Abstract
Background
Androgen deprivation therapy (ADT) is an effective palliation treatment in men with advanced prostate cancer (PC). However, ADT has well documented side effects that could alter the patient’s health-related quality of life (HRQoL). The current study aims to test whether a genetic stratification could provide better knowledge for optimising ADT options to minimize HRQoL effects.
Methods
A cohort of 206 PC survivors (75 treated with and 131 without ADT) was recruited with written consent to collect patient characteristics, clinical data and HRQoL data related to PC management. The primary outcomes were the percentage scores under each HRQoL subscale assessed using the European Organisation for Research and Treatment of Cancer Quality of Life questionnaires (QLQ-C30 and PR25) and the Depression Anxiety Stress Scales developed by the University of Melbourne, Australia. Genotyping of these men was carried out for the aldo-keto reductase family 1, member C3 (AKR1C3) rs12529 single nucleotide polymorphism (SNP). Analysis of HRQoL scores were carried out against ADT duration and in association with the AKR1C3 rs12529 SNP using the generalised linear model. P-values <0 · 05 were considered significant, and were further tested for restriction with Bonferroni correction.
Results
Increase in hormone treatment-related effects were recorded with long-term ADT compared to no ADT. The C and G allele frequencies of the AKR1C3rs12529 SNP were 53·4 % and 46·6 % respectively. Hormone treatment-related symptoms showed an increase with ADT when associated with the AKR1C3 rs12529 G allele. Meanwhile, decreasing trends on cancer-specific symptoms and increased sexual interest were recorded with no ADT when associated with the AKR1C3 rs12529 G allele and reverse trends with the C allele. As higher incidence of cancer-specific symptoms relate to cancer retention it is possible that associated with the C allele there could be higher incidence of unresolved cancers under no ADT options.
Conclusions
If these findings can be reproduced in larger homogeneous cohorts, a genetic stratification based on the AKR1C3 rs12529 SNP, can minimize ADT-related HRQoL effects in PC patients. Our data additionally show that with this stratification it could also be possible to identify men needing ADT for better oncological advantage.
Similar content being viewed by others
Background
Androgen deprivation therapy (ADT) is an effective treatment in men with advanced metastatic PC and those with high risk tumors in combination with radiation therapy (RT) [1]. The main types of medical castration methods used in New Zealand are the luteinizing hormone-releasing hormone (LHRH) agonists and the anti-androgens (AA). The LHRH agonists suppress the gonadotropin-releasing hormone receptors at the hypothalamus. This subsequently affects the production of luteinizing hormone and follicular stimulating hormone at the pituitary resulting in reduced testicular androgen production for up to 97 % [2]. However, Labrie [2] suggests that 41 % of the total androgen pool still remains in the serum after LHRH agonist treatment due to the existence of other androgen sources. Androgen is also produced in the prostate by adrenal derived dehydroepiandrosterone [3]. The type 5 17-hydroxysteroid dehydrogenase [aldo-keto reductase family 1, member C3 (AKR1C3)], which is produced in many tissue types including the adrenal gland also converts androstenedione to androgen [4]. The AAs such as Flutamide and Bicalutamide mainly target the hormone-binding pocket of the androgen receptor (AR) ligand-binding domain [5]. However, in patients with high tumour burden and with metastatic disease, AA monotherapy does not provide castration as with LHRH agonists [6].
Both surgical and medical strategies of ADT have well documented side effects altering patient’s HRQoL [7]. Some of these symptoms are common between LHRH agonists and AAs while some others have more pronounced side effect than others [7, 8].
