Abstract
Background
The forgotten ureteral stents (FUS) is one of the late complications of stent placement. This systematic review summarized different aspects of FUS and focused on the problems and solutions related to FUS.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. PubMed® and Embase® were searched from inception until October 1st, 2022. Eligible studies were those defining FUS as a stent unintentionally left in situ longer than at least 2 months.
Results
Total 147 studies with 1292 patients were finally included. The mean indwelling time of FUS was 33.5 months (range from 3 months to 32 years). The most common initial cause for stent placement was adjunct treatment to urolithiasis (79.2%). The major forgetting reasons were patient-related (83.9%), which included poor compliance, lapse in memory, and misconceptions about the necessity of timely removal. Primary presenting complaints were flank pain (37.3%), lower urinary tract symptoms (33.3%), and hematuria (22.8%). Encrustation (80.8%) and urinary tract infections (40.2%) were the most common complications detected in patients with FUS. Computed tomography evolving as a preferred imaging test (76.1%) was indispensable for evaluating encrustation, migration, fracture and other complicated situations in patients with FUS. Besides, evaluation of kidney function and infection status was also of great importance. Multiple and multimodal procedures (59.0%) were often necessitated to achieve the stent-free status, and were mostly endoscopic procedures. Cystoscope was most commonly used (64.8%). Retrograde ureteroscopy (43.4%) and antegrade stent removal (31.6%) were often used when dealing with more complicated situations. Extracorporeal shockwave lithotripsy (30.4%) was often used as adjunctive to other endoscopic procedures, but it sometimes failed. The decision regarding the choice of treatment is based on the volume and site of encrustation, the direction of migration, the site of fracture, kidney function and other urinary comorbidities.
Conclusions
FUS not only pose hazard to patients’ health, but also impose a huge economic burden on medical care. Thorough preoperative evaluation is fundamental to developing the treatment strategy. The management of FUS should be individualized using different treatment modalities with their advantages to minimize patients’ morbidities. Prevention is better than cure. Strengthening health education and setting a tracking program are of great importance to the prevention of FUS.
Similar content being viewed by others
Background
Ureteral stents, especially double-J stents are currently one of the most widely used surgical tools in the field of urology. The first ureteral stent for long-term retention was used by Zimskind and associates in 1967 [1]. The pigtail shape design described by Finney in 1978 [2] and a variety of innovations and developments [3] laid the foundation for modern stents. Stent insertion is the most efficient way to relieve ureteral obstruction, and it was indispensable to many surgical interventions to promote ureteral healing and to prevent complications. However, some stent-related problems may develop, such as irritative symptoms, urinary tract infections (UTI), encrustation, migration and fracture [4].
Technological advances in stent design, constitutive materials and surface coating allow patients to tolerate stents more easily, and this may cause a decrease in patient compliance for stent removal [5]. Studies have found that stents were forgotten in up to 0.9–12.0% of patients [6]. Ureteral stents as foreign bodies should be removed or replaced after they have served their purposes before the intended maximal stent life (MSL). The forgotten ureteral stent (FUS) with long-term use tends to migrate, encrust and fracture, and can lead to severe sepsis, renal failure and even a life- threatening situation [7, 8]. Therefore, the management of FUS presents a considerable surgical challenge for urologists and an increased morbidity to patients, usually requiring multimodal treatments to render stent-free. In addition to potential legal consequences [9], the cost of removing complicated stents was estimated to be 1.8- to 21-fold higher than a regular stent, and financial burden of FUS management increased in parallel with the duration of the stent retention [10].
This systematic review provides an overview of FUS and pools data regarding patients’ demographics, diagnosis, management and prevention to improve urologist understanding of FUS management.
Methods
Search strategy
A comprehensive search for eligible studies was conducted using PubMed® and Embase® from inception until October 1st, 2022. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement was followed in this review [11]. The search was restricted to English using search terms included ‘forgotten ureteral/ureteric stent’, ‘retained ureteral/ureteric stent’, ‘overlooked ureteral/ureteric stent’, ‘missed ureteral/ureteric stent’ or ‘neglected ureteral/ureteric stent’ in previous literatures.
