Abstract
Background
Pancreatic β-cell dysfunction resulting from inflammation has been recognized to contribute to type 2 diabetes mellitus (T2DM). Netrin-1 is a new indicator of subclinical inflammation and it has a role in β-cell apoptosis. This study evaluated the level of netrin-1 in newly diagnosed T2DM patients and explored whether netrin-1 is a reliable marker or a key factor in the development of T2DM.
Methods
Netrin-1 level was determined using a commercially available human enzyme-linked immune sorbent assay (ELISA) kit. The homeostasis model assessment of insulin resistance (HOMA-IR) was used as an index to measure insulin resistance. The sample consisted of 30 patients with newly diagnosed T2DM who had a glycosylated hemoglobin (HbA1c) level ranging from 7.5 % (58 mmol/mol) to 10.5 % (91 mmol/mol). The control group consisted of 26 healthy individuals matched for age and body mass index.
Results
The netrin-1 level of T2DM patients was significantly lower than that of healthy controls (p < 0.01). Logistic regression analysis showed that the level of netrin-1 was negatively correlated with HOMA-IR, fasting blood glucose, postprandial blood glucose, fasting insulin and HbA1c.
Conclusions
Plasma netrin-1 levels were decreased in patients with newly diagnosed T2DM, and the levels of netrin-1 were negatively associated with IR and glucose homeostasis. Future studies on the precise mechanism will offer new insights into the prevention and treatment of T2DM.
Similar content being viewed by others
Background
Insulin resistance (IR) and pancreatic β-cell dysfunction lead to type 2 diabetes mellitus (T2DM) [1–3]. IR leads to the inability of insulin to act normally in regulating nutrient metabolism in peripheral tissues. In recent years, increasing evidence from human population studies and animal research has pointed to a correlative and causative relationship between inflammation and IR/T2DM [4–6]. IR might promote inflammation by impairing the anti-inflammatory effect of insulin. Meanwhile, cytokines in turn enhance IR in adipose and other tissues, increasing the risk of T2DM [7]. Netrin-1, a neuroimmune guidance cue, plays a major role in the development of embryonic pancreas [8–10]. It associates with integral proteins α6β4 and α3β1 and inhibits pancreatic epithelial cell adhesion and migration [11], which differs from its function in neuronal cell migration. In addition to inhibition of migration, netrin-1 also suppresses inflammatory cytokine and chemokine production [9]. Recently, some studies have suggested that netrin-1 suppresses infiltration and inflammation in sepsis, acute kidney injury, acute lung injury and peritoneal inflammation [12, 13]. Other studies indicate that netrin-1 might regulate cyclooxygenase-2 (COX-2) expression through inhibition of nuclear factor kappa B activation [14] and promote macrophage differentiation to the M2-like phenotype [15].
Therefore, netrin-1 may be involved in the inflammatory mechanism of IR. In this article, we measured plasma netrin-1 levels in newly diagnosed patients with T2DM.
Study population and design
Conducted in the Department of Endocrinology of Nanjing First Hospital, Nanjing Medical University, China from January to June 2013, our study included 30 patients newly diagnosed with T2DM. All of the patients had been diagnosed with T2DM within 6 months and had not received previous anti-hyperglycemic therapy. Diagnosis of T2DM was based on World Health Organization diagnostic criteria from 1999 [16]. Exclusions included: (1) patients prescribed any oral hypoglycemic agents or insulin; (2) impaired glucose tolerance or impaired fasting glucose; (3) a history of acute diabetic complications (such as diabetic ketoacidosis, hyperglycemic hyperosmolar status and diabetic lactic acidosis); (4) severe uncontrolled hypertension (systolic blood pressure ≥180 mmHg and/or diastolic blood pressure ≥110 mmHg); (5) any acute inflammation or infection; (6) current or a history of significant co-morbid diseases, such as cardiovascular (including myocardial infarction, cardiac surgery or revascularization, angina and congestive heart failure), hepatic and renal conditions; and (7) positivity for islet cell autoantibodies (such as glutamic acid decarboxylase autoantibody, islet cell autoantibody or insulinoma-like antigen 2) that indicate the possibility of type 1 diabetes mellitus. The control group comprised 26 aged-matched healthy subjects (all subjects with normal glucose tolerance at the baseline examination). All participants were surveyed on the following: age, sex, height, weight, smoking, drugs used, hyperlipidemia, and dietary compliance.
