Abstract
Background
Lung adenocarcinoma is the most common subtype of non-small cell lung cancer. The surgical strategy of lymph node dissection is controversial because many more patients are diagnosed at an early stage in clinical practice.
Methods
We retrospectively reviewed 622 clinical N0 lung adenocarcinoma patients with 3 cm or less in tumor size who underwent lobectomy or segmentectomy combined with lymph node dissection in our hospital from January 2017 to December 2019. We performed univariate and multivariate analyses to identify preoperative risk factors of lymph node metastasis.
Results
Lymph node metastasis was found in 60 out of 622 patients. On univariate analysis, lymph node metastasis was linked to smoking history, preoperative CEA level, tumor size, tumor location (peripheral or central), consolidation/tumor ratio, pleural invasion, and pathologic type. However, only the preoperative CEA level, tumor size, and consolidation/tumor ratio were independent risk factors in multivariate analysis. The ROC curve showed that the cutoff value of tumor size was 1.7 cm. There was no lymph node metastasis in patients without risk factors.
Conclusions
The preoperative CEA level, tumor size, and consolidation/tumor ratio were independent risk factors of lymph node metastasis in clinical N0 lung adenocarcinoma with tumor size ≤ 3 cm. The lymph node metastasis rate was extremely low in clinical N0 lung adenocarcinoma patients without risk factors and lymph node dissection should be avoided in these patients to reduce surgical trauma.
Similar content being viewed by others
Background
Lung cancer is a common disease with high mortality rate all over the world. According to statistics, lung cancer accounts for 11.4% of the worldwide new cases of cancer and 18% of cancer deaths [1]. Non-small cell lung cancer is the most common type, in which squamous cell carcinoma and adenocarcinoma account for the vast majority. Nowadays, lung adenocarcinoma has become the most common type of non-small cell lung cancer surpassing squamous cell carcinoma.
Lymph node metastasis is an important site for metastasis of lung cancer and directly affects the patient survival. The accurate N stage is important in guiding postoperative treatment and predicting postoperative survival. The standard surgical treatment for lung cancer used to be lobectomy and systematic lymph node dissection. However, with the wide application of high resolution computer tomography (HRCT), many early stage lung cancers have been diagnosed, with ground-grass lesion as the main feature. According to the literature, lung cancer with pure ground grass opacity (GGO) does not present with lymph node metastasis, and the incidence of lymph node metastasis was low in GGO-dominant lung cancer [2, 3]. Systematic lymph node dissection is actually unnecessary and only increases surgical trauma in patients with early stage lung cancer. However, it is hard to know the accurate stage of lymph node by conventional examinations before operation. Liberman et al. reported a sensitivity and specificity of 12.8% and 99.6%, respectively for the diagnosis of lymph node metastasis on plain CT, while the sensitivity and specificity was 22.1% and 90.7%, respectively on contrast CT. The sensitivity and specificity was 39.7% and 80.3%, respectively using positron emission tomography/computed tomography (PET/CT) in the diagnosis of lymph node metastasis [4].
From literature several risk factors are related to lymph node metastasis such as age [5], tumor size [6, 7], carcinoembryonic antigen (CEA) level [8], consolidation/tumor ratio (CTR) [9], tumor location [10], poor differentiation [11], pleural invasion [12], lymphatic vascular invasion [13], micropapillary component [14], and tumor spread through air spaces [15]. However, characteristics such as the micropapillary component, lymphatic vascular invasion, degree of differentiation, and tumor spread through air spaces were often unconfirmed until one week after surgery in paraffin-embedded pathology specimens. Surgeons can only obtain the results of preoperative examinations, intraoperative exploration, and fast frozen pathology during surgery before lymph node dissection. Fast frozen pathology during surgery in our hospital can only reveal the type of lung cancer (e.g. squamous cell carcinoma or adenocarcinoma) and also the invasive degree of the tumor (adenocarcinoma in situ, micro invasive and invasive adenocarcinoma). Lepidic-predominant adenocarcinoma can also be diagnosed by fast frozen pathology during surgery in our hospital.