Study selection
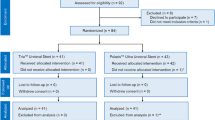
After deduplication of retrieval records, the abstracts were independently screened for eligibility by two authors (X.W. and Z.J.), followed by independent retrieval and scrutiny of full-text articles. Any discrepancies were resolved by discussion or by consulting a third author (P.Y.). Studies defining FUS as a stent unintentionally left in situ longer than at least 2 months were included. The PRISMA flow chart with details of exclusion criterion were shown in Fig. 1.
Data analysis
We reported FUS-related data, such as epidemiology, aetiology, diagnosis, management and prevention. The numerical data obtained from available studies were synthesized and calculated. The mean or median (with standard deviation or range, if available) was reported for continuous variables, while a constituent ratio was reported for each category of categorical variables. All statistical tests were performed by SPSS version 24.0 (IBM, Armonk, NY, USA).
Results
Description of the included studies
Four hundred and fifteen records were initially retrieved from electronic databases. After deduplication, 363 abstracts were screened and 171 full-text articles were reviewed. The exclusion criteria are listed in Fig. 1. Finally, 147 articles were included with 1292 patients. A narrative synthesis rather than a quantified meta-analysis of data was performed. All included 147 articles are shown in Supplementary Material.
Study characteristics
All 147 articles were published from 1985 to 2022 with a rising tendency in publication numbers by year. Seventy-eight (53.1%) articles were published in recent past 10 years. There are 109 case reports including 130 patients with 141 stents and 38 case series (> 3 cases) including 1162 patients with 1182 stents. The most commonly used terms for FUS are ‘forgotten’. The criteria for indwelling time of FUS has been defined in previous studies (case series) as a variable period more than ranging from 3 to 12 months. (Table 1)
Patient demographics, initial causes for placement and forgetting reasons
The mean age of included patients was 41.5 years with age range from 2 to 92 years. Pediatric and adolescent patients accounted for 7.3%. Male to female ratio was 1.85. The initial causes for stent placement in patients with FUS fall into 4 categories (Table 2): Stent placement as an adjunct to the stone treatment was the most common reason (79.2%) for stent placement.
The major forgetting reasons were patient-related (83.9%), which included poor compliance, lapse in memory, and misconceptions about the necessity of timely removal. The second most common reasons were physician-related (24.7%) and attributed to inadequate counseling. Besides the above, objective factors (4.4%) such as individual financial problem, necessity to treat other diseases, low education status and social instability also led to the delay for stent removal.
Clinical manifestations and diagnostic tests
The mean indwelling time was 33.5 months (range from 3 months to 32 years). A 59-year-old woman suffered from heavily encrusted bilateral stents for 32 years, which was the longest indwelling time in previous literatures [12]. The “32-year-old” stents were inserted for a prophylactic use in a hysterectomy. The distribution of the indwelling time of FUS in 130 case reports is detailed in Table 1. Bilateral FUS and FUS in solitary kidneys are both uncommon (2.4% and 2.5% respectively).
The most common primary presenting complaints of FUS were flank pain (37.3%), lower urinary tract symptoms (33.3%) and hematuria (22.8%), which are so-called stent-related symptoms. Encrustation (80.8%) and UTIs (40.2%) were the most common complications. More details are shown in Table 3.
KUB (kidney-ureter-bladder) radiography (96.8%), non-contrast computed tomography (NCCT) (76.1%) and KUB ultrasonography (45.2%) were commonly used for imaging evaluation. Elevated serum creatinine was detected in 24.8% of patients, and the rate increased to 62.5% in patients with bilateral FUS. Renal scintigraphy was preferred in 35.5% of cases to quantitatively estimate the split renal function of affected kidneys.