The study was approved by the appropriate independent ethics committees and regulatory authorities, and was conducted in accordance with the Declaration of Helsinki [17] and Good Clinical Practice guidelines [18]. All subjects provided informed consent prior to being enrolled and after the purpose and procedures of the study were fully explained. Written informed consent was obtained from all patients.
Methods
Baseline clinical and demographic data for all of the study participants were collected from the medical records. Body mass index (BMI) was calculated as weight (kg)/square of height. Venous blood samples were obtained from all of the participants after 8-h fasting and treated separately. Postprandial blood glucose (PBG) and postprandial insulin concentrations (PINS) were assessed 2 h after a test meal. Hematology and biochemistry were determined by routine techniques using an automated analyzer. Blood was collected in 4 mL EDTA containers and was centrifuged within 30 min at 10000 rpm for 10 min. Serum samples were subsequently stored in aliquots without preservatives at −80 °C for an average of 3 months until immediately before analysis of netrin-1. Netrin-1 level was determined using a commercially available human enzyme-linked immune sorbent assay (ELISA) kit, purchased from Wuhan Huamei Biological Engineering Co., Ltd.
Plasma glucose levels, sodium concentrations, insulin levels, blood lipid profiles and glycosylated hemoglobin (HbA1c) levels were assayed by routine methods. The homeostasis model of IR (HOMA-IR) was used as a measure of IR. HOMA-IR was calculated using the following formula: fasting plasma glucose (mmol/L) multiplied by fasting serum insulin (mIU/L) divided by 22.5.
Definitions
Diabetes was diagnosed based on the World Health Organization consulting criteria [16] (i.e., fasting plasma glucose [FPG] of ≥7.0 mmol/L [126 mg/dL] and/or a 2-h post glucose value of ≥11.1 mmol/L [200 mg/dL]).
Statistical analysis
Data were analyzed with the SPSS 20.0 program. For continuous variables with normal distributions, data were expressed as mean ± standard deviation and non-normally distributed variables (FBG, PBG, HbA1c, low-density lipid [LDL] cholesterol) were expressed as median (interquartile range). The Kolmogorov–Smirnov test was used to evaluate the distribution of variables. Student’s t-test (independent-sample t-test) was used for continuous variables with normal distribution. Non-normally distributed variables have been log-transformed. Pearson’s correlation analyses were used to assess the relationships. Logistic regression analysis was used to assess the associations between netrin-1 level and the other parameters evaluated. A value of p < 0.05 was accepted as the level of significance (two-tailed).
Results
The groups were similar in terms of age, sex, smoking history, drugs used and BMI. The T2DM group showed significantly higher FBG, PBG, FINS and HbA1c values than the control group. No significant differences in triglyceride (TG), total cholesterol (TC), high-density lipid (HDL) cholesterol, and LDL cholesterol levels were detected between the two groups. The demographic and laboratory data of the groups is outlined in Table 1.
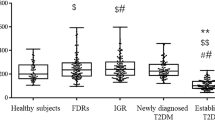
The netrin-1 levels of the T2DM group were significantly lower than those of the control group (Table 1, Fig. 1). Netrin-1 levels showed significant negative correlation with HOMA-IR (r = −0.413, p < 0.001, Fig. 2).
