Abstract
Background
Hypertension (HTN) and non-alcoholic fatty liver disease (NAFLD) frequently coexist and share pathophysiological symptoms. Based on the liver stiffness measurement and controlled attenuation parameter obtained by performing liver transient ultrasound elastography (TUE), we determined the relationship between HTN status and the rates of liver steatosis and fibrosis in this study.
Methods
To perform this cross-sectional study, data were obtained from the National Health and Nutrition Examination Survey for 2017-March 2020 Pre-pandemic cycle. The relationship between HTN and the rates of liver steatosis and fibrosis was analyzed by constructing a multivariate logistic regression model. The VCTE was performed using a FibroScan® system (model 502, V2 Touch), and CAP was measured at ≥ 274 dB/m for liver steatosis, and the LSM result (median, ≥ 8 kPa) confirmed fibrosis. We also conducted subgroup analyses based on the age, sex, ethnicity, and body mass index (BMI) of the patients.
Results
In total, 4,705 participants were recruited, including 2,287 participants with HTN and 2,418 without HTN. After adjusting possible confounders, HTN was positively related to the liver steatosis rate (OR = 1.4, 95% CI: 1.1–1.8). Such HTN-associated prevalence was higher among males (OR = 1.6, 95% CI: 1.1–2.2), non-Hispanic African American individuals (OR = 2.1, 95% CI: 1.1–3.7), and participants with BMI ≥ 25 < 30 kg/m2 (OR = 1.7, 95% CI: 1.1–2.5). Additionally, HTN was positively associated with the fibrosis rate (OR = 2.0, 95% CI: 1.3–3.0), especially among females (OR = 2.6, 95% CI: 1.3–5.2), among individuals who were 40–59 years old (OR = 2.1, 95% CI: 1.0–4.3), 60–80 years old (OR = 2.4, 95% CI:1.3–4.6), non-Hispanic Caucasian (OR = 2.9, 95% CI: 1.5–5.6), among those with BMI ≥ 25 < 30 kg/m2 (OR = 3.0, 95% CI: 1.1–8.2), and those with BMI ≥ 30 kg/m2 (OR = 2.1, 95% CI: 1.4–3.2).
Conclusion
The results of this study revealed that HTN status was associated with higher rates of liver steatosis and fibrosis, particularly in subjects with BMI ≥ 25 kg/m2. The ethnicity of the participants also had an impact on the relationship.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is a common chronic hepatopathy and a major global health concern [1, 2]. It occurs as a result of metabolic syndrome (MetS). NAFLD and hypertension (HTN) have reached epidemic proportions [3]. Some systemic diseases, inflammatory disorders, alcoholism, and infections have a negative impact on the liver and heart. NAFLD is a hepatic manifestation of metabolic disorders that affects the occurrence of cardiovascular diseases (CVDs) [4]. HTN is frequently associated with NAFLD, which affects approximately 40% of the population. NAFLD may increase the likelihood of developing CVDs [5].
Histologically, NAFLD encompasses a disease spectrum ranging from steatosis to mild inflammation (non-alcoholic fatty liver) [6]. NAFLD is divided into two types: non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). While NAFL is defined as the presence of ≥ 5% hepatic steatosis without evidence of hepatocyte injury, NASH is defined as hepatic steatosis with accompanying lobular inflammation and hepatocyte injury (e.g., hepatocyte ballooning), with or without fibrosis [7]. NAFLD is linked to metabolic disorders such as dyslipidemia, hypertension, and hyperglycemia. In addition to increased fat content, the accumulation of pancreatic ectopic dysfunctional adipose tissue, which is primarily associated with insulin resistance and beta cell dysfunction, plays an important role in this context [8, 9]. Insulin resistance is accompanied by compensatory persistent hyperinsulinemia, which is critical for establishing and maintaining an unfavorable metabolic milieu (e.g., increased free fatty acid and glucose levels), whereby the prevailing insulin resistance worsens and promotes the development of cardiometabolic disorder [10]. Insulin resistance is associated with dysregulated neurohumoral activation of the renin–angiotensin–aldosterone system, fibrinolytic dysfunction via increased plasminogen activator inhibitor-1 (PAI-1) levels, cardiac autonomic neuropathy, which may promote the development of systolic and diastolic dysfunction or cardiac arrhythmias, endothelial dysfunction, and subsequent hypertension [10,11,12,13].
