Abstract
Background
Pancreatic ductal carcinoma (PDAC) is an extremely poor prognostic disease. Even though multidisciplinary treatment for PDAC has developed, supportive therapies, such as nutritional therapy or perioperative rehabilitation to sustain and complete aggressive treatment, have not yet been well-established in PDAC. The aim of this study was to elucidate the relationship between the combined index using psoas muscle mass index (PMI) values and controlling nutritional status (CONUT) score and prognosis.
Methods
We included 101 patients diagnosed with PDAC who underwent radical pancreatectomy with regional lymphadenectomy. The cut-off value was set at the first quartile (male, 6.3 cm2/m2; female 4.4 cm2/m2), and patients were classified into high PMI and low PMI groups. A CONUT score of 0 to 1 was classified as the normal nutritional status group, and 2 or more points as the malnutritional status group. Patients were further divided into three groups: high PMI and normal nutrition (good general condition group), low PMI and low nutrition (poor general condition group), and none of the above (moderate general condition group). We performed a prognostic analysis of overall survival (OS), stratified according to PMI values and CONUT scores.
Results
In the poor general condition group, the proportion of elderly people over 70 years of age was significantly higher than that in the other groups (p < 0.001). The poor general condition group had a significantly worse prognosis than the good and moderate general condition groups (p = 0.012 and p = 0.037). The 5-year survival rates were 10.9%, 22.3%, and 36.1% in the poor, moderate, and good general condition groups, respectively. In multivariate analysis, poor general condition, with both low PMI and malnutrition status, was an independent poor prognostic factor for postoperative OS (hazard ratio 2.161, p = 0.031).
Conclusions
The combination of PMI and CONUT scores may be useful for predicting the prognosis of patients with PDAC after radical surgery.
Similar content being viewed by others
Background
Pancreatic ductal carcinoma (PDAC) is the fourth leading cause of cancer-related deaths worldwide [1]. The 5-year survival rate is approximately 6–10% for all patients with PDAC, and even after radical resection, it is approximately 20%, making it an extremely poor prognostic disease [2]. Although multidisciplinary treatment for PDAC, including advanced surgical techniques, adjuvant chemotherapy, and neoadjuvant therapy, has made great strides in improving prognosis [3], supportive therapies, such as nutritional therapy or perioperative rehabilitation to sustain and complete aggressive treatment, have not yet been well-established.
Clinical outcomes of PDAC not only depend on tumor biology and treatment response but are also strongly influenced by the nutrition and performance status of the patients [4]. Body composition is considered important for predicting survival outcomes. Skeletal muscle wasting (i.e., sarcopenia) contributes to poor prognosis in patients with various cancers, such as colorectal, esophageal, and prostate cancer [5,6,7]. Additionally, a meta-analysis reported that sarcopenia is found in 38.7% of patients with PDAC and is an independent prognostic factor in multivariate analysis [8]. The early recognition and objective assessment of nutritional problems are key for PDAC management [9]. The Controlling Nutritional Status (CONUT) score as a tool to evaluate patient nutritional status has been used to predict the prognosis of patients with PDAC [10] and is an effective prognostic factor in patients with PDAC [11].
Although many studies have examined the association between sarcopenia or CONUT score and prognosis, few have examined the relationship between sarcopenia and CONUT score and prognosis. Thus, the aim of this study was to investigate the association between an index defined as combined sarcopenia (specifically, psoas muscle mass index [PMI]) and CONUT score and prognosis in patients with PDAC.
Methods
Patients
We retrospectively analyzed the medical records of 101 patients diagnosed with pancreatic cancer who underwent radical pancreatectomy with regional lymphadenectomy at the Department of Surgery, Obihiro Kosei General Hospital, between January 2009 and April 2021. Patients with intraductal papillary mucinous adenocarcinoma, those who underwent only exploratory laparotomy due to peritoneal dissemination, and those who underwent palliative surgery such as gastrointestinal bypass surgery or choledochojejunostomy were excluded from this study. Clinicopathological data were collected from patient records. All tumors were staged according to the 8th TNM classification system of the Union for International Cancer Control [12]. The present study was approved by the Institutional Review Board of Obihiro Kosei General Hospital (authorization number: 2021-067), and was carried out in compliance with the Helsinki Declaration. The opt-out recruitment method was applied to all patients, with providing an opportunity to decline to take part in the study. Informed consent was obtained from all the patients.
