Abstract
Background
With the introduction of robotic surgery, whether the robot-assisted radical cystectomy (RARC) could reduce the perioperative morbidity compared with Open radical cystectomy (ORC) was unknown.
Methods
Studies reported RARC were reviewed based on all randomized controlled trials (RCTs), which focused on the efficacy of RARC versus ORC.
Results
Of the 201 studies from preliminary screening, four RCTs were included. By pooling these studies, there were significant differences in comparison of operative time (p = 0.007), estimated blood loss (EBL) (p < 0.001) and time to diet (p < 0.001) between the RARC group and ORC groups. There was no significant difference regarding perioperative complications (Clavien 2–5, Clavien 3–5), length of stay (LOS), positive surgical margins (PSM) and lymph node positive.
Conclusion
This meta-analysis presented evidence for a benefit of EBL, time to diet, similar perioperative complications and oncological outcomes, but a longer operative time in RARC. It is noted that RARC was considered as a comparable surgical procedure to ORC.
Similar content being viewed by others
Background
In United States, approximately 74000 new cases of urinary bladder cancer with estimated 16000 deaths were expected in 2015 [1]. Open radical cystectomy (ORC) combined pelvic lymph node dissection (PLND) and urinary diversion (UD) is gold standard surgical intervention for high risk non-muscle invasive and muscle invasive bladder cancer, but accompanied with significant perioperative morbidity. In 2003, Menon reported the first case of robot-assisted radical cystectomy (RARC) [2]. With the introduction of robotic surgery, minimally invasive bladder cancer surgery set off a new climax with the promise of decreasing perioperative morbidity and mortality once again. Since then, a few prospective and retrospective studies had reported lower or comparable rates of complications, quicker recovery, and equivalent oncologic outcomes compared with ORC, however, which did not lead to a conclusive result [3–6]. Furthermore, these non-randomised researchs were accompanied with prominent selection bias. Although several meta-analyses regarding comparison of RARC with ORC had existed [7–11], these reviews incorporated a majority of non-randomized trials. Currently four randomized controlled trials had been publicated, therefore we conducted a systematical review of these literatures comparing surgical outcomes of RARC with those of ORC to provide powerful evidence.
Methods
Literature search
A systematic review of literatures was performed in Dec 2015. The electronic databases including PubMed, Embase, and the Cochrane Library were searched with restriction to English language. The following terms and their combinations were searched in [Title/Abstract]: cystectomy, cystectomies, cystoprostatectomy, bladder resection, robotic, robot, robot*, robot-assisted, and da Vinci. The related articles function was also used to broaden the search. Lists of references from the retrieved articles were manually searched to ensure as many studies as possible. When multiple reports describing the same population were published, the most recent or complete report was used.
Inclusion criteria and exclusion criteria
All available randomized controlled trials comparing RRC and ORC were considered, in addition at least one outcome of interest was mentioned. Studies as follow were excluded: (i) prospective non-randomised trials or retrospective trials comparing RARC and ORC, editorials, letters to the editor, review articles, experimental animal studies, case reports, comments, and conference abstracts, (ii) no outcomes of interest were reported.
Data extraction and outcomes of interest
Two reviewers independently selected studies for inclusion and extracted the following data: first author, year of publication, country, study interval, study design, indications for operation, number of patients who underwent RARC or ORC, rate of conversion from robot-assisted to open technique, matching criteria: age, gender, body mass index, American Society of Anesthesiologists (score), diversion type (conduit or neo-bladder), clinical stage, neoadjuvant chemotherapy, and outcomes of interest.
The primary outcomes were perioperative complication rates including intraoperative complications and postoperative complications classified according to the Clavien-Dindo grading system [12]. If sufficient data was available, perioperative complications were subdivided into 30d and 90d. The secondary outcome variables included operating time, estimated blood loss (EBL), number of patients receiving blood transfusion, length of stay (LOS), time to regular diet, positive margins, number of lymph nodes and pathologic stage. All disagreements were resolved by discussion until a consensus was reached. On condition that the data was incomplete, the corresponding authors were contacted.