Presently, the necessity of lymph node dissection of clinical early stage lung cancer remains controversial and lacks a consensus in clinical practice [16, 17]. We analyzed the preoperative characteristics of lung adenocarcinoma combined with tumor invasive degree which can be revealed by fast frozen pathology during surgery, and we tried to find the risk factors of lymph node metastasis of lung adenocarcinoma and provide reference data for clinical practice.
Methods
Patient selection
We retrospectively reviewed the clinical data of patients operated for lung cancer in our hospital from January 2017 to December 2019. We included (1) All patients should receive conventional preoperative examinations to exclude distant metastasis. High resolution CT should be performed one month before surgery; (2) The pathology was confirmed as lung adenocarcinoma; (3) The clinical N stage before operation should be N0 and the maximum tumor diameter on CT image should be 3 cm or less; and (4) Lobectomy or segmentectomy combined with lymph node dissection was performed. The number of patients received segmentectomy was increasing in recent years in our center. At present, surgeons in our hospital tend to perform sublobar resection instead of lobectomy for clinical early stage patients with GGO-dominant lension and tumor size ≤ 2 cm. The surgical margin should not be less than 2 cm or the maximum diameter of the tumor. Patients with poor pulmonary function who cannot bear lobectomy also receive sublobar resection. Lymph node dissection should include at least three N1 stations (always contain stations 10, 11 and 12) and at least three N2 stations (always contain subcarinal station) in accordance with the criteria raised by the European Society of Thoracic Surgeons [18]. Lobe specific lymph node stations should also be included. We excluded: (1) patients who had received neoadjuvant therapy before surgery (chemotherapy, immunotherapy or targeted therapy); (2) patients with multiple malignant lesions; (3) carcinoma in situ; (4) parietal pleura or pericardium invaded; (5) more than one lobe invaded; (6) history of any other malignant tumors; and (7) lung adenocarcinoma associated with cystic airspaces which was hard to measure the exact GGO or solid component. We included 622 patients in the study.
Data collection
Patient information was collected from the electronic database in our hospital. We collected the clinical data such as gender, age, smoking history, preoperative CEA level, surgery type, tumor location, pathologic characteristic, tumor size, consolidation/tumor ratio, and lymph node metastasis for analysis. Consolidation/tumor ratio was calculated as the proportion of the maximum consolidation diameter divided by the maximum tumor diameter. The invasive degree of the tumor was divided into adenocarcinoma in situ, micro invasive, and invasive adenocarcinoma. In our study, invasive adenocarcinoma was further divided into lepidic-predominant adenocarcinoma and other types of invasive adenocarcinoma. Clinical stage N0 was defined as no hilar or mediastinal lymphadenopathy. Lymphadenopathy was defined as the shortest axis of lymph node ≥ 1 cm. The lung cancer staging was according to the 8th edition of TNM classification issued by International Union Against Cancer (UICC).
Statistical analysis
Continuous variables were expressed as mean ± SD, and compared by Student’s t test. Categorical variables were compared by Chi-squared test or Fisher exact test. Multivariate analysis by logistic regression was done using variables with statistical significance in univariate analysis. The cutoff value of tumor size was determined by the receiver operating characteristic (ROC) curve. P values less than 0.05 were considered statistically significant. Statistical analysis was performed by the SPSS software (version 19, IBM, USA). This research was permitted by the ethical committee of the first affiliated hospital of Zhejiang University (Hangzhou, China).
Results
In the study, we included 221 men and 401 women. The median age was 61 years (range from 26 to 85 years), 74 patients benefitted from segmentectomy while 548 patients from lobectomy. Most patients had tumors located at the right upper lobe (202 patients) while 33 patients had tumors located at the right middle lobe, which was the least. Sixty patients presented with lymph node metastasis (28 patients at N1 stage and 32 patients at N2 stage). No lymph node metastasis was found in the pure GGO group. In the GGO-predominant group, 1 patient had N1 lymph node metastasis. The general characteristics of the patients were listed in Table 1.
Univariate analysis showed that the smoking history, preoperative CEA level, tumor size, tumor location (peripheral or central), consolidation/tumor ratio, pleural invasion and pathologic type were related to lymph node metastasis. Gender, age, and lobe distribution of the tumor did not correlate with lymph node metastasis (Table 2). Preoperative CEA level, tumor size, and consolidation/tumor ratio were found to be independent risk factors in multivariate analysis (Table 3). ROC curve was used to determine the cutoff value of tumor size to predict lymph node metastasis (Fig. 1). The result showed that the cutoff value of tumor size was 1.7 cm and the area under curve (AUC) were 0.74 (p < 0.001, 95% CI 0.68–0.80).
