Abstract
Background
Thyroid cancer is the most common malignant tumor of the endocrine system. There have been some reports on kidney cancer with thyroid metastasis. However, kidney cancer has rarely been detected during thyroid cancer surgery.
Case presentation
We present a rare case of kidney cancer with thyroid metastasis, combined with thyroid carcinoma. A 66-year-old woman was admitted to our hospital in September 2021 due to enlarged left thyroid nodules for two years. The patient was diagnosed with a left thyroid nodule on physical examination in 2012. Extended radical resection of the thyroid cancer was performed. Intraoperatively, two thyroid lesions were identified. Thus, the patient was definitively diagnosed with kidney cancer with thyroid metastasis and papillary thyroid carcinoma. Furthermore, two metastatic nodules due to kidney cancer and one metastatic lymph node lesion due to thyroid cancer were found in the loose connective tissue adjacent to the thyroid.
Conclusions
Kidney cancer with thyroid metastasis and thyroid carcinoma rarely co-occur, and it is difficult to identify the primary tumor. Although clinical examination methods are increasingly updated, the past medical history and physical examination are still very important.
Similar content being viewed by others
Background
Thyroid cancer is the most common malignancy of the endocrine system. The incidence of papillary thyroid cancer has increased rapidly [1, 2]. Surgery plays an important role in the treatment of thyroid patients, while clinical follow-up observation is also feasible, especially for pantiens with low-risk papillary thyroid cancer [3, 4]. Despite the advancements in the preoperative diagnostic tools, papillary thyroid cancer can still be misdiagnosed, even with an ultrasound-guided fine-needle aspiration biopsy, the gold standard [2, 5, 6]. Some patients are preoperatively diagnosed with papillary carcinoma. However, rapid freezing of the pathological specimen yielded a postoperative diagnosis of follicular carcinoma, medullary carcinoma, or even metastatic tumors. Some articles have reported kidney cancer metastases to the thyroid, but kidney cancer is rarely an incidental finding in thyroid cancer surgery [7, 8].We present a rare case of kidney cancer with thyroid metastasis combined with thyroid carcinoma. The patient’s clinical characteristics, which aided in the clinical diagnosis and treatment, are summarized in this study.
Case presentation
A 66-year-old woman was admitted to our hospital in September 2021 for enlarged left thyroid nodules, lasting two years. The patient was diagnosed with a left thyroid nodule on physical examination in 2012. In the following nine years, the patient developed no symptoms, such as hoarseness of the voice, difficulty breathing, and fever.
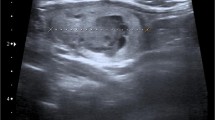
An outpatient ultrasound demonstrated a solid mass in the left thyroid gland (Thyroid Imaging Reporting and Data System 4b). The hypoechoic nodule (shown in Fig. 1A) grew horizontally and was composed of fused lesions, measuring 61.6 × 21.4 mm. It had an irregular shape, poorly defined boundaries, and heterogeneous internal echo. There was no significant internal echo, and no significant changes in the posterior echo. Abundant blood flow signals were appreciated on color doppler flow imaging. Moreover, there were several anechoic, hypoechoic, and mixed echoic nodules (Thyroid Imaging Reporting and Data System 3) in the left and right thyroid glands. One of the upper and middle poles in the left thyroid measured approximately 7.7 × 5.3 mm. One of the lower poles in the right thyroid measured approximately 12.8 × 7.1 mm. Both horizontally growing nodules had an elliptical shape, clear edges, and poor internal echoes. There was no obvious change in the posterior echoes, and the blood supply of color doppler flow imaging was low. Three-dimensional color and power Doppler ultrasound showed no large and twisted blood vessels. There were no apparent abnormalities in the left and right parathyroid regions and cervical lymph nodes.
Abnormal imaging findings of patients. A The hypoechoic nodule grew horizontally and was composed of fused lesions, measuring 61.6 × 21.4 mm. B The preoperative computed tomography (CT) revealed bilateral thyroid nodules with unclear boundaries. C Three weeks after the surgery, positron emission tomography-CT revealed increased metabolism in the surgical site of the thyroid
The preoperative computed tomography (CT) revealed bilateral thyroid nodules with unclear boundaries (shown Fig. 1B). One nodule had a diameter of 2.5 cm, and a small grainy, high-density shadow. Furthermore, the trachea was slightly deviated to the right. No apparent abnormalities were observed in the other neck soft tissues. The laboratory test results (including free triiodothyronine, thyroxine, thyroid-stimulating hormone, parathyroid hormone) were within the normal ranges (Shown Table 1).