Methodological quality
An evaluation of the methodological quality of the eligible studies was performed according to the Cochrane handbook [13]. For risk of bias assessment, the selection bias, performance bias and detection bias, attribution bias, reporting bias and other potential sources of bias were assessed in each of the included studies. The intention-to-treat analyses were described in the majority of studies.
Statistical analysis
The meta-analyses were conducted using Review Manager Version 5.3.
Dichotomous variables were presented as odds ratios (ORs) with 95 % confidence intervals (CIs), continuous variables as weighted mean differences (WMD) with 95 % CI. For studies that presented some continuous data as median and range values, the means and standard deviations were derived by statistical algorithms decribed by Wan et al. [14]. The p-Value was considered significant if <0.05. Statistical heterogeneity between studies was assessed using the chi-square test with p < 0.10 used for statistical significance. Statistical heterogeneity was also assessed using the I2 test: I2 values of 25 % (low), 50 % (medium), and 75 % (high). With I2 values of 50 % or less, heterogeneity was acceptable referring to Cochrane handbook and in case that high levels of heterogeneity with I2 values of 50 % or larger, we adopted a random-effects model.
Results
Characteristics of eligible studies
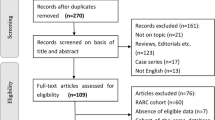
A thorough review of the potentially relevant studies resulted in 201 articles of which 4 were selected in the final analysis including 239 cases (121 cases in RARC group and 118 controls in ORC group (Fig. 1). Participant characteristics were presented as follows (Table 1). All 4 RCTs scored level 2b. 3 RCTs reported extracorporeal urinary diversion method with the similar percentage of neobladder [15–17]. Only one patient converted to open surgery due to equipment failure [16]. Of these excluded studies, 5 lacked controls, 25 non-original publications, 6 non-randomized controlled trial, 1 ongoing trial, 1 shared overlapping populations with no outcomes of interest.
The risk of bias summary about each risk of bias item was available in Fig. 2. Nix et al. adopted inappropriate randomization, and allocation concealment could not be judged insufficient information. On account of different surgical approaches, blinding could not be achieved in four RCTs comparing RARC and ORC. Few data missing and ITT (intention-to-treat) analysis other than one RCT reduced attribution bias. Then all preestablished outcomes were entirely reported. Moreover, limited cases brought out relatively weaker Statistical power.
Demographic and clinical characteristics
The mean or median age in the included studies acquired without sufficient standard variation for pooling data was quite close. Pooled data showed no difference in the male/female ratio or BMI. Clinical stage T4 was excluded, and no difference was found in distribution of T2-3 stage in three RCTs. There was also no difference in neochemotherapy applied in three studies. Likewise, the proportion of neobladder showed no difference in urinary conversion.
Perioperative outcomes
Pooled data from 4 studies evaluated operative time and estimated blood loss showed significantly longer OP (WMD: 71.72; 95 % CI, 19.74 to 123.70; p = 0.007) (Fig. 3) and less EBL (WMD:−241.99; 95 % CI,−332.55 to−151.43; p < 0.00001) (Fig. 4) in the RARC than the ORC group.
All RCTs compared complications using the Clavien system. Pooled data from 3 RCTs showed no significance in perioperative complications between RARC vs. ORC regarding to Clavien 2–5 (OR: 1.18; 95 % CI, 0.66 to 2.11; p = 0.58) (Fig. 5) or Clavien 3–5 (OR: 1.2; 95 % CI, 0.57 to 2.5; p = 0.63) (Fig. 6).
Data of time to flatus was extracted from 2 studies. Postoperative flatus was significantly shorter in RARC group (WMD:−0.79; 95 % CI,−1.28 to−0.30; p = 0.002) (Fig. 7). Likewise, time to regular diet from 3 studies was significantly shorter in RARC group (WMD:−1.14; 95 % CI,−1.71 to−0.75; p < 0.0001) (Fig. 8). There was no significant difference for length of stay between RARC and ORC from 4 RCTs (WMD:−0.54; 95 % CI,−1.44 to−0.35; p = 0.23) (Fig. 9).