Measurement and assessment of psoas muscle mass index
We measured the cross-sectional areas of the bilateral psoas muscles at the umbilical level using preoperative computed tomography (CT) images by the manual tracing method, and PMI was calculated as previously reported [13]. Cut-off values for PMI were established using the quartile method.
Assessment of nutritional status
To assess preoperative nutritional status, we used the CONUT score, calculated from serum albumin, total lymphocyte count, and total cholesterol [14]. In the present study, we defined CONUT score > 2 as the “malnutritional” group and CONUT score < 2 as the “normal” group (Table 1).
Statistical analysis
Differences between the groups were analyzed using the Mann-Whitney U-test. Categorical variables were compared using Fisher’s exact test or χ2 -test. OS was defined as the time from surgery to death due to any cause. The proportion of OS was calculated using the Kaplan-Meier method. Comparisons between groups were performed using the log-rank test. Univariate and multivariate analyses were performed using a Cox proportional hazards model. Differences were considered statistically significant at p < 0.05 [15]. Statistical analyses were performed using JMP Pro 17 (SAS Institute Inc., Cary, NC, USA).
Results
Comparison of PMI values by sex
The median PMI was significantly higher for male patients than for female patients (7.2 cm2/m2 [range, 3.4–10.7 cm2/m2] and 4.9 cm2/m2 [range, 2.3-8.0 cm2/m2], respectively; p < 0.001). The first quartile, 6.3 cm2/m2 for male and 4.4 cm2/m2 for female patients, was set as the cut-off value (Fig. 1). The patients were then classified into high and low PMI groups based on the cut-off values.
Evaluation of nutritional status according to CONUT score
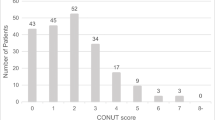
According to the assessment of nutritional status using the CONUT score, 47 patients (46.5%) were classified into the “normal” group and 54 patients (53.5%) were classified into the “malnutrition” group.
Prognostic analysis
We performed a prognostic analysis of OS, stratified according to PMI values and CONUT scores. The high PMI group had better prognosis than the low PMI group; however, the difference was not significant (p = 0.1160). Similarly, the normal nutritional status group had a better prognosis than the malnutrition status group, but the difference was not significant (p = 0.1129) (Fig. 2).
Based on these results, we stratified the patients into three groups based on the combined PMI values and nutritional status based on the CONUT score. Patients with both low PMI and in the malnutritional group were defined as in “poor” general condition (n = 16), those with high PMI or in the normal nutritional group (but not both) were defined as in “moderate” general condition (n = 46), and those with both high PMI and normal nutritional status were defined as in “good” general condition (n = 39). The relationships between the clinicopathological characteristics and each of the three groups are summarized in Table 2. In the poor general condition group, the proportion of elderly people over 70 years of age was significantly higher than that in the other groups (p < 0.001). There were no significant differences in sex; tumor location; presence or absence of portal vein resection; pathological T, N, and M factors with or without adjuvant chemotherapy; or postoperative complications.
The median follow-up period was 733 days. Figure 3 shows the Kaplan-Meier curves for the patients’ general condition based on the combination of PMI values and CONUT scores. The poor general condition group had a significantly worse prognosis than the moderate general condition group (p = 0.037) and, thus, the good general condition group (p = 0.012). The 5-year survival rates were 10.9%, 22.3%, and 36.1% in the poor, moderate, and good general condition groups, respectively. Univariate and multivariate analyses were performed by adding the patients’ general condition to the clinicopathological factors. In multivariate analysis, poor general condition (hazard ratio [HR]: 2.161, 95% confidence interval [CI]: 1.071–4.359, p = 0.031), with both low PMI and malnutrition status, was determined to be an independent prognostic factor of a poor outcome (Table 3).
Discussion
The purpose of the present study was to investigate the relationship between the combined index, according to the PMI and CONUT scores, and prognosis in patients with PDAC. We demonstrated that patients with a poor general condition, defined as both decreased skeletal muscle (i.e., low PMI) and malnutrition (i.e., CONUT score > 2), had a significantly worse prognosis than other patients, and this was found to be an independent unfavorable prognostic factor in multivariate analysis.