The patient underwent a left nephrectomy in 2013, and the postoperative paraffin pathology confirmed clear cell carcinoma (grade II, 5.0 × 4.0 × 3.5 cm). After the kidney surgery, routine outpatient follow-up was performed, without any radiotherapy or chemotherapy. During the clinical follow-up observation, no other metastases were found in other organs. The patient had no history of hypertension, diabetes, and heart disease. The patient's complete past medical history was shown in Fig. 2.
Extended radical resection of the thyroid cancer was conducted (left thyroidectomy, right partial thyroidectomy, and left lateral lymph node dissection). Intraoperatively, two thyroid lesions (measuring 3.5 × 2.5 × 1.5 cm, 0.2 × 0.2 × 0.2 cm, respectively) were found. The former lesion was subsequently confirmed as metastatic kidney cancer by a pathologist. On immunohistochemical examination, the metastatic kidney cancer was CA9( +), Claudin-7(partially positive), CD10 ( +), vimentin ( +), PAX-8( +), PAX-2 (weakly positive), CK19 (partially positive), Galectin-3 ( +), Ki-67 (high expression area 30%), HBME-1 (-), TTF-1 (-), TG (-), CK7 (-), and CD117 (-). The latter lesion was subsequently confirmed as papillary thyroid carcinoma by the pathologist.
Furthermore, two metastatic nodules (0.2–0.4 cm in diameter) secondary to kidney cancer and one metastatic lymph node lesion (0.2–0.4 cm in diameter) secondary to thyroid cancer were found in the loose connective tissue adjacent to the thyroid. Given the patient's tumor stage (Papillary thyroid cancer, T1aN1bM0, ATA intermediate risk), only endocrine therapy (Levothyroxine Sodium Tablets, Euthyrox) was performed. The patient was discharged from the hospital postoperatively. Three weeks after the surgery, positron emission tomography-CT revealed increased metabolism in the surgical site of the thyroid. It was likely caused by metastasis or postoperative inflammatory changes, but it was not determined (shown in Fig. 1C). Based on the tumor characteristics, the patient’s long-term prognosis remains undetermined. However, no thyroid and kidney tumor metastasis has occurred in the patient so far.
Discussion and conclusions
Renal cell carcinoma (RCC) is a malignant tumor that originates from the inner wall of the proximal tubule. It is the third-most common genitourinary cancer site after prostate and bladder cancer [7]. RCC accounts for approximately 90% of renal tumors, and its pathological types mainly include clear cell carcinoma. Approximately 30% of patients have metastatic disease at the time of RCC diagnosis, and 25% develop metastatic disease following nephrectomy [8]. The most common sites of metastasis are the lungs (75%), followed by the regional lymph nodes (65%), liver (40%), and bone (40%). Thyroid metastasis secondary to RCC rarely occurs [8, 9]. However, RCC is the most common primary tumor in metastatic thyroid cancer [9, 10]. The five-year relative survival for European RCC patients, diagnosed between 2000 and 2007, was 60.6%. This rate was reportedly 72.4% according to the Surveillance, Epidemiology, and End Results program database from 2004 to 2010 [11]. The timing of the onset for thyroid metastasis in RCC is prolonged, and the median time from the diagnosis of RCC to the discovery of thyroid metastasis is estimated to be 8.8 years [12]. Compared to those with other single-site metastases, patients with thyroid metastasis have a better survival after thyroidectomy. Total thyroidectomy was not associated with a better survival than partial thyroidectomy [9]. For the treatment of RCC metastatic to thyroid, the current clinical guidelines do not give clear treatment recommendations. In view of the particularity of this patient, that is, remote metastasis was found by chance eight years after kid surgery, the patient was given Outpatient follow-up after multidisciplinary consultation [13, 14].