NAFLD is typically diagnosed after liver steatosis is discovered through a liver biopsy, histological analysis, and imaging examinations in the absence of causes of abnormal transaminase values or secondary causes of liver fat accumulation as determined by a medical history or laboratory tests [14, 15]. As a non-invasive imaging method, vibration controlled transient elastography (VCTE) can be used to accurately diagnose liver steatosis and advanced hepatopathy in adults [16]. VCTE was included as a method for detecting liver steatosis and hepatic fibrosis in the most recent cycle of the National Health and Nutrition Examination Survey (NHANES) based on the liver stiffness measurement (LSM) and controlled attenuation parameter (CAP). Using the NHANES database, we examined the relationship between HTN and liver steatosis and fibrosis in adult participants, as measured by CAP and LSM.
Methods
Participants
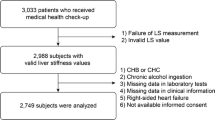
This cross-sectional study obtained data from the NHANES database (2017-March 2020 Pre-pandemic cycle). In the NHANES, health data on the US population were collected objectively. The data collection methodology is available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm) [17]. Of the 9,232 adults (≥ 20 years old) for whom information was available in the database mentioned above, unqualified adults were eliminated as follows, one individual for whom blood pressure values were unavailable; 1,310 for whom LSM or CAP information was unavailable; 3,025 individuals positive for hepatitis C antibody, hepatitis B surface antigen, or with a history of alcoholism (≥ 3 and ≥ 4 drinks/day for women and men, respectively) [18]; 59 individuals for whom information on body mass index (BMI) was unavailable; 132 individuals for whom information on IQR/Median was unavailable, or IQR/Median > 30%. Overall, data on 4,705 participants were included in the analysis. A flow chart describing the outline of our study is presented in Fig. 1.
The National Health and Nutrition Examination Survey serves as the foundation for our survey strategy. All participants provided written informed consent for data collection and information use. Following the guidelines of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), our study maintained transparency [19, 20].
Variables in the study
Hypertension status was investigated in this study and was defined based on the following criteria: first, the questionnaire item that stated “ever told you had high blood pressure” represented the self-reported status of HTN; second, mean diastolic pressure > 90 mmHg and mean systolic pressure > 140 mmHg were determined four times; third, the participants with HTN were identified based on their response to the questionnaire item “taking prescribed medication for hypertension”[21]. A FibroScan® system (model 502, V2 Touch) was used for performing VCTE, and CAP was measured at ≥ 274 dB/m for liver steatosis, which indicated steatosis on liver ultrasound [22]. The result of the LSM (median, ≥ 8 kPa) confirmed fibrosis [23], which was measured using the FibroScan® model 502 V2 Touch inVCTE that possessed an extra-large or moderate probe. Besides recording data on clinical and demographic factors, we extracted the data on several variables to be used as covariates, including age, sex, ethnicity, education level, BMI, family income-to-poverty ratio, smoked ≥ 100 cigarettes during the lifetime, and the levels of blood urea nitrogen (BUN), serum glucose, total cholesterol (TC), triglyceride (TG), serum uric acid (SUC), LDL cholesterol, aspartic acid transferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), and glycohemoglobin.
Statistical analysis
EmpowerStats (X&Y Solutions; Boston, MA) and R (version 3.4.3) were used for conducting statistical analyses, and P < 0.05 represented statistical significance. We constructed a multivariate logistic regression model to determine the association between liver steatosis and fibrosis and HTN status. Three statistical models were considered for data analysis, including model 1 with unadjusted covariates, model 2 with adjusted age, sex, and ethnicity, and model 3 with adjusted covariates shown in Table 1. We also conducted subgroup analyses based on age, sex, ethnicity, and BMI and used Full sample interview weight as the sampling weight for statistical analysis.
Results
As shown in Table 1, the participants were characterized by their HTN status. Of the 4,705 participants enrolled, 2,287 were placed in the HTN group, while the remaining 2,418 participants were placed in the non-HTN group. The HTN patients were older, had higher BMI, higher ALP, GGT, TG, glycohemoglobin, serum glucose, BUN, and uric acid levels, higher LSM and CAP values, and elevated liver steatosis and fibrosis rates than the non-HTN patients, but they had lower TC and LDL cholesterol levels than non-HTN patients.