Management
Multiple sessions or modalities were necessitated to render stent-free status for most patients (59.0%). Simple cystoscopic stent removal (SCSR) with or without endoscopic cystolithotripsy (EnCL) was the most commonly used procedures (64.8%) for FUS removal and associated stones. Retrograde ureteroscopic stent removal with or without intracorporeal lithotripsy (43.4%) and antegrade stent removal with or without percutaneous nephrolithotomy (PCNL) (31.6%) were often used when SCSRs failed. Extracorporeal shockwave lithotripsy (ESWL) (30.4%) was often used followed by other endoscopic procedures. Pretreatment with percutaneous nephrostomy (3.6%) and antibiotics may be needed for complicated UTIs. Laparoscopy and open surgeries such as pyelolithotomy, ureterolithotomy, cystolithotomy, ureteral reimplantation, pyeloureteroplasty and even nephrectomy was performed to deal with more complicated situations (6.7%) which included a huge stone burden, a non-functioning kidney and comorbid stricture or malformation. Postoperative complications were uncommon, however, not a few complications were severe or even lethal. More details are shown in Table 4.
Discussion
Study characteristics
Technically, FUS is defined as a stent of which the indwelling time exceeds the MSL. In a broad sense, FUS is also defined as a stent unintentionally left in situ despite the physician’s recommendation. MSL was recommended for different products provided by various companies [13]. However, in previous literatures, the term “MSL” was not always applied and a strict definition for “forgotten” did not exist. We recommended to use the term “forgotten”, and report the MSL or the intended indwelling time recommended by the physician.
Forgetting reasons and prevention strategies
The best treatment for FUS is prevention. Meticulous patient education which increases patient’s insight into the importance of both stent insertion and its timely removal includes explaining possible complications of indwelling stents, highlighting the importance of drinking enough water and avoiding excessive exercise, minimizing patient-specific lithogenic factors, undergoing appropriate antimicrobial treatment and making an appointment with patients to ensure their timely follow-up.
Despite extensive counseling, up to 10% of patients with retained stents were lost to follow-up and failed to have their stents removed [14]. Therefore, patient education alone could not be solely relied upon, and it is necessitated to set a monitoring program for tracking the patients with long-term indwelling stents. Computerized monitorization programs, stent removal software and reminder short message or e-mail services have been recommended [9].
The exact interval for changing or removing an indwelling ureteral stent was controversial. An optimum interval is usually 2–6 months, but it should be sooner in patients with risk factors, such as stone history, pregnancy, recurrent encrustation and UTIs [15, 16]. Novel stent coatings and degradable stents have also been investigated as a strategy to prevent bacterial adherence, encrustation and subsequent FUS [17, 18].
Clinical manifestations and diagnostic tests
Encrustation was thought to be the result of ionic deposition on the biofilm, and it usually begins to agglomerate at both ends. Encrustation along the stent in mid-ureter is relatively uncommon and mild [16, 19]. Common risk factors for stent encrustation are long indwelling time, UTIs, chronic renal failure, recurrent or residual stones, lithogenic history, metabolic abnormalities, congenital renal anomalies and ureteral obstruction, of which indwelling time and history of urolithiasis were major contributing factors [17, 19]. The FECal (forgotten, encrusted, calcified) grading system described by Acosta-Miranda et al. [20] and the KUB grading system defined by Arenas et al. [21] are commonly used to evaluate encrusted stents, and are also useful tools to help urologists to make decisions in the management of FUS.
The migration and the spontaneous fracture (fragmentation) were uncommonly seen. Mild or moderate migration manifested as the proximal or distal end migrating into the ureter. Severe migration manifested as the stent totally migrating into the renal pelvis or bladder, and even the proximal or distal end protruding into the retroperitoneum or out of urethra [22, 23]. The reason for migration is primarily due to the short stent for the ureter [24]. An appropriate length of stents should be chosen, and full loops should be kept in both the pelvic and bladder, especially in children. Long indwelling time is the leading risk factor for broken stents. Fracture could also occur during the extraction of FUS; therefore, gentle traction should be used and the integrity of the stent should be examined and confirmed after stent removal [17]. Fracture was thought to be the result of loss of tensile strength, which was due to the hardening and degeneration of stent polymers [25]. The risk of both fracture and encrustation is dependent on the type of stent material. Silicone was found to be less prone to fracture and encrustation than polyurethane [18].