Pathologic outcomes
Four studies reported the rates of positive surgical margin. There was no significant statistical difference in PSM between RARC group and ORC group (OR: 0.98; 95 % CI, 0.30 to 3.19; p = 0.98) (Fig. 10). Pathological stage in detail was reported in 4 studies. No significance was found in part of ≤ pT2 (OR: 1.21; 95 % CI, 0.71 to 2.05; p = 0.49) (Fig. 11), or ≥ pT3 (OR: 0.93; 95 % CI, 0.53 to 1.62; p = 0.8) (Fig. 12). Data of lymph node positive was available in 3 studies. Similarly, there was no significant statistical difference between RARC group and ORC group (OR: 0.84; 95 % CI, 0.42 to 1.72; p = 0.64) (Fig. 13).
Discussion
ORC with pelvic lymph node dissection still remains the gold standard approach for management of high grade non muscle-invasive and muscle-invasive bladder cancer. Notwithstanding, RARC became prevailing, especially in the U.S, which increased from 0.6 to 12.4 % of all RC cases from 2004 to 2010 [18]. Previous meta-analyses included both retrospective and prospective studies, which inevitably gave rise to selection bias. Fortunately, 4 RCTs comparing RARC and ORC could be achieved. For this reason, we conducted a meta-analysis with higher level of evidence. According to the analysis result, RARC had an advantage of less EBL and more rapid return to regular diet, but accompanied by longer operative time. Interestingly, this review showed similar rate of perioperative complications, which differed from what proposed by previous researchers. Besides, RARC was comparable with ORC in PSM, LOS.
On account of the 3 dimensional visual effect and elaborate operation, blood loss was much less in RARC group. Another important reason was the tamponade effect from the pneumoperitoneum used during RARC [19]. There is no doubt that it is the distinct advantage of RARC in terms of bleeding control, regardless of study types [5–8, 11, 15–17, 20]. Less EBL signified less blood transfusion. Unfortunately, only one RCT reported the rate of transfusion with no statistical difference [20], therefore pooling data could not be carried out.
Pooled data of operative time showed that RARC took longer time compared with ORC as with previous results [4, 21]. No matter docking Da Vinci system or conversion to open urinary diversion adopted was time-consuming. Parekh et al. reported the similar operative time for both surgical procedures, and uniquely depicted the definition of operative time (defined as incision to closure), however, the data of urinary diversion was not available [20]. In addition, a better comparison would be drawn in case that the time of radical cystectomy, PLND and UD could be set apart. Prior studies demonstrated that learning curve was significantly associated with shorter operative time [22, 23]. In this meta-analysis, 3 RCTs reported that surgeons performed approximately 50–110 RARCs to eliminate the impact of learning curve. However, Bochner et al. only gave a vague statement that surgeons were experienced in extensive robotic pelvic surgery experience.
Previous meta-analyses demonstrated a lower complications [7–11], however no significance was found in perioperative complications between RARC and ORC in this review. Less blood loss failed to bring about lower complications in RARC groups. Moreover, the study conducted by Bochner et al. terminated, because the primary objective that the rate of grade 2–5 complications would be 20 % lower for RARC compared with ORC was not reached. Hautmann et al. reported that the majority of complications after radical cystectomy were correlated with urinary diversion [24]. Recently, totally robotic-assisted radical cystectomy with intracorporeal diversion was increasingly adopted, which brought potential benefits to the patients. Ahmed et al. demonstrated a 32 % reduction in complications at 90d comparing open with robotic urinary diversion [25]. For overall complication rates, Koupparis et al. reported a trend to lower complication rate (31 % vs 48 %) in the RARC group vs ORC group. Introducing robotic-assisted radical cystectomy with intracorporeal diversion in the future RCTs might show the potential advantage for perioperative complications. Remarkably, extracorporeal urinary diversion was adopted in 3 RCTs, which might be the reason why lower perioperative complications could not be found in RARC group.