Relationship between HTN status and CAP
After adjusting all confounders, our results showed that HTN status positively correlated with CAP (β = 9.1, 95% CI: 4.3–14.0; Table 2). The results of the subgroup analysis showed a positive relationship among women participants (β = 8.8, 95% CI: 1.7–16.0), among men participants (β = 8.5, 95% CI, 1.8–15.2) and also among participants who were 40–59 years old (β = 12.8, 95% CI: 5.4–20.2), non-Hispanic black (β = 17.0, 95% CI: 4.8–29.1), Non-Hispanic White (β = 9.6, 95% CI: 2.0–17.2), and those who had BMI < 25 kg/m2 (β = 13.6, 95% CI: 3.0–24.3) and BMI ≥ 25 kg/m2 (β = 11.6, 95% CI: 3.7–19.4).
Relationship between HTN status and the prevalence of liver steatosis
As determined by the model adjusted for all covariates (Table 3), HTN status showed a positive relationship with the liver steatosis rate (OR = 1.4, 95% CI: 1.1–1.8). In the subgroup analysis, a positive relationship was found among men (OR = 1.6, 95% CI: 1.1–2.2), and also among participants with BMI ≥ 25 < 30 kg/m2 (OR = 1.7, 95% CI: 1.1–2.5), and among those who were non-Hispanic Black (OR = 2.1, 95% CI: 1.1–3.7).
Relationship between the HTN status and LSM
After adjusting the model for all covariates, HTN status was positively associated with LSM (β = 0.5, 95% CI: 0.1–1.0; Table 4). In the subgroup analysis, a positive relationship was found among women (β = 0.4, 95% CI: 0.0–0.8) and also among participants who were 40–59 years old (β = 0.5, 95% CI: 0.0–1.0) and those with BMI ≥ 30 kg/m2 (β = 1.2, 95% CI: 0.3–2.0).
Relationship between HTN status and liver fibrosis
After adjusting the model for all covariates, HTN status showed a positive relationship with liver fibrosis (OR = 2.0, 95% CI: 1.3–3.0) (Table 5). In subgroup analysis, a positive relationship was recorded among women (OR = 2.6, 95% CI: 1.3–5.2) and also among participants who were 40–59 years old (OR = 2.1, 95% CI: 1.0–4.3), 60–80 years old (OR = 2.4, 95% CI: 1.3–4.6), non-Hispanic White (OR = 2.9, 95% CI: 1.5–5.6), and those who had BMI ≥ 30 kg/m2 (OR = 2.1, 95% CI: 1.4–3.2) and BMI ≥ 25 < 30 kg/m2 (OR = 3.0, 95% CI: 1.1–8.2).
Discussion
This study looked at the link between HTN status and the prevalence of liver steatosis and fibrosis in adults. Our findings revealed that HTN was linked to an increased risk of liver steatosis, which was more prevalent in men, non-Hispanic Black participants, and those with BMI ≥ 25 < 30 kg/m2. HTN status also showed a positive relationship with the prevalence of fibrosis, and it was more prominent among women, non-Hispanic White participants, and participants who were older and those with BMI ≥ 25 kg/m2.
Several epidemiological studies have discovered a bidirectional and mutual relationship between HTN and NAFLD, which means that the risk of developing NAFLD increases when people have HTN, and the risk of developing HTN increases when people have NAFLD [24, 25]. Ciardullo et al. conducted a meta-analysis of 11 longitudinal studies. They discovered that NAFLD cases had a 66% higher risk of developing HTN (HR: 1.66, CI: 1.38–2.01), though its prevalence varied with the patients’ age and BMI [26]. Ciardullo et al. found that NAFLD prevalence increased progressively from optimal (16.5%) to normal (34.5%), high normal (39.9%), and elevated blood pressure in another cross-sectional study of 11 489 adults from the 2005 to 2016 National Health and Nutrition Examination Survey (50.2%, P < 0.001). Hypertensive patients also had a higher prevalence of advanced fibrosis (3-9%, based on the specific biomarker used) [27]. Ciardullo et al. also used the National Health and Nutrition Examination Survey data from the 2017–2018 cycle for cross-sectional analysis, and the findings show that blood pressure status was associated with a progressively higher risk of steatosis. In contrast, obesity and diabetes were consistently associated with both steatosis and fibrosis. At the same time, their findings show that there is no significant link between blood pressure and liver fibrosis [28]. This differs slightly from our conclusion, which could be due to differences in inclusion, and exclusion criteria, as well as statistical methods. When compared to NAFLD cases without hypertension, NAFLD cases with HTN have a higher risk of progression [29]. HTN was linked to cardiovascular and all-cause mortality in NAFLD patients in another study (NHANES III) [5].