We have previously assessed androgen pathway related gene polymorphisms for their association with the serum PSA level, which is a downstream product of androgen binding to androgen receptor (AR) [9]. This has shown that compared with controls among PC patients an increase in the AKR1C3 rs12529 G allele is associated with a suppression of the serum PSA level when influenced by confounders including smoking [10]. Therefore, it could be possible that men carrying the AKR1C3 rs12529 G allele also carry lower androgen levels; when interacting with confounders. The AKR1C3 gene located at chromosomal position 10p15 [11], records a non-synonymous SNP rs11551177 [11] associated with serum testosterone levels [12]. The non-synonymous SNP rs12529 located in exon 1 of the AKR1C3 gene [13] is in linkage disequilibrium with the promoter SNP rs1937845. Additionally, rs12529 SNP is located closer to transcription factor binding sites for the antioxidant responsive element and an activator protein-1 [14]. The flanking region of −104 to + 65 of this gene contains a reverse CCAAT and a GC box which are known for transcription regulation; mutation of the GC box is affected by SP3 transcription factor regulated reduction of AKR1C3 activity by 70 % [15]. Meanwhile, the polymorphism rs3763676 located at −138 is reported to produce a 2.2 fold increase of promoter activity by dihydrotestosterone [15]. Together these facts provide a possibility for the AKR1C3 gene to produce differential expression levels and thus alter subsequent contribution to total androgen production. The AKR1C3 rs12529 has not appeared under genome-wide association studies (GWAS) related to PC, possibly due to its pro-cancer modulation effects getting pronounced only after interaction with confounders [10, 16]. An alternate approach to GWAS has shown that the AKR1C3 rs4881400 SNP is associated with risk of sporadic PC among Caucasians [17].
The current analysis is an attempt to assess whether the HRQoL impacts with ADT are associated with the AKR1C3 rs12529 SNP.
Methods
Patient recruitment
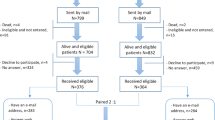
Men with confirmed clinical diagnoses of PC and registered with the Urology Department databases at hospitals managed under the District Health Boards of Auckland, Waitemata, and Counties Manukau, and patients attending private Urology and Oncology clinics from Auckland and Hamilton, in New Zealand (NZ) were invited to take part in this study. They were invited directly at recruitment to urology studies or subsequent to being in our database since 2006. A total of 206 interested patients were enrolled in this study with their written consent. A flowchart indicating the procedure for collection of survey data is presented in Fig. 1.
Flowchart to show patient recruitment and data collection process for the Quality of Life Survey. The HRQoL data collection was carried out with new recruits from March 2013- November 2014. In May 2013 a list of National Health Index numbers of all patients in our Urology study database was sent to the Analytical Services of the MOH, New Zealand, to check for any recorded mortalities among patients recruited. This check was carried out subsequent to approval from the HDEC, the MOH, New Zealand (ethics reference NTY/05/06/037/AM02). In November 2013, upon receiving this information from the Analytical Services of the MOH, all PC survivors in our patient cohort who have not yet completed the PC treatment/HRQoL survey, were invited to take part. Therefore, the final number of patients that took part in this survey was dependent on patient response to these invitations. (*Number assumed to have received the invitation includes PC survivors who completed the survey, those that declined and those assumed to have received the invitation.)
Patient characteristics
At recruitment, self-reported ethnicity, age at PC diagnosis, body mass index (BMI), tobacco smoking and alcohol consumption status, Gleason score, cancer staging data (if available) and PC treatment type/s were recorded at a clinical setting. Classification into Caucasian-associated or Maori, Pacific and East Asian-associated groups was based on our previous studies [18].
Summary of questionnaire administered
The survey questionnaire consisted of parts A, B1 and B2
Part A recorded PC management options received and details on treatment duration. Management options included active surveillance (AS), watchful waiting (WW), radical prostatectomy (RP), RT including brachytherapy (BT) and ADT. The ADT options provided in the questionnaire were LHRH agonists, surgical orchiectomy, AA and estrogen therapy. The final analyses considered LHRH agonists, AA and estrogen treatments under ADT and were further stratified as short-term (less than six months) or long-term (over six months) treatments. If a patient had any ADT combined with other treatments (RT/BT), they were placed under ADT. All other PC management methods were considered under no ADT category. Patient reported treatment types were verified against available clinical records.
