Abstract
Background
Patients with giant ovarian tumor often have severe symptoms, such as abdominal distention, and the tumor tends to grow rapidly; therefore, sufficient preoperative assessments are difficult to perform. It is not always easy to differentiate between primary and metastatic ovarian cancer, especially when the ovarian tumor is huge, since a precise diagnosis of ovarian tumor depends on the histopathological findings of the excised specimen. Although metastatic ovarian tumors account for over 20% of all malignant ovarian tumors, preoperative colonoscopy is not considered a routine examination before surgery for giant ovarian tumor.
Case presentation
We herein report 3 cases of giant (> 25 cm) ovarian tumor with colorectal cancer. All three patients visited the clinic with progressing abdominal distention, and were referred with primary ovarian malignancy. Case 1: Rectal tumor was suspected by a digital examination at the outpatient clinic, and rectal cancer was diagnosed preoperatively by colonoscopy. Computed tomography revealed a single-nodule liver tumor. Ovariectomy, rectal resection, and partial hepatectomy were performed. A histological examination revealed both primary mucinous ovarian carcinoma and rectal carcinoma with liver metastasis. Case 2: Initially, the ovarian tumor was diagnosed as primary carcinoma based on the histological findings of an incision biopsy at the previous hospital. Chemotherapy for ovarian cancer was administered without remission, and subsequently, the patient was referred to our hospital. Since the CEA level was high (142 ng/ml), colonoscopy was performed and cecal cancer was diagnosed. Ovariectomy and right colectomy were performed, and the ovarian tumor was histologically diagnosed as metastatic adenocarcinoma. Case 3: Initial ovariectomy was performed, and rectal cancer was suspected at intra-operative surveillance. Colonoscopy was performed after surgery, and rectal cancer was diagnosed. The ovarian tumor was diagnosed as metastatic adenocarcinoma. After six cycles of FOLFOX, rectal resection was performed.
Conclusion
Regrettably, two of three cases in the current series were not diagnosed with colorectal cancer at the start of treatment. This experience suggests that screening colonoscopy should be considered before treatment for every case of giant ovarian tumor.
Similar content being viewed by others
Background
Giant ovarian tumor includes not only primary ovarian cancer but also metastatic malignancy, such as that originating from colorectal cancer. Metastatic ovarian tumors account for over 20–30% of all malignant ovarian tumors [1, 2]. In recent reports, colorectal cancer accounted for 65% of ovarian metastases, with an increased percentage of cases in recent years [3]. Metastatic colorectal cancer may have the same presentation as advanced ovarian cancer, including pelvic mass and ascites [4]. A preoperative diagnosis between primary ovarian cancer and metastatic tumor is important to determine treatment strategies, but often challenging and difficult [5]. Despite advancements in imaging modalities, including computed tomography (CT), positron emission tomography (PET), ultrasonography (US), and magnetic resonance imaging (MRI), the final diagnosis of ovarian malignancy depends on a histological examination, particularly the findings of immunohistochemical staining [6].
Colonoscopy is the most sensitive examination for evaluating the presence of colorectal malignancy; however, screening colonoscopy is not considered a required preoperative investigation for primary ovarian cancer [5, 7]. In actual clinical practice, patients with metastatic ovarian cancer originating from colorectal cancer often undergo surgery based on a misdiagnosis of primary ovarian malignancy.
We herein report three cases of giant ovarian carcinoma with colorectal carcinoma, two of which were metastatic ovarian carcinoma and the other was primary ovarian cancer.
Case presentation
Below are described three cases of giant (> 25 cm) ovarian tumor with colorectal cancer. All three patients visited the clinic with progressing abdominal distention, and were referred with primary ovarian malignancy. The detailed profiles of the patients are described in Table 1.