Non-alcoholic fatty liver disease (NAFLD) is linked to metabolic comorbidities such as obesity [30], type 2 diabetes mellitus (T2DM) [31], or dyslipidemia [32], and thus may be a hepatic manifestation of a metabolic disorder. NAFLD can causeclinical or subclinical CVDs in addition to hepatic morbidity and mortality. Patients with NAFLD have an increased risk of HTN, cardiac arrhythmias, cardiomyopathy, and coronary heart disease (CHD), as well as increased cardiovascular morbidity and mortality in the clinic. Patients with advanced NAFLD, such as those with non-alcoholic steatohepatitis (NASH) and advanced fibrosis, are at the highest risk of developing CVDs [7].
A liver biopsy is the most accurate method of diagnosing and staging the severity of NASH. However, it is costly and invasive and may result in complications and interobserver variability among various pathological characteristics. Several non-invasive methods for diagnosing NASH and staging liver fibrosis have been proposed, including TE, which can be used to estimate liver stiffness as a surrogate for liver fibrosis [33, 34]. An NHANES study found that HTN is independently related to NAFLD fibrosis; however, race-dependent differences exist [35]. Our findings also revealed that HTN status was significantly related to CAP or LSM among individuals of a specific ethnicity but not to CAP or LSM in the Mexican-American population.
Non-alcoholic fatty liver disease (NALFD) might develop into cirrhosis, which might include complications such as malignant tumors and is associated with CVDs or metabolic diseases [36, 37]. Genetic factors with susceptibility to NAFLD have an important effect on inflammation and lipid metabolism, thus affecting hypertension status [38,39,40]. Metabolic dysfunction is strongly related to the complicated mechanism involving the development of NAFLD; therefore, NAFLD might be called metabolic dysfunction-associated fatty liver disease (MAFLD). In this condition, metabolic dysfunction includes obesity, T2DM, hypertension, metabolic syndrome, and dyslipidemia [40,41,42]. NAFLD is an underdiagnosed metabolic disorder that is linked to a high prevalence of prehypertension and hypertension [43]. HTN and NAFLD share risk factors and have synergistic effects on the development and complications of the disorders. Therefore, routine screening for HTN in NAFLD cases and people undergoing lifestyle changes, such as physical activity and dietary changes, is required to prevent and manage HTN and NAFLD [44].
Our research had some limitations. First, because this was a cross-sectional study, causal relationships could not be established. Second, the participants’ blood pressures were measured at a single point in time, which may not accurately reflect blood pressure variation. Thus, hypertension was defined using a variety of criteria. Third, the CAP value used to define liver steatosis in various studies based on the NAHENS 2017–2018 database was inconsistent with the LSM value used to define obvious [23, 45, 46]. Therefore, the sensitivity and specificity of the VCTE test varied depending on the cut-off value. Fourth, different measurements were obtained due to the different FibroScan probes [47, 48]. Elastography, on the other hand, was performed by qualified and trained technicians following specific protocols [49]. Finally, self-reported confounders may have caused individual bias, which can be reduced by using NHANES data extracted by trained personnel using appropriate procedures.
Conclusion
Overall, HTN was associated with higher rates of liver steatosis and fibrosis, which was stronger in subjects with BMI ≥ 25 kg/m2 and was influenced by the participants’ ethnicity. Our findings suggested that screening for HTN in NAFLD patients could aid in preventing and managing both HTN and NAFLD.