Part B recorded HRQoL effects post-treatment or post-diagnosis for those who were on AS or WW. Questionnaires were modified with a variation to fit in the requirements of this study by changing the period of the HRQoL data recording from that of the ‘past week’ as mentioned in the original to ‘period of worst HRQoL effects post-diagnosis’. Part B1 contained Quality of Life Core Questionnaire, EORTC -QLQ-C30, (Version 3 · 0) [19] and EORTC QLQ PR-25 (phase IV) [20] with permission from the EORTC. The total score for each domain was transformed to a percentage scale using respective guidelines [20, 21]. All questions in Part B.2 were adapted and scored based on the Common Assessment Measures: Depression Anxiety Stress Scales (DASS), the Australian Centre for Posttraumatic Mental Health, University of Melbourne [22]. Scoring was carried out only where >60 % of questions under each HRQoL subscale tested were answered.
Genotype analysis
At patient recruitment, non-fasting blood samples were collected into coded 6.00ml EDTA BD Vacutainer® blood collection tubes and placed on ice before being transported to our laboratory. A total of 195 patients have supplied a blood sample at recruitment. DNA was extracted using the QIAamp genomic DNA kit (from Qiagen, Hilden, Germany) using the QIAcube (from Qiagen, Hilden, Germany). Initial SNP genotyping was carried out using the Sequenom MassArray system according to manufacturer’s instructions (Sequenom, San Diego, CA, USA) as described in Ferguson et al. [23]. The TaqMan® SNP Genotyping Assay using allele-specific, dual-labelled hybridization probes from apredesigned assay on demand (C__8723970_1_) from the Applied Biosystems was used for subsequent genotyping as described before [23]. Additional details on SNP genotyping procedure are given in Additional file 1: Table S1.
Statistical analysis
As HRQoL effects can change with age, BMI, tobacco smoking and clinical Gleason score, statistical variability of these parameters between ADT and no ADT groups was assessed and considered for HRQoL data correction. Prostate cancer management types including RP, RT, ADT and AS have all been reported as having a bias towards HRQoL effects compared to men without PC [24]. Therefore, treatment types were not considered for correction in this analysis.
Categorical variables were described as frequency and percentage between those that received ADT and those that did not. These variables were compared between the two groups using the Fisher’s exact test. Continuous variables were described as mean and standard deviation or median and 75th percentile. Medians were compared using the Mann–Whitney U statistics. The generalised linear model was used to test variables between those that received ADT and those that did not. The ADT group was standardised against the no ADT group.
The generalised linear model was also used to test the association between the HRQoL scores and ADT treatment duration as well as for testing the AKR1C3 rs12529 G allele association with and without ADT treatment. For testing association of the HRQoL scores between ADT duration, short- and long-term treatment scores were standardised against no ADT group. The relative differences between the no ADT and ADT groups were given as estimates with 95 % CI. Changes in the HRQoL scores as the G allele number increased (from 0 to 1 to 2) were given as estimates with 95 % CI for both ADT and no ADT groups. The HRQoL results were presented as before and after adjustments for significant confounding variables between the ADT and no ADT groups and will be hereafter referred to as before and after corrections. There was not enough response data to analyse dyspnoea and appetite loss when the data were stratified between, short- and long-term ADT and no ADT and were excluded from analysis between the HRQoL scores and ADT duration. For the analysis of gene association related to the HRQoL scores with combined types of ADT, all the HRQoL subscales were included.
Statistical significance for variation of patient characteristics was set at p < 0 · 05. To avoid false positives with multiple testing, statistical significance was restricted by Bonferroni correction under each HRQoL subscale. Analyses were carried out using SAS (v9 · 4 SAS Institute, Cary, NC, USA) or the R statistical software [25].