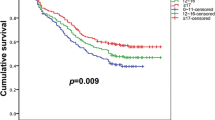
According to the result of multivariate analysis, patients were divided into risk factors positive (R+) and risk factors negative (R−) groups. The R− group was patients with preoperative CEA levels ≤ 5 ng/ml, CTR ≤ 0.5, and tumor size less than 1.7 cm. We included 185 patients in the R− group, and no lymph node metastasis was found. We classified 427 patients into the R+ group and 10 patients were excluded due to lack of CEA level. We found 60 patients with lymph node metastasis in R+ group, 28 patients were staged as N1 while 32 patients were staged as N2. The lymph node metastasis rate was 14% in the R+ group (Fig. 2).
Discussion
Our research showed that the preoperative CEA level, tumor size, and consolidation/tumor ratio were independent risk factors of lymph node metastasis in lung adenocarcinoma at clinical N0 stage with tumor size ≤ 3 cm, which was in accordance with previous reports. Koike et al. reviewed 894 patients with peripheral clinical stage IA non-small cell lung cancer and reported that the preoperative serum CEA level, tumor size and consolidation/tumor ratio were risk factors. They found patients with preoperative CEA level more than 5 ng/ml had higher rate of lymph node metastasis. The cutoff point of preoperative serum CEA level was 3.5 ng/ml [5]. Simon et al. performed a meta-analysis which included 12 studies with 4666 patients who were clinical stage I involved. They found that higher preoperative CEA level was associated with higher rate of occult lymph node metastasis [8]. Chen et al. retrospectively reviewed 10,885 non-small cell lung cancer patients staged as clinical T1. They divided patients into three groups according to the tumor size (> 0 and ≤ 1 cm; > 1 and ≤ 2 cm; > 2 and ≤ 3 cm). They found that with the increase of tumor size, the rate of lymph node metastasis also increased [19]. In our research, we found that the best cutoff value of tumor size was 1.7 cm. The preoperative CEA level was classified into normal group (≤ 5 ng/ml) or abnormal group (> 5 ng/ml). Consolidation/tumor ratio was also divided into two groups according to the proportion of GGO component (CTR ≤ 0.5 and CTR > 0.5). Preoperative CEA > 5 ng/ml, CTR > 0.5, and tumor size ≥ 1.7 cm were independent risk factors of lymph node metastasis according to the result of multivariate analysis.
The tumor invasive degree was an important pathologic characteristic and is related with the stage of lung cancer. Wang et al. retrospectively analyzed the clinical data of 327 patients with clinical stage IA peripheral lung cancer and the pathologic type was divided as adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IA). Patients in the AIS and MIA group had no lymph node metastasis, and 26 (10.6%) patients had positive lymph nodes in IA group. Tumor invasive degree was found to be an independent risk factor of lymph node metastasis [20]. In our study, we excluded patients with adenocarcinoma in situ because it was impossible for patients in this group to have lymph node metastasis. Lepidic-predominant adenocarcinoma (LPA) was reported to have lower tumor metastasis and better survival rate than other types of invasive adenocarcinoma, such as acinar predominant adenocarcinoma (APA), papillary predominant adenocarcinoma (PPA), micropapillary predominant adenocarcinoma (MPA) and solid predominant adenocarcinoma (SPA) [21]. We divided patients with invasive adenocarcinoma into LPA group and other types of invasive adenocarcinoma. In our study, patients in the MIA and LPA group had no lymph node metastasis and the lymph node metastasis rate was significantly lower than for other types of invasive adenocarcinomas. However, the tumor invasive degree was not an independent risk factor in multivariate metastasis. This may be because the tumor invasive degree correlated with consolidation/tumor ratio. In pure GGO and GGO-predominant group (CTR ≤ 0.5), 79 (25.6%) patients had LPA and 33 (10.7%) patients had MIA, while in solid predominant and solid group (CTR > 0.5), 10 (3.2%) patients were LPA and only 1 (0.3%) patient with MIA, which was significantly less than the former group (p < 0.001). The tumor invasive degree was not found to be an independent risk factor in multivariate analysis when analyzed together with the consolidation/tumor ratio.