In overall surgical margin, we draw a conclusion of an equivalent in RARC group and ORC group. The total rate of PSM in RARC groups was 5 % (range, 0–15 %), which was similar to 6.8 % raised by the International Robotic Cystectomy Consortium [26]. Higher pathological T stage was significantly correlated with an increased likelihood of a positive margin [26]. In this review, pooled data of extravesical disease (≥ pT3) showed no significance difference. However, PSM could not be detailed according to stage (<pT2 vs > pT3) for insufficient data. Only Bochner et al. reported PSM in subgroup of patients ≥ pT3 (RARC versus ORC: 12 %, 16 %, p = 0.7) [15]. PLND served as an indicator of surgical quality of RC [27]. Parekh et al. reported an appearance of fewer median LNs in RARC with no significance. Nix et al. described a similar mean LNs between RARC and ORC. These data was insufficient for pooling data. Lymph node positive available in 3 studies was similar.
For long-term oncologic outcomes in RARC from International Robotic Cystectomy Consortium, after median follow-up of 67-months, 5 year recurrence-free survival, cancer-specific survival, and overall survival were 67 %, 75 %, and 50 %, respectively [28]. In this meta-analysis, only short term oncological outcomes were reported by Khan et al. that disease recurrence (11 %, 16 %) and disease-specific mortality (0 %, 5 %) was equivalent between RARC and ORC at 12 months.
With regard to LOS, although several retrospective studies and meta-analysis had demonstrated a significantly shorter LOS in RARC that may derive from the severe selection bias [4, 21, 29], but no significant difference was found in RARC group versus ORC group in our review, only modest trends of shorter LOS. The less bleeding and shorter time to regular diet had not brought about shorter LOS in our analysis. Above all, the similar complications might mainly contribute to longer hospital stay.
Outcomes of interest were mainly acquired, and incomplete data was depicted. The heterogeneity of variables was mostly low (I2 ≤ 50 %) except operative time (I2 = 90 %), for which random effects were taken.
Potential selection biases could be eliminated by the randomized trials, but surgeon’s experience might introduce an important confounder to results. Patients unable to bear the pneumoperitoneum and steep trendelenburg position were excluded in RARC, coupled with improper randomization method applied in Nix’s study. Hence, selection bias still existed.
Conclusion
Four RCTs comparing RARC and ORC were included in this meta-analysis. Based upon analysis, a benefit of less EBL, shoter time to diet, similar perioperative complications and oncological outcomes, but a longer operative time could be seen in RARC group. We might draw a conclusion that RARC was a safe and effective surgical procedure noninferior to the open approach, meanwhile randomized controlled trial comparing RARC and ORC was feasible. Despite a rigorous methodological review, restrictions were exerted to draw a straightforward conclusion for limitations of the included studies and patients. Certainly, further prospective, multicentric and large sample randomized control trials should be undertaken to confirm our findings. It’s expected that Parekh et al. paved the way toward a phase III, multi-institutional, randomized trial that can offer more definitive answers.
Abbreviations
- Cis:
-
Confidence intervals
- EBL:
-
Estimated blood loss
- LOS:
-
Length of stay
- ORC:
-
Open radical cystectomy
- ORs:
-
Odds ratios
- PLND:
-
Pelvic lymph node dissection
- PSM:
-
Positive surgical margins
- RARC:
-
Robot-assisted radical cystectomy
- UD:
-
Urinary diversion
- WMD:
-
Weighted mean differences
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92(3):232–6.
Atmaca AF, Canda AE, Gok B, Akbulut Z, Altinova S, Balbay MD. Open versus robotic radical cystectomy with intracorporeal Studer diversion. JSLS. 2015;19(1):e2014–e00193.
Kader AK, Richards KA, Krane LS, Pettus JA, Smith JJ, Hemal AK. Robot-assisted laparoscopic vs open radical cystectomy: comparison of complications and perioperative oncological outcomes in 200 patients. BJU Int. 2013;112(4):E290–4.
Styn NR, Montgomery JS, Wood DP, et al. Matched comparison of robotic-assisted and open radical cystectomy. Urology. 2012;79(6):1303–8.
Sung HH, Ahn JS, Seo SI, et al. A comparison of early complications between open and robot-assisted radical cystectomy. J Endourol. 2012;26(6):670–5.