In this case, the preoperative examination did not match the final diagnosis. The solid mass in the left thyroid gland (61.6 × 21.4 cm, Thyroid Imaging Reporting and Data System 4b) was considered a primary thyroid cancer. However, the postoperative findings confirmed that it was a a metastasis from RCC. Review the patient's history, the reported thyroid nodes had not been further investigated in 2012, while in 2019 outpatient examination reported an enlarged left thyroid nodule, and it was the same where metastasis of renal cancer was subsequently diagnosed. Another small nodule was identified, and it was diagnosed as a papillary thyroid cancer, instead of a benign tumor. As for the discrepance in size between US and CT, we think it is due to the difference in work principle of machines. The CT scan shows the transverse axis of the mass, so the value is not the maximum diameter. However, on account of the mass effect and the squeezing effect of the sonographer, ultrasound examination may identify the tumor and its surrounding inflammatory tissue as the entire mass, which increase the size of the mass. Hence, both the surgeon and sonographer should take a complete medical history for patients presenting with a thyroid nodule, even though it is a common disease.
Upon reviewing the patient’s previous diagnoses and treatment plans, he was previously diagnosed with a left thyroid nodule in 2012, and he underwent a left nephrectomy (clear cell carcinoma) in 2013. He was admitted to our hospital for enlarged left thyroid nodules two years later. Kidney cancer rarely occurs with thyroid metastasis and thyroid carcinoma. Moreover, it is difficult to identify the primary tumor. This point of medical relevance is worth considering.
Availability of data and materials
All data generated and analyzed during this study are included in this published article and are available from the corresponding author upon reasonable request.
Abbreviations
- CT:
-
Computed tomography
- RCC:
-
Renal cell carcinoma
References
Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ, et al. 2021 American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2021;31:337–86.
Deng Y, Li H, Wang M, Li N, Tian T, Wu Y, et al. Global burden of thyroid cancer from 1990 to 2017. Jama Netw Open. 2020;3:e208759.
Jerkovich F, Abelleira E, Bueno F, Guerrero L, Pitoia F. Active Surveillance of small metastatic lymph nodes as an alternative to surgery in selected patients with low-risk papillary thyroid cancer: a retrospective cohort study. Thyroid. 2022;32:1178–83.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1.
Kiblut NK, Cussac J-F, Soudan B, Farrell SG, Armstrong JA, Arnalsteen L, et al. Fine needle aspiration and intraparathyroid intact parathyroid hormone measurement for reoperative parathyroid surgery. World J Surg. 2004;28:1143–7.
Ospina NS, Iñiguez-Ariza NM, Castro MR. Thyroid nodules: diagnostic evaluation based on thyroid cancer risk assessment. BMJ. 2020;368:l6670.
Garcia JA, Cowey CL, Godley PA. Renal cell carcinoma. Curr Opin Oncol. 2009;21:266–71.
Duggal NM, Horattas M. Metastatic renal cell carcinoma to the thyroid gland. Endocr Pract. 2008;14:1040–6.
Beutner U, Leowardi C, Bork U, Lüthi C, Tarantino I, Pahernik S, et al. Survival after renal cell carcinoma metastasis to the thyroid: single center experience and systematic review of the literature. Thyroid. 2015;25:314–24.
Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H. Metastasis to the thyroid gland: report of a large series from the mayo clinic. Am J Clin Oncol. 2015;38:338–42.
Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–30.
Sindoni A, Rizzo M, Tuccari G, Ieni A, Barresi V, Calbo L, et al. Thyroid metastases from renal cell carcinoma: review of the literature. Sci World J. 2010;10:590–602.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399–410.
Rathmell WK, Rumble RB, Veldhuizen PJV, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957–95.
Acknowledgements
We thank Dr. TW, Dr. WW and Dr.MW for the help in this clinical research.
Funding
This study was supported by the Shanghai Municipal Health Commission (Grant No. 20214Y0223) and Shanghai Huangpu District Municipal Health Commission (Grant No. HKM201906).
Author information
Authors and Affiliations
Contributions
JS and LZ diagnosed the patient, provided acquired clinical data. RX, DT, BL, XH, XJ and DS conducted investigations, reviewed literature. RX, DT and LZ drafted the manuscript. JS reviewed the manuscript for final publication. All of the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of Ruijin hospital Lu Wan Branch. Written informed consent was obtained prior to the study.
Consent for publication
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, R., Tan, D., Liu, B. et al. Kidney cancer with thyroid metastasis combined with thyroid carcinoma, a case report. BMC Endocr Disord 23, 95 (2023). https://doi.org/10.1186/s12902-023-01332-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01332-3