Results
Demographic, lifestyle and clinical characteristics
Patient characteristics between ADT and no ADT groups are given in Table 1. Age at diagnosis (69 · 9 ± 7 · 9y vs 65 · 3 ± 7 · 7y), p < 0 · 0001) and alcohol consumption (48 % vs 67 %, p = 0 · 0068) were significantly different between the groups. A higher proportion (56 · 7 %) of patients with Gleason score ≥7(4 + 3) have received ADT, while only 43.2 % of patients with Gleason score ≤7(3 + 4) have received this treatment (p = 0 · 0005). There was a significant difference between ADT and no ADT groups with regards to staging data between ≤ T3a, >T3a and the group with no available staging data (p = 0·0005).
Table 2 presents a stratification of patient-declared treatment types at the time of the survey. Those who reported taking ADT have selected the options of either LHRH agonists or AA as monotherapies or in combination. The ADTs have been received on their own or in association with RT. Among this cohort, 63 · 6 % have had no ADT while 25 · 7 % had it long-term, and 10 · 7 % short-term. Among ADT recipients, 85 · 3 % have used AA. The majority of those who have not received ADT have undergone RP (35 · 4 % without further treatment and 9 · 2 % with RT). RT or BT on their own was used by 7 · 3 % while 11 · 7 % have indicated being on AS or WW.
Genotyping
Genotype data is given for 191 (Additional file 2:Table S2) participants (CC = 55, CG = 94, GG = 42) with C and G allele frequencies being 53·4 % and 46·6 % respectively.
Quality of life assessment
Survey compliance
The valid response rates of questionnaire completion were, 88.9 % on direct administration at recruitment and 38 · 8 % and 34 · 2 % respectively by postal and electronic mail (Figure 1). The HRQoL survey compliance rates are given in Table 3.
HRQoL outcomes
The mean percentage HRQoL scores estimated for this cohort are given in Table 4. The HRQoL data evaluations for short-and long-term ADT groups compared to those without ADT are given in Table 5. The cognitive function effects were significantly attenuated [−27.76 (95 % CI −42.84 to −12.67), p = 0 · 0017) while depression was significantly increased [7 · 56 (95 % CI 4 · 51-10 · 60), p = 0 · 0002] with long-term ADT compared to no ADT before corrections. Long-term ADT showed significant increases of hormone treatment-related effects compared to no ADT both before [24 · 81 (95 % CI 14 · 64-34 · 98), p = 0 · 0002] and after [36 · 12 (95 % CI 18 · 59-53 · 65), p = 0 · 0046] corrections.
HRQoL outcomes in association with the AKR1C3 rs12529 SNP
The association of the AKR1C3 rs12529 G allele with HRQoL data between ADT and no ADT groups is given in Table 6. Hormone treatment-related symptoms showed a significant increase with ADT when associated with the AKR1C3 rs12529 G allele, both before [5 · 40 (95 % CI 1 · 56-9 · 24), p = 0 · 0061] and after [4 · 86 (95 % CI 1 · 08-8 · 64), p = 0 · 0120] corrections.
Discussion
ADT related HRQoL effects in PC patients are well known [26]. Management strategies of such issues including psychosocial and lifestyle interventions, consideration of comorbidities before ADT and intermittent ADT (iADT), have been reviewed by Rhee et al. [7]. After controlling for false positives, our analysis indicates a significant gain of cognitive functions and increased depression with long-term ADT when interacting with confounders. The most prominent feature after long-term ADT was the hormone treatment-related effects which were significantly higher both before and after corrections. This latter feature was significantly associated with the AKR1C3 rs12529 G allele both before and after corrections for confounders. The data suggest that with each increase in the G allele, hormone treatment-related effects increase by a score of 5 · 4 before and 4 · 9 after corrections for confounders. Therefore those with the AKR1C3 rs12529 GG genotype will have a score increase of 10 · 8 and 9 · 8 as compared to the CC genotype before and after corrections respectively. Therefore, the AKR1C3 rs12529 GG genotype associated scores for our study are 65 % and 59 % before and after corrections respectively of the mean hormone treatment-related effects reported in our study (Table 4). If the same ADT regime is used for men with the AKR1C3 rs12529 CC, CG or GG genotypes, it could be possible that those carrying the G allele/s receive an over-treatment and hence increased side effects. The current findings add possibilities for better utilisation of existing ADT drugs. It will be advantageous to study whether the AKR1C3 rs12529 G allele carriers could benefit with iADT usage for oncological and HRQoL benefits; while complementing previous findings on iADT [27]. This genotypic association of the hormone treatment-related effects is novel and prove our hypothesis.