Occult lymph node metastasis is not rare in clinical N0 lung cancer [14, 22]. The preoperative radiologic examinations were not reliable in predicting lymph node metastasis according to the research reported by Liberman et al. [4]. However, most patients with clinical N0 stage do have no lymph node metastasis. Wang et al. reported that 7.95% of the clinical stage IA patients had lymph node metastasis [20]. In our study, only 60 of 622 patients had lymph node metastasis and the lymph node metastasis rate was 9.6% in patients with clinical N0 stage. Lymph node dissection was unessential in many patients with clinical early stage for the low lymph node metastasis rate. According to the result of multivariate analysis, we further divided patients into risk factors positive (R+) group and risk factors negative (R−) group. Patients with preoperative CEA level ≤ 5 ng/ml, CTR ≤ 0.5, and tumor size less than 1.7 cm were classified into R− group. There were 185 patients in the R− group and no lymph node metastasis was found. 60 out of 427(14.1%) patients were found to have lymph node metastasis in R+ group. According to the results, we considered that the lymph node metastasis rate was extremely low in clinical N0 lung adenocarcinoma patients with preoperative CEA level ≤ 5 ng/ml, CTR ≤ 0.5 and tumor size less than 1.7 cm. Lymph node dissection was unessential for these patients and should be avoided to reduce surgical trauma because lymph node dissection prolonged operative time, increased blood loss and might lead to postoperative complications such as lymphatic leakage.
This study had some limitations. First, it was a retrospective study and conducted in a single center. Second, PET-CT was performed in only a small number of patients for its high cost. If further multicenter randomized trial including routine PET-CT examination can be conducted, the results will be more convinced.
Conclusions
Our study demonstrated that preoperative CEA level, tumor size and consolidation/tumor ratio were independent risk factors of lymph node metastasis in clinical N0 lung adenocarcinoma with tumor size ≤ 3 cm. Lymph node metastasis rate was extremely low in clinical N0 lung adenocarcinoma patients without risk factors and lymph node dissection should be avoided in these patients to reduce surgical trauma.
Availability of data and materials
The clinical data can be achieved in the electronic medical record system in the first affiliated hospital of Zhejiang university.
Abbreviations
- CT:
-
Computer tomography
- PET/CT:
-
Positron emission tomography/computed tomography
- GGO:
-
Ground grass opacity
- CEA:
-
Carcinoembryonic antigen
- CTR:
-
Consolidation/tumor ratio
- MIA:
-
Micro invasive adenocarcinoma
- LPA:
-
Lepidic-predominant adenocarcinoma
- IA:
-
Invasive adenocarcinoma
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under curve
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, Okada M. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. 2014;145(1):66–71.
Jianlong B, Pinyi Z, Xiaohong W, Su Z, Sainan P, Jinfeng N, Shidong X. Risk factors for lymph node metastasis and surgical scope in patients with cN0 non-small cell lung cancer: a single-center study in China. J Cardiothorac Surg. 2021;16(1):304.
Frechet B, Kazakov J, Thiffault V, Ferraro P, Liberman M. Diagnostic accuracy of mediastinal lymph node staging techniques in the preoperative assessment of nonsmall cell lung cancer patients. J Bronchol Interv Pulmonol. 2018;25(1):17–24.
Koike T, Yamato Y, Yoshiya K, Toyabe S. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol. 2012;7(8):1246–51.
Zhao F, Zhou Y, Ge PF, Huang CJ, Yu Y, Li J, Sun YG, Meng YC, Xu JX, Jiang T, et al. A prediction model for lymph node metastases using pathologic features in patients intraoperatively diagnosed as stage I non-small cell lung cancer. BMC Cancer. 2017;17(1):267.
Zhang YK, Chai ZD, Tan LL, Wang ZY, Chen ZJ, Le HB, Zhu WY. Association of lymph node involvement with the prognosis of pathological T1 invasive non-small cell lung cancer. World J Surg Oncol. 2017;15(1):64.