A logistic regression analysis was also carried out using the enter method to evaluate the impact of netrin-1 on modulation of glucose homeostasis. The measurement of TG (r = −0.248, p = 0.032), FBG (r = −0.408, p = 0.001), PBG (r = −0.299, p = 0. 013), HbA1c (r = −0.346, p = 0.005), FINS (r = −0.293, p = 0.014) and PINS (r = 0.357, p = 0.003) were dependent parameters, whereas age, sex, BMI, TC, HDL -C and LDL -C were independent parameters. The results showed that netrin-1 was independently related to IR and glucose homeostasis. Data are shown in Table 2.
Discussion
The present study showed that the level of netrin-1 in patients with T2DM was significantly lower than that of the control group (p < 0.001). In addition, the level of netrin-1 was negatively related with FBG, PBG, HbA1c, FINS, TG and HOMA-IR.
Netrin-1 level was reduced and negatively correlated with blood glucose levels, IR and TG in T2DM patients. There may be three possible explanations for this finding. First, netrin-1 has a role in inflammation, which is implicated in the pathogenic mechanism of T2DM. Natura r [14] showed that netrin-1 could regulate inflammation, which might negatively regulate insulin secretion and contribute to β-cell dysfunction. Ranganathan et al. [19] also reported that netrin-1 regulates COX-2 expression at the transcriptional level. At the same time, overexpression of netrin-1 promotes macrophage differentiation to the “alternative” or M2-like phenotype and promotes islet remodeling [15]. Second, netrin-1 is also associated with islet dysfunction in diabetes and negatively correlated with hyperglycemia. It is understandable that repeated and prolonged exposure to hyperglycemia leads to β-cell degradation, reduces glucose-stimulated insulin secretion and eventually causes β-cell apoptosis [20]. De Breuck et al. [15] had suggested that netrin-1 is expressed and secreted in the pancreas where it plays a major role in pancreatic morphogenesis in the regenerating pancreas. In patients with newly diagnosed T2DM, secretion of netrin-1 in the injured and apoptotic β cells is significantly reduced, in turn promoting β-cell function failure. Third, IR disrupts the circulation of netrin-1. Ramkhelawon et al. [21] have suggested that netrin-1 is only selectively modestly upregulated in the visceral white adipose tissue, and that significant reductions in circulating levels of netrin-1 occur in obese individuals compared with lean individuals. They also studied obese rats and showed that netrin-1 is a macrophage retention signal in adipose tissue during obesity, which possibly promotes the chronic inflammation and insulin resistance that subsequently occurs in T2DM.
Data from this study suggest that netrin-1 is negatively correlated with blood glucose levels and IR. In addition, De Breuck et al. [15] also suggested that netrin-1 increases pancreatic islet cell mass and density in T2DM, which showed that netrin-1 possibly has a protective role in β-cell to delay the progression of this disease. If supplied with exogenous netrin-1 to patients could increase insulin sensitivity and improve insulin resistance. In the event of this regard, netrin-1 may be useful in treating patients with T2DM or without diabetes but with insulin resistance.
Our study has some limitations that should be considered in the interpretation of these results. First, the sample size is relatively modest. Second, we detected netrin-1 levels only in humans but not in animals. The receptors of netrin-1 have been widely shown to affect the inflammatory response, and changes in the expression levels of netrin-1 receptors could have confounded the results. Lastly, HOMA-IR is difficult to elucidate, causal relationships cannot be identified in cross-sectional studies, and there are no comparative patient groups, such as pre-diabetes. Despite these limitations, the safety and feasibility results of this study support further investigations to evaluate the influence of netrin-1 in the pathogenesis of T2DM.
Conclusions
This study provides new evidence that netrin-1 participates in the development of T2DM and facilitates future studies to focus on the precise mechanism. Further study of netrin-1 could offer new insights into the prevention and treatment of T2DM.