Sarcopenia is defined as a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, with a risk of adverse outcomes such as physical disability, poor quality of life, and death. It is recommended that the definition of sarcopenia should include not only low muscle mass but also low muscle function [16]; however, most previous studies have investigated only skeletal muscle mass to define sarcopenia. Because we investigated only PMI in this study, we did not use the term sarcopenia but instead expressed it as decreased skeletal muscle. Based on the recent consensus of the Asian Working Group for Sarcopenia, CT imaging at the level of the third lumbar vertebra is an effective imaging modality for the clinical detection of sarcopenia [17], but we measured the cross-sectional area of the bilateral psoas muscles at the umbilical level using preoperative CT imaging and calculated the PMI following a previous report [13]. Interestingly, the cut-off values of the PMI obtained from this study (male, 6.3 cm2/m2; female, 4.4 cm2/m2) were approximately the same as those used in the sarcopenia criteria proposed by the Japan Society of Hepatology (male, 6.36 cm2/m2; female, 3.92 cm2/m2) [18]. Several studies have shown that the low PMI group has a significantly worse prognosis and is an independent prognostic factor after curative surgery, previously [13, 19]. Measurement of the psoas muscle area is a simple and convenient method, and PMI values may be useful for predicting the postoperative prognosis of patients with PDAC.
Malnutrition is prevalent in patients with cancer and can affect short- and long-term outcomes. Nutritional status has a prognostic capacity for post-treatment long-term outcomes, including disease progression and survival [20]. The CONUT score is a newly proposed index for objectively assessing patients’ nutritional status [14], which has prognostic value with respect to poor survival in patients with cancer [9,10,11, 21]. The CONUT score was calculated using serum albumin, total lymphocyte count, and total cholesterol values. The predictive effect of the CONUT score can be attributed to several factors. First, serum albumin has long been regarded as an important marker of nutritional status, and low albumin levels are associated with advanced cachexia and perioperative outcomes [22]. Second, total lymphocyte count is an immunological indicator. The decrease in lymphocyte and primary T lymphocyte levels indicates an inadequate immune response against a tumor [23]. Third, cholesterol is essential for the maintenance of cell membrane function, which is crucial for signal transduction. Decreased cholesterol levels can affect the antitumor activity of immunocompetent cells [24]. These findings suggest that the CONUT score has higher prognostic accuracy and is superior in predicting survival in different types of cancer [21].
Despite the availability of the PMI or CONUT score reported previously, this study did not find the PMI or CONUT score alone to be effective in predicting prognosis. This may have been owing to the small sample size. Therefore, we devised a new evaluation method, a combination of PMI values and CONUT scores, and proposed it as a prognostic indicator for the first time. We revealed that patients with poor general condition, defined as both low PMI and malnutrition, expressed as a CONUT score > 2, had significantly worse prognosis, and was found to be an independent prognostic indicator in PDAC following radical surgery. A combined assessment of decreased skeletal muscle mass and nutritional impairment may accurately reflect a patient’s general condition. Indeed, in patients with PDAC, poor oral nutritional intake, catabolism due to malignancy, and reduced intestinal absorption due to obstruction can synergistically affect the nutritional status and lead to malnutrition and loss of muscle mass [25]. Pancreatic exocrine insufficiency also contributes to malnutrition and weight loss. Pancreatic enzymes are essential for the degradation and absorption of fat and liposoluble vitamins; thus, pancreatic enzyme deficiency results in steatorrhea and severe maldigestion [26]. As respects the relationship between a combined index of skeletal muscle mass and malnutrition and patients’ prognosis, there was another report that the combination of the geriatric nutritional risk index (GNRI) and psoas muscle volume (PMV) might be useful to predict prognosis in older patients with pancreatic cancer. Previously, it has demonstrated that patients with low GNRI and low PMV had the worst pancreatic cancer prognosis [27]. Our findings strongly suggest that poor nutritional status and decreased skeletal muscle mass negatively affect long-term clinical outcomes after pancreatic surgery. Prehabilitation regimens based on exercise such as aerobic and resistant activity and nutritional support focused on maximizing energy and protein intake should be required for these patients to improve their prognosis.
This study has several limitations. First, this was a single-institute, retrospective cohort study with a small population of Asian patients. This might have resulted in a selection bias that limited the generalizability of our findings to patients worldwide. Second, the PMI values of each patient were measured using a manual tracing method, which led to measurement errors by the examiner. To avoid measurement errors, the use of more objective instruments such as bioelectrical impedance analysis or dual-energy X-ray absorptiometry to measure skeletal muscle mass is necessary. Third, the cut-off value of PMI obtained from this study was originally defined using the quartile method without consensus. For universalization, cut-off values based on international standards should be used. Numerous multi-institutional prospective studies involving various ethnicities are necessary to confirm our findings.
Conclusions
In conclusion, we described, for the first time, the usefulness of the combination of the PMI and CONUT scores in predicting the prognosis of patients with PDAC after radical surgery. Preoperative management, including rehabilitation and nutritional support, might impact patients with a poor general condition and contribute to improving their prognosis.
