(5):641–54. https://doi.org/10.1002/jrsm.1429
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. https://doi.org/10.1177/0962280216669183
Zhou X, Cao Q, Orfila C, Zhao J, Zhang L. Systematic review and Meta-analysis on the Effects of Astaxanthin on Human skin ageing. Nutrients. 2021;13(9). https://doi.org/10.3390/nu13092917
Wang R, Yao Q, Chen W, Gao F, Li P, Wu J, et al. Stem cell therapy for Crohn’s disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. 2021;12(1):463. https://doi.org/10.1186/s13287-021-02533-0
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17(8):841–56.
Huang M, Luo J, Luo G, Berahmand F, Zhang X. The effect of gum consumption on anthropometric characteristics and cardiac disorders: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2020;54:102578.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2
Ibáñez L, Potau N, Chacon P, Pascual C, Carrascosa A. Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia. 1998;41(9):1057–63. https://doi.org/10.1007/s001250051030
Guven A, Cinaz P, Bideci A. Is premature adrenarche a risk factor for atherogenesis? Pediatr Int. 2005;47(1):20–5. https://doi.org/10.1111/j.1442-200x.2004.02006.x
Sorensen K, Mouritsen A, Mogensen SS, Aksglaede L, Juul A. Insulin sensitivity and lipid profiles in girls with central precocious puberty before and during gonadal suppression. J Clin Endocrinol Metabolism. 2010;95(8):3736–44. https://doi.org/10.1210/jc.2010-0731
Boyne MS, Thame M, Osmond C, Fraser RA, Gabay L, Reid M, et al. Growth, body composition, and the onset of puberty: longitudinal observations in afro-caribbean children. J Clin Endocrinol Metab. 2010;95(7):3194–200. https://doi.org/10.1210/jc.2010-0080
Boyne MS, Osmond C, Fraser RA, Reid M, Taylor-Bryan C, Soares-Wynter S, et al. Developmental origins of cardiovascular risk in jamaican children: the vulnerable Windows Cohort study. Br J Nutr. 2010;104(7):1026–33. https://doi.org/10.1017/s0007114510001790
Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche–normal variant or forerunner of adult disease? Endocr Rev. 2000;21(6):671–96. https://doi.org/10.1210/edrv.21.6.0416
Ibañez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinyé M, et al. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76(6):1599–603. https://doi.org/10.1210/jcem.76.6.8501168
Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obes (Silver Spring). 2015;23(4):713–9. https://doi.org/10.1002/oby.21033
Lazar L, Lebenthal Y, Yackobovitch-Gavan M, Shalitin S, de Vries L, Phillip M, et al. Treated and untreated women with idiopathic precocious puberty: BMI evolution, metabolic outcome, and general health between third and fifth decades. J Clin Endocrinol Metab. 2015;100(4):1445–51. https://doi.org/10.1210/jc.2014-3748
Lorenzo C, Delgado P, Busse CE, Sanz-Bravo A, Martos-Folgado I, Bonzon-Kulichenko E, et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature. 2021;589(7841):287–92. https://doi.org/10.1038/s41586-020-2993-2
Keidar S, Angiotensin. LDL peroxidation and atherosclerosis. Life Sci. 1998;63(1):1–11. https://doi.org/10.1016/s0024-3205(98)00014-9
Al-Aubaidy HA, Jelinek HF. Oxidative stress and triglycerides as predictors of subclinical atherosclerosis in prediabetes. Redox Rep. 2014;19(2):87–91. https://doi.org/10.1179/1351000213y.0000000080
Alessandri SB, Pereira Fde A, Villela RA, Antonini SR, Elias PC, Martinelli CE Jr., et al. Bone mineral density and body composition in girls with idiopathic central precocious puberty before and after treatment with a gonadotropin-releasing hormone agonist. Clin (Sao Paulo). 2012;67(6):591–6. https://doi.org/10.6061/clinics/2012(06)08
Chiocca E, Dati E, Baroncelli GI, Mora S, Parrini D, Erba P, et al. Body mass index and body composition in adolescents treated with gonadotropin-releasing hormone analogue triptorelin depot for central precocious puberty: data at near final height. Neuroendocrinology. 2009;89(4):441–7. https://doi.org/10.1159/000197862
de Zegher F, García Beltrán C, López-Bermejo A, Ibáñez L. Metformin for rapidly maturing girls with Central Adiposity: Less Liver Fat and slower bone maturation. Horm Res Paediatr. 2018;89(2):136–40. https://doi.org/10.1159/000479369
Bassols J, Martínez-Calcerrada JM, Osiniri I, Díaz-Roldán F, Xargay-Torrent S, Mas-Parés B, et al. Effects of metformin administration on endocrine-metabolic parameters, visceral adiposity and cardiovascular risk factors in children with obesity and risk markers for metabolic syndrome: a pilot study. PLoS ONE. 2019;14(12):e0226303. https://doi.org/10.1371/journal.pone.0226303
Laakso M, Sarlund H, Mykkänen L. Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose tolerance. Arteriosclerosis. 1990;10(2):223–31. https://doi.org/10.1161/01.atv.10.2.223
Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003;79(4):956–62. https://doi.org/10.1016/s0015-0282(02)04925-7
Tosca L, Chabrolle C, Uzbekova S, Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5’ monophosphate-activated protein kinase (AMPK). Biol Reprod. 2007;76(3):368–78. https://doi.org/10.1095/biolreprod.106.055749
Velazquez EM, Mendoza SG, Wang P, Glueck CJ. Metformin therapy is associated with a decrease in plasma plasminogen activator inhibitor-1, lipoprotein(a), and immunoreactive insulin levels in patients with the polycystic ovary syndrome. Metabolism. 1997;46(4):454–7. https://doi.org/10.1016/s0026-0495(97)90066-4
Glueck CJ, Rorick MH, Schmerler M, Anthony J, Feibel J, Bashir M, et al. Hypofibrinolytic and atherogenic risk factors for stroke. J Lab Clin Med. 1995;125(3):319–25.
Acknowledgements
Not applicable.
Funding
This work was supported by grant from the National Natural Science Foundation of China (No. 81904240)); and Beijing University of Chinese Medicine vertical research and development fund (No. 2023-ZXFZJJ-058).
Author information
Authors and Affiliations
Contributions
MJ and KW: literature search, screening, and data extraction. LH and YG: data analysis and results visualization. MJ and LH: manuscript draft. LH: manuscript modification. All authors reviewed the final version of the manuscript and approve it for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, M., Gao, Y., Wang, K. et al. Lipid profile in girls with precocious puberty: a systematic review and meta-analysis. BMC Endocr Disord 23, 225 (2023). https://doi.org/10.1186/s12902-023-01470-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01470-8