Abbreviations
FBG, fasting blood glucose; PBG, postprandial blood glucose; HbA1c, glycosylated hemoglobin; TG, triglyceride; TC, total cholesterol; HDL-C cholesterol, high-density lipid cholesterol; LDL-C cholesterol, low-density lipid cholesterol; FINS, fasting insulin; PINS, postprandial insulin; HOMA-IR, homeostasis model assessment of insulin resistance; BMI, body mass index; IR, insulin resistance; T2DM, type 2 diabetes mellitus; COX-2, cyclooxygenase-2
References
Farmer A, Fox R. Diagnosis, classification, and treatment of diabetes. BMJ. 2011;342:d3319.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, American Diabetes A. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203.
Heine RJ, Diamant M, Mbanya JC, Nathan DM. Management of hyperglycaemia in type 2 diabetes: the end of recurrent failure? BMJ. 2006;333(7580):1200–4.
Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, Plebani M, Fioretto P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. Am Soc Nephrol: JASN. 2005;16 Suppl 1:S78–82.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30.
King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–34.
Jayakumar C, Mohamed R, Ranganathan PV, Ramesh G. Intracellular kinases mediate increased translation and secretion of netrin-1 from renal tubular epithelial cells. PLoS One. 2011;6(10):e26776.
Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185(6):3750–8.
Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–69.
Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5(5):695–707.
DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–94.
Kruszynska YT, Olefsky JM. Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J Invest Med. 1996;44(8):413–28.
Natura G, Bar KJ, Eitner A, Boettger MK, Richter F, Hensellek S, Ebersberger A, Leuchtweis J, Maruyama T, Hofmann GO et al. Neuronal prostaglandin E2 receptor subtype EP3 mediates antinociception during inflammation. Proc Natl Acad Sci U S A. 2013;110(33):13648–53.
De Breuck S, Lardon J, Rooman I, Bouwens L. Netrin-1 expression in fetal and regenerating rat pancreas and its effect on the migration of human pancreatic duct and porcine islet precursor cells. Diabetologia. 2003;46(7):926–33.
Colman PG, Thomas DW, Zimmet PZ, Welborn TA, Garcia-Webb P, Moore MP. New classification and criteria for diagnosis of diabetes mellitus. The Australasian Working Party on Diagnostic Criteria for Diabetes Mellitus. N Z Med J. 1999;112(1086):139–41.
Cosentino M, Picozzi M. The declaration of Helsinki and post-study access to effective drug treatments for subjects participating in clinical trials. Bioethics. 2012;26(7):393–4.
Sinclair AJ, Task, Finish Group of Diabetes UK. Good clinical practice guidelines for care home residents with diabetes: an executive summary. Diabetic Med. 2011;28(7):772–7.
Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am J Physiol Renal Physiol. 2013;304(7):F948–957.
Kim JW, Yoon KH. Glucolipotoxicity in Pancreatic beta-Cells. Diabetes Metab J. 2011;35(5):444–50.
Ramkhelawon B, Hennessy EJ, Menager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20(4):377–84.
Acknowledgments
We would like to thank the members of the Central Laboratory of Nanjing First Hospital Affiliated to Nanjing Medical University and the Department of Endocrinology for sharing ideas.
Funding
This research was supported by grants from National Natural Science Foundation of China (81200594), Jiangsu Planned Projects of Postdoctoral Research Funds, the Peak of Six Personnel in Jiangsu and Nanjing Medical Science and Technique Development Foundation.
Availability of data and materials
Our data will not be shared due to it was involved in individual privacy and needed further study.
Authors’ contributions
CL, with the most contribution to the study, was responsible for the study design, data collection and manuscript writing. XK, YW, XF and Qi Li helped with the acquisition and interpretation of data and with manuscript revisions. YZ and JZ analyzed the data. Qian Li guaranteed this work, provided academic guidance, and took responsibility for the accuracy of the data analysis. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the appropriate independent ethics committees and regulatory authorities, and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, C., Ke, X., Wang, Y. et al. The level of netrin-1 is decreased in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord 16, 33 (2016). https://doi.org/10.1186/s12902-016-0112-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-016-0112-z