Data availability
The datasets used and/or analysis during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemel A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Mizrahi JD, Surana R, Valle JW, Shoff R. Pancreatic cancer. Lancet. 2020;395:2008–20.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Choi MH, Yoon SB. Sarcopenia in pancreatic cancer: Effect on patient outcomes. World J Gastrointest Oncol. 2022;14:2302–12.
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, et al. Can Sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1419–27.
Deng HY, Zha P, Peng L, Hou L, Huang KL, Li XY. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta-analysis. Dis Esophagus. 2019; 32.
Meyer HJ, Wienke A, Surov A. CT-defined low-skeletal muscle mass as a prognostic marker for survival in prostate cancer: a systematic review and meta-analysis. Urol Oncol. 2022;40:e9–10316.
Thormann M, Hinnerichs M, Ordonez FB, Saalfeld S, Perrakis M, Croner R et al. Sarcopenia is an independent prognostic factor in patients with pancreatic cancer- a meta-analysis. Acad Radiol. 2022; 1–10.
Mao YS, Hao SJ, Zou CF, Xie ZB, Fu DL. Controlling nutritional score is superior to prognostic nutritional index score in predicting survival and complication in pancreatic ductal adenocarcinoma: a Chinese propensity score matching study. Br J Nutr. 2020;124:1190–97.
Kato Y, Yamada S, Suenaga M, Takami H, Niwa Y, Hayashi M, et al. Impact of the controlling nutritional status score on the prognosis after curative resection of pancreatic ductal adenocarcinoma. Pancreas. 2018;47:823–9.
Ma X, Zou W, Sun Y. Prognostic value of pretreatment controlling nutritional score for patients with pancreatic cancer: a meta-analysis. Front Oncol. 2022;11:1–8.
Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Hoboken: Wiley; 2017.
Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088–98.
Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45.
Kuwabara S, Tsuchikawa T, Nakamura T, Hatanaka Y, Hatanaka CK, Sasaki K, et al. Prognostic relevance of tertiary lymphoid organs following neoadjuvant chemotherapy in pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110(6):1853–62.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39:412–23.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeuing TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101.
Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016; 46: 951–63.
Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of Sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrrointest Surg. 2012;16:1478–86.
Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69:1151–76.
Kheirouri S, Alizadeh M. Prognostic potential of the preoperative controlling nutritional status (CONUT) score in predicting survival of patients with cancer: a systematic review. Adv Nutr. 2021;12:234–50.
Delitto D, Judge SM, George TJ Jr, Sarosi GA, Thomas RM, Behrns K, et al. A clinically applicable muscular index predicts long-term survival in resectable pancreatic cancer. Surgery. 2017;161:930–8.
Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–8.
Chimento A, Casaburi I, Avena P, Trotta F, Luca AD, Rago V, et al. Cholesterol and its metabolites in tumor growth: therapeutic potential of statins in cancer treatment. Front Endocrinol (Lausanne). 2018;9:807.
Rovesti G, Varoriani F, Rimini M, Bardasi C, Ballarin R, Benedetto FD et al. Clinical implications of Malnutrition in the management of patients with pancreatic cancer: introducing the concept of the Nutritional Oncology Board. Nutrients. 2021; 13.
Vujasinovic M, Valente R, Del Chiaro M, Permert J, Lohr JM. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients. 2017; 9.
Sakamoto T, Yagyu T, Uchinaka E, Miyatani Kozo, Hanaki T, Kihara K, et al. The prognostic significance of combined geriatric nutritional risk index and psoas muscle volume in older patients with pancreatic cancer. BMC Cancer. 2021;21:342.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
The authors have no funding to declare.
Author information
Authors and Affiliations
Contributions
SK, YT, OS, TM, MI, KM, and KO collected data.SK evaluated data, wrote the manuscript, and prepared figures.YA, KI, and SH revised the manuscripts, and SH provided comments on the structure and details of the article.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of Obihiro Kosei General Hospital (authorization number: 2021-067), and was carried out in compliance with the Helsinki Declaration. The opt-out recruitment method was applied to all patients, with providing an opportunity to decline to take part in the study. Informed consent was obtained from all the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kuwabara, S., Takeuchi, Y., Sato, O. et al. Prognostic value of combined psoas muscle mass and controlling nutritional status in patients with pancreatic ductal adenocarcinoma: a retrospective cohort study. BMC Surg 24, 116 (2024). https://doi.org/10.1186/s12893-024-02395-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-024-02395-2