Case 1
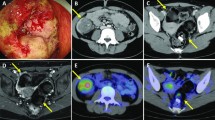
A 53-year-old woman was referred to the gynecology department with a 2-month history of increasing abdominal distention. Enhanced CT showed a cystic ovarian mass with an irregularly shaped solid component measuring 34 × 29 cm (Fig. 1A). CT also revealed a 38 mm single-nodule liver tumor in segment 2 (Fig. 1B). A rectal tumor was suspected based on a digital examination at the outpatient clinic, and lower rectal cancer was diagnosed preoperatively by colonoscopy (Fig. 1C). Ovariectomy, abdominoperineal rectal excision, and partial hepatectomy were performed. A histopathological evaluation of the ovarian tumor showed mucinous adenocarcinoma forming cystic lesions containing mucin (Fig. 1D). Immunohistochemistry staining showed that the ovarian tumor was CK7-positive (Fig. 1E), CK20-negative (Fig. 1F) and CDX2-negative (Fig. 1G). The histopathological findings of the liver tumor showed adenocarcinoma consisting of atypical columnar epithelium with necrosis (Fig. 1H). Immunohistochemistry staining showed that the liver tumor was CK7-negative (Fig. 1I) and CK20-positive (Fig. 1J). Primary mucinous ovarian carcinoma and rectal carcinoma with liver metastasis were diagnosed.
A, B Enhanced CT revealed a cystic ovarian mass with an irregularly shaped solid component measuring 34 × 29 cm and single-nodule liver tumor measuring 3.8 cm in segment 2. C Colonoscopy showed type 2 cancer in the lower rectum. D A histopathological evaluation of the ovarian tumor showed mucinous adenocarcinoma forming a cystic lesion containing mucin (Hematoxylin and eosin (HE) 100×). Immunohistochemistry staining showed that the ovarian tumor was CK7-positive (E), CK20-negative (F), and CDX2-negative. (H) Histopathological findings of the liver tumor showed adenocarcinoma consisting of atypical columnar epithelium with necrosis (HE 200×). (G) Immunohistochemistry staining showed that the liver tumor was CK7-negative (I) and CK20-positive (J)
Case 2
A 58-year-old woman was referred to a hospital with lower abdominal pain. Enhanced CT revealed a cystic ovarian mass with an irregularly shaped septum and solid component measuring 34 × 28 cm (Fig. 2A). Initially, the ovarian tumor was diagnosed as primary carcinoma based on histological findings of a specimen from an incision biopsy. Chemotherapy for ovarian cancer was administered without remission, and subsequently, the patient was referred to our hospital. Since the CEA level was high (142 ng/ml), colonoscopy was performed, and cecal cancer was diagnosed (Fig. 2B). Ovariectomy and right colectomy were performed, and the ovarian tumor was histologically diagnosed as adenocarcinoma consisting of atypical columnar epithelium with severe necrosis (Fig. 2C). Immunohistochemistry staining showed that ovarian tumor was CK7-negative (Fig. 2D), CK20-positive (Fig. 2E), and CDX2-positive (Fig. 2F), suggesting metastasis of cecal cancer.
A Enhanced CT revealed a cystic ovarian mass with an irregularly shaped septum and a solid component measuring 34 × 28 cm. B Colonoscopy showed type 2 cecal cancer. C A histopathological evaluation of the ovarian tumor showed adenocarcinoma consisting of atypical columnar epithelium with severe necrosis (HE 200×). Immunohistochemistry staining showed that the ovarian tumor was CK7-negative (D), CK20-positive (E), and CDX2-positive (F)
Case 3
A 61-year-old woman was referred to our hospital with progressive abdominal distension and severe constipation. Enhanced CT showed 29 × 26 cm polycystic mass with an irregularly shaped septum, fed by the left ovarian artery (Fig. 3A). Ovariectomy was performed, and rectal cancer was suspected at intra-operative surveillance. Colonoscopy was performed after surgery, and rectal cancer was diagnosed (Fig. 3B). A histopathological evaluation of the ovarian tumor showed adenocarcinoma consisting of atypical stratified columnar epithelium with necrosis (Fig. 3C). Immunohistochemistry staining showed that the ovarian tumor was CK7-negative (Fig. 3D), CK20-positive (Fig. 3E), and CDX2-positive (Fig. 3F). The ovarian tumor was diagnosed as metastatic adenocarcinoma. After six cycles of FOLFOX, rectal resection was performed.