Continuous variables were presented as the mean ± SD, and P-values were determined by performing the Kruskal-Wallis H test (skewed distribution) and one-way ANOVA (normal distribution). Categorical variables were presented as a percentage, and the P-values were determined by performing a Chi-squared test.
Data Availability
All data utilized or analyzed in this work can be obtained from NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- HTN:
-
Hypertension
- TE:
-
Transient elastography
- NHANES:
-
National Health and Nutrition Examination Survey
- CAP:
-
Controlled attenuation parameter
- LSM:
-
Liver stiffness measurement
- HbA1c:
-
Glycohemoglobin
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- GGT:
-
Gamma-glutamyl transpeptidase
- ALT:
-
Alanine amino transferase
- ALP:
-
Alkaline phosphatase.
References
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018 Jan;15(1):11–20.
Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16.
Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018 Feb;68(2):335–52.
Park J, Kim G, Kim H, Lee J, Lee Y-B, Jin S-M, et al. The association of hepatic steatosis and fibrosis with heart failure and mortality. Cardiovasc Diabetol. 2021 Sep;20(1):197.
Ng CH, Wong ZY, Chew NWS, Chan KE, Xiao J, Sayed N, et al. Hypertension is prevalent in non-alcoholic fatty liver disease and increases all-cause and cardiovascular mortality. Front Cardiovasc Med. 2022;9:942753.
Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. Lancet Lond Engl. 2021 Jun;397(10290):2212–24.
Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol Off J Ger Card Soc. 2021 Jul;110(7):921–37.
Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014 Sep;371(12):1131–41.
Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance.Physiol Rev. 2018Oct;98(4):2133–223.
Lechner K, McKenzie AL, Kränkel N, Von Schacky C, Worm N, Nixdorff U, et al. High-risk atherosclerosis and metabolic phenotype: the roles of ectopic adiposity, atherogenic Dyslipidemia, and inflammation. Metab Syndr Relat Disord. 2020 May;18(4):176–85.
Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: Cardiovascular Disease in Diabetes Mellitus: atherosclerotic Cardiovascular Disease and Heart failure in type 2 diabetes Mellitus - Mechanisms, Management, and clinical considerations. Circulation. 2016 Jun;133(24):2459–502.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014 May;10(5):293–302.
Engin A. Endothelial dysfunction in obesity. Adv Exp Med Biol. 2017;960:345–79.
Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr. 2019 Apr;207:64–70.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of Liver Diseases. Hepatol Baltim Md. 2018 Jan;67(1):328–57.
Ramírez-Vélez R, García-Hermoso A, Correa-Rodríguez M, Izquierdo M. Defining values for controlled attenuation parameter and liver stiffness in youth without liver disease.Pediatr Res. 2022Mar;91(4):912–20.
Cl J, R P-R, Cl O, Md C, D K-M, Sm D et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013 Sep [cited 2022 Oct 11].;(161). Available from: https://pubmed.ncbi.nlm.nih.gov/25090154/
Rattan P, Penrice DD, Ahn JC, Ferrer A, Patnaik M, Shah VH, et al. Inverse Association of Telomere length with Liver Disease and Mortality in the US Population. Hepatol Commun. 2022 Feb;6(2):399–410.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet Lond Engl. 2007 Oct;370(9596):1453–7.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of Observational Studies in Epidemiology using mendelian randomization: the STROBE-MR Statement. JAMA. 2021 Oct;326(16):1614–21.
Zhang Y, He Q, Zhang W, Xiong Y, Shen S, Yang J, et al. Non-linear Associations between visceral Adiposity Index and Cardiovascular and Cerebrovascular Diseases: results from the NHANES (1999–2018). Front Cardiovasc Med. 2022;9:908020.
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan Controlled Attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019 May;156(6):1717–30.
Liu X, Shen H, Chen M, Shao J. Clinical relevance of vitamins and carotenoids with liver steatosis and fibrosis detected by transient elastography in adults. Front Nutr. 2021;8:760985.
Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH et al. An Observational Data Meta-analysis on the Differences in Prevalence and Risk Factors Between MAFLD vs NAFLD.Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2021 Dec;S1542-3565(21)01276-3.
Targher G, Corey KE, Byrne CD. NAFLD, and cardiovascular and cardiac diseases: factors influencing risk, prediction and treatment. Diabetes Metab. 2021 Mar;47(2):101215.