Our approach of considering correction for multiple testing however could be overly conservative due to possible non-independence of factors assessed [28, 29] under each HRQoL scale. For instance Engstrom has pointed out the interdependence of many HRQoL effects subsequent to hot flush experience [29]. Therefore, for this study it is beneficial to discuss HRQoL effects that were recorded under a significance level of p < 0.05 without Bonferroni restriction as well. In this regard additional recording of long-term ADT related increase in insomnia, depression and anxiety after correction for confounders are worth mentioning. Additionally, the AKR1C3 rs12529 G allele associated increased effects on emotional functioning, and decreased effects on fatigue, dyspnoea, appetite loss, bowel symptoms, hormone treatment-related symptoms and sexual interest (or reverse associated with the C allele) among those not having ADT are important recordings. This could mean that those opting for PC management methods that do not involve ADT have a higher risk of retaining these symptoms possibly due to untreated metastasis associated with the C allele. This could be due to higher androgen levels associated with the C allele [10], supporting metastasis that requires ADT. In addition to findings from Yu et al., where increased prostate cancer- specific mortality after ADT was observed among the AKR1C3 rs12529 CC genotype could mean that patients with this genotype require extended ADT treatments for better oncological benefits. The AKR1C3 rs12529 polymorphism has been previously shown to be associated with emotional control of lifestyle behaviour [30]. AKR1C3 is considered as an endogenous neuroactive steroid that has shown to mediate effects at the GABAA receptors in the brain [31]. The trends of association of the AKR1C3 rs12529 G allele with increased emotional functioning effects and increased sexual interest in our cohort with no ADT could have some relevance to the GABAA receptor modulation.
Although ADT is supportive of survival advantage for men diagnosed with advanced PC for some men, long-term ADT may not necessarily promote a better life; and this HRQoL suppression may even extend beyond 18 months of treatment [32]. As commented by Nguyen [33], evaluating short-term side effects vs long-term oncological benefits is important in this dialogue. Our study is adding another paradigm to this dialogue through a genetic stratification for ADT. If patients can be stratified based on the AKR1C3 rs12529 SNP, physicians might be able to organise ADT regimes that will be more beneficial for both oncological outcomes and HRQoL [7]. However, this hypothesis needs testing in large prospective studies with homogeneous patient cohorts.
Our study has the following shortcomings. The variation in HRQoL data recording from that of the ‘past week’ to ‘worst HRQoL effects post-diagnosis could have caused a symptoms recall bias. Besides HRQoL is a complicated outcome to measure due to many confounding variables. However, as the outcome of symptom scores aligns well with such quality of life studies done with original questionnaires [32], it shows that this exercise was not likely to have affected at least the long-term recall of hormonal treatment-related HRQoL effects. We have removed the questions on use of incontinence aid and sexual activity data from the analysis. However, the former is represented by urinary symptom score and the latter by sexual interest score as shown in Table 3. The study cohort was mainly Caucasian based and therefore, the results cannot be extended to other ethnicities. We have not defined the ADT usage between curative and palliative requirements. Our protocol also did not define those using AA only for flare protection. As 85.3 % of ADT users have received AAs, our findings could be biased towards these treatments. The grouping of LHRH agonist and AA treatments as a single ADT group was unavoidable due to the small sample size which itself is a shortcoming. The need for grouping all with short- and long-term ADT as one group for genetic analysis is also a shortcoming of this analysis. As staging data availability was limited we were not able to correct our results with this clinical feature although adjustments were carried out based on the Gleason score data.