A thorough preoperative imaging evaluation is crucial to decide on the treatment strategy. KUB radiography was performed to preliminarily evaluate the degree and site of encrustation, the associated stone burden and the location information of migration or fracture. KUB ultrasonography was usually used as an ancillary examination, however, it should be the first choice for the pregnant. NCCT could help assess the exact stone burden and the extent of encrustation, which are underestimated by KUB radiography [16, 19, 26]. In addition, since bowel preparation is hard to achieve in children, intensive gas on KUB could mask visualization of the actual stone burden [27]. NCCT is also useful in evaluating comorbidities in urinary system and adjacent organs (such as colon, rectum, and uterus). Therefore, NCCT has become a preferred and indispensable modality in recent years to diagnose FUS, especially the one with severe encrustation and other complicated situations.
Kidney function was mainly focused, especially in patients with a solitary kidney or bilateral severely encrusted stents. It is demonstrated that patients with FUS are at increased risk for loss of renal function [28]. The estimated glomerular filtration rate at diagnosis of FUS was significantly lower than that at the time of stent insertion [29]. UTIs and urosepsis also cause great attention of physicians. Long-term indwelling stents offer an ideal surface for bacterial colonization and biofilm formation. The adherent bacteria which hydrolyze the urea to ammonia increase the urinary pH which leads to precipitation of minerals [30]. Bacteriuria is a strong contributing factor for stent encrustation and stone formation.
FUS was detected incidentally in 10.8% of patients, and more than a few patients carried stents for years and decades until symptoms occurred. It was described that FUS was more common in patients who tolerated stents well than in those who had discomfort [5].
Management
There are currently no formal guidelines but several treatment algorithms in the management of FUS. Single-session removal is often discouraged, and it is better to stage the procedures to avoid long intraoperative time and resultant complications [18]. With improvements in surgical position [34] and techniques [5], removing fractured or encrusted FUS in a single endourologic session could be achieved with reasonable operating time and acceptable morbidity. At an experienced center, combined endourological procedures can achieve safe and successful management even in the pediatric group [29]. A complex situation often involves the kidney, ureter and bladder, necessitating multimodal endoscopic procedures and even a more invasive surgery that may be performed either simultaneously, sequentially or separately. Each treatment modality has its advantages and disadvantages, and therefore a treatment strategy should be devised individually. The strategy is mainly based on the volume and site of encrustation, the direction of migration, the site of fracture, kidney function and other urinary comorbidities. It is recommended to deal with the distal ends first in order to facilitate subsequent procedures such as ESWL and PCNL [15], and also facilitate placing a ureteral access catheter or a parallel stent [7].
SCSR or semi-rigid ureteroscopes alone could be performed for distal ends with no or minimally encrustation and simply downward migrated FUS. Although severely or circular encrustation completely encasing the distal end could be management by SCSR + EnCL [31], the semi-rigid ureteroscope combined with lithotripsy devices has an advantage in dealing with ureteral part, broken stent pieces left in situ after retrograde traction, and upward migrated FUS in the ureter [14, 16].
It is demonstrated that ESWL cannot be successful alone, and may offer less help in cases with severe encrustation and a large stone burden. However, as a noninvasive treatment, ESWL may increase the potential success of subsequent endourological procedures [32]. Therefore, in cases with failure of retrograde removal, the initial adjunctive use of ESWL (1–3 sessions) on proximal ends may be efficacious, and ESWL is also useful in disintegrate the encrustation on the ureteral part [5, 16].
Proximal stone burden is described as a main determining factor in the management of FUS, and correlated with multiple sessions, multimodal procedures and complications [28, 33]. Antegrade stent removal (with PCNL) alone was performed when FUS was evaluated only having encrusted proximal coil, associate renal stones or upward migration. However, PCNL is usually combined with other retrograde procedures, or performed when ESWL failed [19]. Flexible ureteroscopy is used in some selected cases having uncoiled proximal ends with encrustation, and it is also useful to manage upper ureteral and renal stones that are not accessible by PCNL [34]. Sometimes, a ureteral access sheath or even a guidewire cannot be placed beside FUS, and thus a parallel stent for pre-stenting or an additional lithotripsy with semi-rigid ureteroscopes will be needed [15].