Ciardullo S, Grassi G, Mancia G, Perseghin G. Nonalcoholic fatty liver disease and risk of incident hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2022 Apr;34(4):365–71.
Ciardullo S, Monti T, Sala I, Grassi G, Mancia G, Perseghin G. Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in US Adults Across Blood Pressure Categories. Hypertens Dallas Tex 1979. 2020 Aug;76(2):562–8.
Ciardullo S, Monti T, Grassi G, Mancia G, Perseghin G. Blood pressure, glycemic status and advanced liver fibrosis assessed by transient elastography in the general United States population. J Hypertens. 2021 Aug;39(8):1621–7.
Paik JM, Deshpande R, Golabi P, Younossi I, Henry L, Younossi ZM. The impact of modifiable risk factors on the long-term outcomes of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020 Jan;51(2):291–304.
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012 Feb;482(7384):179–85.
Chen J, Hu P, Wang Y, Zhu Z. Association between type 2 diabetes status and prevalence of liver steatosis and fibrosis among adults aged ≥ 40 years. BMC Endocr Disord. 2022 May;22(1):128.
Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016 Aug;65(8):1109–23.
Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatol Baltim Md. 2018 Jul;68(1):349–60.
Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014 Jul;5(3):211–8.
Zhang X, Heredia NI, Balakrishnan M, Thrift AP. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: results from NHANES 2017–2018. PLoS ONE. 2021;16(6):e0252164.
Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613.
Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. 2018 Jul;3(7):509–17.
Abenavoli L, Pellicano R, Boccuto L. Role of genetics and metabolism in non-alcoholic fatty liver disease. Panminerva Med. 2018 Jun;60(2):41–3.
Boccuto L, Abenavoli L. Genetic and Epigenetic Profile of patients with alcoholic liver disease. Ann Hepatol. 2017 Aug;16(4):490–500.
Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019 Mar;92:98–107.
Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of insulin resistance in MAFLD. Int J Mol Sci. 2021 Apr;22(8):4156.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020 Jul;73(1):202–9.
Zhao Y-C, Zhao G-J, Chen Z, She Z-G, Cai J, Li H. Nonalcoholic Fatty Liver Disease: An Emerging Driver of Hypertension. Hypertens Dallas Tex 1979. 2020 Feb;75(2):275–84.
Abenavoli L, Boccuto L, Federico A, Dallio M, Loguercio C, Di Renzo L, et al. Diet and non-alcoholic fatty liver disease: the Mediterranean Way. Int J Environ Res Public Health. 2019 Aug;16(17):E3011.
Gangireddy VGR, Pilkerton C, Xiang J, Tinajero R, Ashcraft AM. Hepatic fibrosis and steatosis in metabolic syndrome. J Obes Metab Syndr. 2022 Mar;31(1):61–9.
Heredia NI, Zhang X, Balakrishnan M, Hwang JP, Thrift AP. Association of lifestyle behaviors with non-alcoholic fatty liver disease and advanced fibrosis detected by transient elastography among Hispanic/Latinos adults in the U.S. Ethn Health. 2022 Jan;1–14.
S O HT, K I YS, Y O MM, et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol. 2020 Apr;55(4). https://doi.org/10.1007/s00535-019-01635-0.
Chan W-K, Nik Mustapha NR, Wong GL-H, Wong VW-S, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an asian population. United Eur Gastroenterol J. 2017 Feb;5(1):76–85.
2018_Liver_Ultrasound_Elastography_Procedures_Manual.pdf. [cited 2022 Oct 11]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2018_Liver_Ultrasound_Elastography_Procedures_Manual.pdf
Acknowledgements
Our thanks should go to all subjects from the NHANES project for their efforts and time in data collection.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HJF, HY, YSZ, and JHC were responsible for collecting, analyzing data and writing manuscript. ZCL was in charge of designing this study and editing manuscript. All author(s) read and approved our eventual version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NHANES protocols gained approval from Ethics Committee of National Center for Health Statistics. Each participant provided informed consent before participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, H., Yu, H., Zhao, Y. et al. Association between hypertension and the prevalence of liver steatosis and fibrosis. BMC Endocr Disord 23, 85 (2023). https://doi.org/10.1186/s12902-023-01318-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01318-1