Nasralla A, Lee J, Dang J, Turner S. Elevated preoperative CEA is associated with subclinical nodal involvement and worse survival in stage I non-small cell lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg. 2020;15(1):318.
Cho JY, Leem CS, Kim Y, Kim ES, Lee SH, Lee YJ, Park JS, Cho YJ, Lee JH, Lee CT, et al. Solid part size is an important predictor of nodal metastasis in lung cancer with a subsolid tumor. BMC Pulm Med. 2018;18(1):151.
Zhang Y, Fu F, Wen Z, Deng L, Wang S, Li Y, Chen H. Segment location and ground glass opacity ratio reliably predict node-negative status in lung cancer. Ann Thorac Surg. 2020;109(4):1061–8.
Chen B, Wang X, Yu X, Xia WJ, Zhao H, Li XF, Liu LX, Liu Y, Hu J, Fu XN, et al. Lymph node metastasis in Chinese patients with clinical T1 non-small cell lung cancer: a multicenter real-world observational study. Thorac Cancer. 2019;10(3):533–42.
Wang Y, Qian F, Hu M, Chen Y, Yang Z, Han B. Clinical significance of visceral pleural and lymphovascular invasion in surgically resected adenosquamous lung cancer. Eur J Cardiothorac Surg. 2020;59(3):617–23.
Liu J, Li J, Lin G, Long Z, Li Q, Liu B. Risk factors of lobar lymph node metastases in non-primary tumoR−bearing lobes among the patients of non-small-cell lung cancer. PLoS ONE. 2020;15(9): e0239281.
Moon Y, Choi SY, Park JK, Lee KY. Risk factors for occult lymph node metastasis in peripheral non-small cell lung cancer with invasive component size 3 cm or less. World J Surg. 2020;44(5):1658–65.
Vaghjiani RG, Takahashi Y, Eguchi T, Lu S, Kameda K, Tano Z, Dozier J, Tan KS, Jones DR, Travis WD, et al. Tumor spread through air spaces is a predictor of occult lymph node metastasis in clinical stage IA lung adenocarcinoma. J Thorac Oncol. 2020;15(5):792–802.
Huang X, Wang J, Chen Q, Jiang J. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9(10): e109979.
Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of pathologic nodal staging quality with survival among patients with non-small cell lung cancer after resection with curative intent. JAMA Oncol. 2018;4(1):80–7.
Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, Zielinski M, Lerut T, Weder W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30(5):787–92.
Chen B, Xia W, Wang Z, Zhao H, Li X, Liu L, Liu Y, Hu J, Fu X, Li Y, et al. Risk analyses of N2 lymph-node metastases in patients with T1 non-small cell lung cancer: a multi-center real-world observational study in China. J Cancer Res Clin Oncol. 2019;145(11):2771–7.
Wang Y, Jing L, Wang G. Risk factors for lymph node metastasis and surgical methods in patients with early-stage peripheral lung adenocarcinoma presenting as ground glass opacity. J Cardiothorac Surg. 2020;15(1):121.
Yu W, Zhao Q, Xia C, Dong M, Liu J, Li X, Zhao H, Chen G, Liu H, Chen J. Validation of stage groupings in the eighth edition of the tumor node metastasis classification for lung adenocarcinoma. Thorac Cancer. 2019;10(3):483–91.
He XQ, Luo TY, Li X, Huo JW, Gong JW, Li Q. Clinicopathological and computed tomographic features associated with occult lymph node metastasis in patients with peripheral solid non-small cell lung cancer. Eur J Radiol. 2021;144: 109981.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
CF collected the clinical data. CF and YX performed statistical analysis. CF conducted a literature search and drafted this manuscript. WH revised the manuscript. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study followed the ethical standards of the World Medical Association Declaration of Helsinki and all methods were performed in accordance with relevant guidelines and regulations. This was a retrospective study, informed consent was waived (approved by Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University).
Consent for publication
Not applicable.
Competing interests
We declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fang, C., Xiang, Y. & Han, W. Preoperative risk factors of lymph node metastasis in clinical N0 lung adenocarcinoma of 3 cm or less in diameter. BMC Surg 22, 153 (2022). https://doi.org/10.1186/s12893-022-01605-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01605-z