Conclusion
Our aim was to test whether ADT-related HRQoL effects in PC patients have an association with the AKR1C3 rs12529 SNP. Our results indicate that the hormone treatment-related effects have a positive association with the AKR1C3 rs12529 G allele. Therefore, it is advantages to assess in larger prospective studies with better patient homogeneity whether those with the AKR1C3 rs12529 G allele/s perform well with iADT; and to evaluate whether the CC genotype can tolerate extended ADT treatment, for better oncological advantage. If proven successful, PC patients may be genetically stratified for optimal ADT effects for both survival benefits, and to maintain HRQoL.
Abbreviations
AA, Antiandrogens; ADT, Androgen deprivation therapy; AKR1C3, Aldo-keto reductase family 1, member C3; AR, Androgen receptor; AS, Active surveillance; BD, Becton, Dickinson and Company; BT, Brachytherapy; BMI, Body mass index; CEPH, Centre d’Etude du polymorphism Human; DASS, Depression Anxiety Stress Scales; ED, Erectile dysfunction; EDTA, Ethylenediaminetetraacetic acid; EORTC, European Organisation for Research and Treatment of Cancer; GABAA, γ-aminobutyric acid type A; HDEC, Health and Disability Ethics Committee; iADT, Intermittent ADT; LHRH, Luteinizing hormone-releasing hormone; MALDI, TOF Matrix-assisted laser desorption/ionization-Time of Flight; MOH, Ministry of Health; NTC, no-template controls; PSA, Prostate-specific antigen; HRQoL, health-related Quality of life; QLQ, Quality of Life questionnaires; RP, Radical prostatectomy; RT, Radiation therapy; SNP, Single nucleotide polymorphism; TURP, Transurethral resection of the prostate; WW, Watchful waiting
References
Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schroder FH, Sternberg CN, Studer UE. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol. 2012;61(1):11–25.
Labrie F. Blockade of testicular and adrenal androgens in prostate cancer treatment. Nat Rev Urol. 2011;8(2):73–85.
Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Belanger A, Vandenput L, Mellstrom D, Ohlsson C. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J Steroid Biochem Mol Biol. 2009;113(1–2):52–6.
Penning TM, Steckelbroeck S, Bauman DR, et al. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;2006(248):182–91.
Tian X, He Y, Zhou J. Progress in antiandrogen design targeting hormone binding pocket to circumvent mutation based resistance. Front Pharmacol. 2015;6:57.
Wirth MP, Hakenberg OW, Froehner M. Antiandrogens in the treatment of prostate cancer. Eur Urol. 2007;51(2):306–13. discussion 314.
Rhee H, Gunter JH, Heathcote P, Ho K, Stricker P, Corcoran NM, Nelson CC. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2015;115 Suppl 5:3–13.
Walker LM, Tran S, Robinson JW. Luteinizing hormone--releasing hormone agonists: a quick reference for prevalence rates of potential adverse effects. Clin Genitourin Cancer. 2013;11(4):375–84.
Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002;16(11):1429–31.
Karunasinghe N, Lange K, Han D, Goudie M, Zhu S, Wang A, Bishop K, Ferguson LR, Masters J. Androgen pathway related gene variants and prostate cancer association in Auckland men. Curr Pharmacogenomics Person Med. 2013;11(1):22–30.
Soderhall C, Korberg IB, Thai HT, Cao J, Chen Y, Zhang X, Shulu Z, van der Zanden LF, van Rooij IA, Frisen L, et al. Fine mapping analysis confirms and strengthens linkage of four chromosomal regions in familial hypospadias. Eur J Hum Genet. 2015;23(4):516–22.
Jakobsson J, Palonek E, Lorentzon M, Ohlsson C, Rane A, Ekstrom L. A novel polymorphism in the 17beta-hydroxysteroid dehydrogenase type 5 (aldo-keto reductase 1C3) gene is associated with lower serum testosterone levels in caucasian men. Pharmacogenomics J. 2007;7(4):282–9.