Some new techniques have been described to remove FUS in selective cases. Yeh et al. introduced a method using a silk loop to assist ureteroscopic lithotripsy and stent removal [35]. Mistry et al. managed mildly to moderately (< 10 mm) encrustation with insertion of a second stent next to the original stent in order to use frictional forces between the two stents causing disruption of encrustation, and then both stents were removed after 2 to 4 weeks [28].
Conclusion
The widespread use of ureteral stents mandates updated knowledge about the management and prevention of FUS. Although FUS is uncommon, it is likely to cause troublesome and severe complications. The indications for stent insertion, especially for long-term placement, should be carefully considered in each patient. Thorough preoperative evaluation for FUS-related complications, especially the extent of encrustation, kidney function and UTIs is fundamental to developing the treatment strategy. The management of FUS should be individualized using different treatment modalities with their advantages to minimize patients’ morbidities. Patient education on timely removal of stents must be provided throughout the perioperative period. Registry and monitoring systems should be maintained for easy tracking of stents, especially in patients with poor compliance. Since the pooled data of FUS trend to be underestimated, it must be realized that it still has have a long way to go to improve the whole-process management of the ureteral stent and to strengthen the prevention of FUS.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. All data were shown in our supplementary material.
References
Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of Long-Term Indwelling Silicone Rubber Ureteral splints Inserted Cystoscopically. J Urol. 1967;97(5):840–4.
Finney RP. Experience with new double J ureteral catheter stent. 1978. J Urol. 2002;167(2 Pt 2):1135–8.
Mosayyebi A, Manes C, Carugo D, et al. Advances in Ureteral Stent Design and materials. Curr Urol Rep. 2018;19(5):35.
Sali GM, Joshi HB. Ureteric stents: overview of current clinical applications and economic implications. Int J Urol. 2020;27(1):7–15.
Ulker V, Celik O, Endoscopic. Single-Session Management of Encrusted, Forgotten Ureteral stents. Med (Kaunas), 2019,55(3).
Distler FA, Veelken R, Wagner A, et al. A Forgotten Ureteral Stent: potential risks for the urinary function. Urol Int. 2022;106(2):209–12.
Aron M, Ansari MS, Singh I, et al. Forgotten ureteral stents causing renal failure: multimodal endourologic treatment. J Endourol. 2006;20(6):423–8.
Puhse G, Piechota H, Scheffold C, et al. Multiorgan failure 17 years after initial stone therapy: forgotten ureteral stent in a horseshoe kidney. Eur Urol. 2007;52(6):1784–7.
Javier-DesLoges JF, Johnson KK, Kenney PA, et al. Novel use of the Epic Electronic Medical Record platform to identify lost Ureteral stents. J Endourol. 2019;33(10):858–62.
Sancaktutar AA, Soylemez H, Bozkurt Y, et al. Treatment of forgotten ureteral stents: how much does it really cost? A cost-effectiveness study in 27 patients. Urol Res. 2012;40(4):317–25.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89.
Darlington D, Anitha FS. Single Session Endoscopic removal of bilateral Ureteric stents retained for three decades: a Case Report. Cureus. 2019;11(3):e4294.
Lin TF, Lin WR, Chen M, et al. The risk factors and complications of forgotten double-J stents: a single-center experience. J Chin Med Assoc. 2019;82(10):767–71.
Tang C, Qu G, Yang G, et al. Case Report: a Calculus-free Ureteral Stent Forgotten for 29 years. Front Surg. 2022;9:878660.
Lam JS, Gupta M. Tips and tricks for the management of retained ureteral stents. J Endourol. 2002;16(10):733–41.
Adanur S, Ozkaya F. Challenges in treatment and diagnosis of forgotten/encrusted double-J ureteral stents: the largest single-center experience. Ren Fail. 2016;38(6):920–6.
Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications–where we are and where we are going. Nat Rev Urol. 2015;12(1):17–25.