Fonseka T, Ahmed K, Froghi S, Khan SA, Dasgupta P, Shamim Khan M. Comparing robotic, laparoscopic and open cystectomy: a systematic review and meta-analysis. Arch Ital Urol Androl. 2015;87(1):41–8.
Ishii H, Rai BP, Stolzenburg JU, et al. Robotic or open radical cystectomy, which is safer? A systematic review and meta-analysis of comparative studies. J Endourol. 2014;28(10):1215–23.
Li K, Lin T, Fan X, et al. Systematic review and meta-analysis of comparative studies reporting early outcomes after robot-assisted radical cystectomy versus open radical cystectomy. Cancer Treat Rev. 2013;39(6):551–60.
Tang K, Xia D, Li H, et al. Robotic vs. open radical cystectomy in bladder cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2014;40(11):1399–411.
Xia L, Wang X, Xu T, et al. Robotic versus open radical cystectomy: an updated systematic review and meta-analysis. PLoS ONE. 2015;10(3), e0121032.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Higgins, J. and S. Green, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: The Cochrane Collaboration; 2011. Available at http://training.cochrane.org/handbook.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial. Eur Urol. 2015;67(6):1042–50.
Khan MS, Gan C, Ahmed K, et al. A Single-centre Early Phase Randomised Controlled Three-arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). Eur Urol. 2016;69(4):613–21.
Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010;57(2):196–201.
Leow JJ, Reese SW, Jiang W, et al. Propensity-matched comparison of morbidity and costs of open and robot-assisted radical cystectomies: a contemporary population-based analysis in the United States. Eur Urol. 2014;66(3):569–76.
Farnham SB, Webster TM, Herrell SD, Smith Jr JA. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67(2):360–3.
Parekh DJ, Messer J, Fitzgerald J, Ercole B, Svatek R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J Urol. 2013;189(2):474–9.
Musch M, Janowski M, Steves A, et al. Comparison of early postoperative morbidity after robot-assisted and open radical cystectomy: results of a prospective observational study. BJU Int. 2014;113(3):458–67.
Hayn MH, Hellenthal NJ, Seixas-Mikelus SA, et al. Is patient outcome compromised during the initial experience with robot-assisted radical cystectomy? Results of 164 consecutive cases. BJU Int. 2011;108(6):882–7.
Richards KA, Kader K, Pettus JA, Smith JJ, Hemal AK. Does initial learning curve compromise outcomes for robot-assisted radical cystectomy? A critical evaluation of the first 60 cases while establishing a robotics program. J Endourol. 2011;25(9):1553–8.
Hautmann RE, De Petriconi RC, Volkmer BG. Lessons learned from 1,000 neobladders: the 90-day complication rate. J Urol. 2010;184(3):990–4. quiz 1235.
Ahmed K, Khan SA, Hayn MH, et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2014;65(2):340–7.
Hellenthal NJ, Hussain A, Andrews PE, et al. Surgical margin status after robot assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol. 2010;184(1):87–91.
Buscarini M, Josephson DY, Stein JP. Lymphadenectomy in bladder cancer: a review. Urol Int. 2007;79(3):191–9.
Raza SJ, Wilson T, Peabody JO, et al. Long-term oncologic outcomes following robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2015;68(4):721–8.
Khan MS, Challacombe B, Elhage O, et al. A dual-centre, cohort comparison of open, laparoscopic and robotic-assisted radical cystectomy. Int J Clin Pract. 2012;66(7):656–62.
Acknowledgements
Zhongquan Sun and Zhiyuan Shen conducted the conception and design of the study, acquisitionand interpretation of data, drafting the article, final approval of the version to be published.
Funding
No funding.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Authors’ contributions
ZQS have made substantial contributions to conception and revisied it critically for important intellectual content. ZYS participated in acquisition of data, analysis, interpretation of data and drafting the manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Given that this manuscript was a meta-analysis, ethics and consent was not required for our study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, Z., Sun, Z. Systematic review and meta-analysis of randomised trials of perioperative outcomes comparing robot-assisted versus open radical cystectomy. BMC Urol 16, 59 (2016). https://doi.org/10.1186/s12894-016-0177-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-016-0177-z