Figueroa JD, Malats N, Garcia-Closas M, Real FX, Silverman D, Kogevinas M, Chanock S, Welch R, Dosemeci M, Lan Q, et al. Bladder cancer risk and genetic variation in AKR1C3 and other metabolizing genes. Carcinogenesis. 2008;29(10):1955–62.
Ciaccio PJ, Walsh ES, Tew KD. Promoter analysis of a human dihydrodiol dehydrogenase. Biochem Biophys Res Commun. 1996;228(2):524–9.
Schulze JJ, Karypidis H, Ekstrom L. Basal and Regulatory Promoter Studies of the AKR1C3 Gene in Relation to Prostate Cancer. Front Pharmacol. 2012;3:151.
Sampson JN, Wheeler WA, Yeager M, Panagiotou O, Wang Z, Berndt SI, Lan Q, Abnet CC, Amundadottir LT, Figueroa JD, et al. Analysis of heritability and shared heritability based on genome-wide association studies for thirteen cancer types. J Natl Cancer Inst. 2015;107(12):djv279.
Kwon EM, Holt SK, Fu R, Kolb S, Williams G, Stanford JL, Ostrander EA. Androgen metabolism and JAK/STAT pathway genes and prostate cancer risk. Cancer Epidemiol. 2012;36(4):347–53.
Karunasinghe N, Han DY, Goudie M, Zhu S, Bishop K, Wang A, Duan H, Lange K, Ko S, Medhora R, et al. Prostate disease risk factors among a New Zealand cohort. J Nutrigenet Nutrigenomics. 2013;5(6):339–51.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
van Andel G, Bottomley A, Fossa SD, Efficace F, Coens C, Guerif S, Kynaston H, Gontero P, Thalmann G, Akdas A, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44(16):2418–24.
Fayers PM, Aaronson NK AN, Bjordal K, Groenvold M, Curran D, Bottomley A, on, Group botEQoL. The EORTC QLQ-C30 Scoring Manual, 3rd edn. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
Lovibond SH, Lovibond PF: Manual for the Depression Anxiety Stress Scales, 2nd edn. Sydney; 1995
Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, Duan H, Karunasinghe N. Genetic factors in chronic inflammation: single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn’s disease in a New Zealand population. Mutat Res. 2010;690(1–2):108–15.
Carlsson S, Drevin L, Loeb S, Widmark A, Lissbrant IF, Robinson D, Johansson E, Stattin P, Fransson P: Population-based study of long-term functional outcomes after prostate cancer treatment. BJU Int 2015
R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011.
Crawford ED, Moul JW. ADT risks and side effects in advanced prostate cancer: cardiovascular and acute renal injury. Oncology (Williston Park). 2015;29(1):55–8. 65–56.
Sciarra A, Abrahamsson PA, Brausi M, Galsky M, Mottet N, Sartor O, Tammela TL, Calais da Silva F. Intermittent androgen-deprivation therapy in prostate cancer: a critical review focused on phase 3 trials. Eur Urol. 2013;64(5):722–30.
Rice TK, Schork NJ, Rao DC. Methods for handling multiple testing. Adv Genet. 2008;60:293–308.
Engstrom C. Hot flash experience in men with prostate cancer: a concept analysis. Oncol Nurs Forum. 2005;32(5):1043–8.
Milivojevic V, Kranzler HR, Gelernter J, Burian L, Covault J. Variation in genes encoding the neuroactive steroid synthetic enzymes 5alpha-reductase type 1 and 3alpha-reductase type 2 is associated with alcohol dependence. Alcohol Clin Exp Res. 2011;35(5):946–52.
Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68(8):1515–25.