Murthy KVR, Reddy SJ, Prasad DV. Endourological management of forgotten encrusted ureteral stents. Int Brazilian J Urol. 2010;36(4):420–9.
Polat H, Yucel MO, Utangac MM, et al. Management of Forgotten Ureteral stents: Relationship between Indwelling Time and required treatment approaches. Balkan Med J. 2017;34(4):301–7.
Acosta-Miranda AM, Milner J, Turk TM. The FECal Double-J: a simplified approach in the management of encrusted and retained ureteral stents. J Endourol. 2009;23(3):409–15.
Arenas JL, Shen JK, Keheila M, et al. Kidney, Ureter, and bladder (KUB): a Novel Grading System for Encrusted Ureteral stents. Urology. 2016;97:51–5.
Shivde SR, Joshi P, Jamkhandikar R. Extrusion of double J stent: a rare complication. Urology. 2008;71(5):814–5.
Murtaza B, Niaz WA, Akmal M, et al. A rare complication of forgotten ureteral stent. J Coll Physicians Surgeons–Pakistan. 2011;21(3):190–2.
Breau RH, Norman RW. Optimal prevention and management of proximal ureteral stent migration and remigration. J Urol. 2001;166(3):890–3.
Murtaza B, Alvi S. Forgotten Ureteral stents: an avoidable morbidity. J Coll Physicians Surg Pak. 2016;26(3):208–12.
Weedin JW, Coburn M, Link RE. The impact of Proximal Stone Burden on the management of encrusted and retained ureteral stents. J Urol. 2011;185(2):542–7.
Sancaktutar AA, Adanur ^, Reşorlu B, et al. The Forgotten Ureteral Stent in Children: from diagnosis to treatment. J Urol. 2013;189(3):1054–60.
Mistry K, Pal P, Chitale S. A simple two-stage bailout technique for the removal of an unyielding ureteric stent. Urology. 2013;82(1):242–4.
Zahran MH, Harraz AM, Taha D, et al. Studying the morbidity and renal function outcome of missed Internal Ureteral stents: a matched pair analysis. J Endourol. 2015;29(9):17–075.
Zumstein V, Betschart P, Albrich WC, et al. Biofilm formation on ureteral stents - incidence, clinical impact, and prevention. Swiss Med Wkly. 2017;147:w14408.
Coulier B, Lefebvre G. Forgotten Ureteral Double-J stent complicated by severe encrustation in the bladder. J Belg Soc Radiol. 2016;100(1):77.
Irkilata L, Ozgur BC, Sancaktutar AA, et al. Extracorporeal shock wave lithotripsy in the primary treatment of encrusted ureteral stents. Urolithiasis. 2015;43(4):379–84.
Kartal IG, Baylan B, Gok A, et al. The Association of Encrustation and Ureteral Stent Indwelling Time in Urolithiasis and KUB Grading System. Urol J. 2018;15(6):323–8.
Tawfeek AM, Elmoazen M, Saafan A, et al. Simultaneous antegrade and retrograde endourological approach in Galdakao-modified supine Valdivia position for the management of missed stents associated with complex renal stones: a non-randomized pilot study. Int Urol Nephrol. 2021;53(2):211–7.
Yeh C, Chen C, Lin C, et al. A new technique for treating Forgotten Indwelling Ureteral stents: Silk Loop assisted ureterorenoscopic lithotripsy. J Urol. 2004;171(2, Part 1):719–21.
Acknowledgements
Not applicable.
Funding
This article was sponsored by Capital’s Funds for Health Improvement and Research (2022-2-1102) and Beijing Natural Science Foundation (No.7232027).
Author information
Authors and Affiliations
Contributions
X Wang: Data collection/Data analysis/Manuscript writing; Z Ji: Data collection; P Yang: Data collection; J Li: Project development/Manuscript editing; Y Tian: Project development/Manuscript editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Ji, Z., Yang, P. et al. Forgotten ureteral stents: a systematic review of literature. BMC Urol 24, 52 (2024). https://doi.org/10.1186/s12894-024-01440-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-024-01440-9