Denham JW, Wilcox C, Joseph D, Spry NA, Lamb DS, Tai KH, Matthews J, Atkinson C, Turner S, Christie D, et al. Quality of life in men with locally advanced prostate cancer treated with leuprorelin and radiotherapy with or without zoledronic acid (TROG 03.04 RADAR): secondary endpoints from a randomised phase 3 factorial trial. Lancet Oncol. 2012;13(12):1260–70.
Nguyen PL. Harms versus benefits with duration of androgen suppression. Lancet Oncol. 2012;13(12):1182–3.
Acknowledgements
We are grateful to the European Organisation for Research and Treatment of Cancer for their permission to use the EORTC QLQ30 and PR25 questionnaires and the scoring manuals for our study. We also wish to acknowledge support provided by Dale Robison from the Analytical Services, the Ministry of Health, New Zealand for checking recorded mortality information of patients in our database. Finally, the patient volunteers who made this study possible are sincerely acknowledged.
Funding
The following grantees supported this study by way of salaries for staff and costs related to patient recruitment and laboratory procedures since 2006- the Mad Butcher’s Charitable Trust, New Zealand, the A+ Trust, Auckland District Health Board, Auckland New Zealand (Grant No. 5461-PG-1204-007), the GoodFellow Trust, Urology Department, Auckland Hospital, Auckland, New Zealand (Grant No. +3192), and the Cancer Society New Zealand (Grant No.10/08). We also wish to thank the Auckland Cancer Society, New Zealand for funding the work related to HRQoL survey and laboratory procedures mentioned in this manuscript. We also wish to acknowledge the University of Auckland for providing a Summer Studentship Grant in 2013 that supported our initial work on the HRQoL study.
Availability of data and materials
The raw data supporting this analysis mentioned under ‘Additional files’ can be requested from the corresponding author.
Authors’ contributions
NK carried out literature review; designed the HRQoL study; organised ethical approvals and contacted the Analytical Services of the Ministry of Health NZ to get a list of deceased patients; interpreted the analysed data and wrote this manuscript. LRF and JM were the Principal Investigators for the main Urology study funded by the Cancer Society, NZ. JM was also the Principal Investigator for the Urology study component funded by the A+ Trust Grant, Auckland District Health Board, Auckland, NZ and GoodFellow Trust grant, Urology Department, Auckland Hospital, Auckland, NZ. JM, BB and MH supported with patient recruitment and clinical advice. MG supported with sending invites to eligible patients for the Urology Study, collection of signed consent from patients, patient enrolment, blood sample collection and extracting clinical data from hospital databases. JK supported with producing the HRQoL questionnaire. KL, AW, YZ and SE supported with the genotyping. YZ supported with contacting patient survivors registered with the Urology study for the HRQoL survey; data entry and for the interim data analysis. SZ supported with database management. DYH did the statistical analysis. All authors have reviewed the manuscript and provided suggestions for improvement and approved the final submission.
Competing interests
We declare that none of the authors have any financial and non-financial competing interest with regards to this manuscript.
Consent to publish
Not Applicable.
Ethics approval and consent to participate
This study was conducted under the ethics approval reference NTY/05/06/037, Health and Disability Ethics Committee, the Ministry of Health, New Zealand. Patient recruitment was carried out with written consent from each participant.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
Summary of details related to batch genotyping for the AKR1C3 rs12529 SNP. Description of data-Details provided as a requirement of the Standard Strengthening the Reporting of Genetic Association Studies (STREGA)–An Extension of the STROBE Statement. (DOCX 62 kb)
Additional file 2: Table S2.
Data used in the analysis -Karunasinghe et al. BMC Urology 2016. All parameters used in the analysis except for age data (that has been removed for patient anonymity). (XLSX 52 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Karunasinghe, N., Zhu, Y., Han, D.Y. et al. Quality of life effects of androgen deprivation therapy in a prostate cancer cohort in New Zealand: can we minimize effects using a stratification based on the aldo-keto reductase family 1, member C3 rs12529 gene polymorphism?. BMC Urol 16, 48 (2016). https://doi.org/10.1186/s12894-016-0164-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-016-0164-4