A Enhanced CT showed 29 × 26 cm polycystic mass with an irregularly shaped septum, fed by the left ovarian artery. B Colonoscopy after ovariectomy showed type 2 sigmoid colon cancer. C A histopathological evaluation of the ovarian tumor showed adenocarcinoma consisting of atypical stratified columnar epithelium with necrosis (HE 100×). Immunohistochemistry staining showed that the ovarian tumor was CK7-negative (D), CK20-positive (E), and CDX2-positive (F)
Discussion and conclusions
We encountered three cases of giant ovarian tumor with coexisting colorectal cancer. Two of them had metastatic ovarian carcinoma of colorectal origin, and the other had primary ovarian cancer. Regrettably, two of the three cases had not been diagnosed with colorectal cancer at the start of treatment.
Making a preoperative diagnosis of ovarian tumor is often challenging and difficult, since patients with giant ovarian tumor include not only those with primary ovarian carcinoma but also those with metastatic tumor and pathologically benign tumor mimicking malignancy [8,9,10]. Metastatic ovarian tumors have been reported to account for over 20–30% of all malignant ovarian tumors [1, 2]. Colorectal cancer account for 65% of ovarian metastases, with an increasing percentage reported in recent years [1, 3, 11]. Conversely, ovarian metastases occur in 5–10% of women with metastatic colorectal cancer [12]. Most of the giant ovarian tumors reported were more than 25 cm in diameter [6, 9, 10, 13]. A pre-treatment differential diagnosis between primary ovarian cancer and metastatic tumor is more difficult when the ovarian tumor is huge [5], since severe symptoms such as abdominal distention and progressive tumor growth, may hinder further examinations and limit the time for a preoperative assessment. Some radiologists claim that a mixed cystic and solid ovarian mass should be regarded as a metastatic tumor, especially in patients with a history of colorectal cancer [14]; however, other authors insist that depending on radiographic studies is inadequate for differentiating between primary and metastatic ovarian tumors [12]. Presently, a precise diagnosis of ovarian tumor depends on the histopathological findings of excised specimen. An immunohistochemical evaluation is essential for distinguishing between primary and metastatic ovarian carcinoma [6]. Colorectal carcinomas are generally negative for CK7 but positive for CK20 and CDX2, whereas primary ovarian cancers are mostly (> 90%) positive for CK7 and negative for CK20 and CDX2 [11].
Although resection of malignant ovarian tumor can provide a survival benefit for both primary ovarian carcinoma and metastatic ovarian carcinoma originating from colorectal cancer, the operative procedures differ greatly, depending on whether the case is one of primary or metastatic ovarian cancer. Extended surgery, including hysterectomy, omentectomy, and lymph node dissection is needed for primary ovarian cancer surgery [15]. Furthermore, neoadjuvant chemotherapy, is often administered prior to surgery for ovarian cancer [2, 16]. Therefore, pre-treatment detection of colorectal cancer is crucial for deciding on a treatment strategy, so adequate chemotherapy regimens should be chosen depending on the primary tumor [17]. Case 2 in the present study was initially misdiagnosed as primary ovarian carcinoma based on the histological findings of an incision biopsy made at the previous hospital without an immunohistochemical study. Screening colonoscopy was not performed despite the elevated serum CEA level. As a result, neoadjuvant chemotherapy for ovarian cancer was mistakenly administered, without remission. The patient was treated improperly for 6 months before she was referred to our hospital and underwent colonoscopy. These facts indicate that an adequate diagnosis at the start of treatment is essential for achieving the best treatment outcomes.
Regrettably, two of the three cases in the current case series had not been diagnosed with colorectal cancer at the start of treatment. Although colonoscopy is a gold standard in evaluating the presence of colorectal malignancy, screening colonoscopy is not considered required as a preoperative investigation for primary ovarian cancer [5, 7]. As a result, in actual clinical practice, patients with metastatic ovarian cancer originating from colorectal cancer often undergo surgery based on a misdiagnosis of primary ovarian malignancy. Saltzman et al. reported that 5 of 212 (2%) gynecologic oncology patients had been diagnosed with colorectal cancer at pre-treatment screening colonoscopy; however, they concluded that colon screening was not necessary in the preoperative workup of gynecologic oncology patients [7]. Renaud et al. reported that 7% had a primary GI cancer in their case series of 71 ovarian malignancies [3]. Ravizza et al. concluded that colonoscopy identified a not insignificant number of patients requiring colorectal surgery. In their prospective study of 144 consecutive patients with a supposed primary ovarian cancer, 6 (4%) patients were diagnosed with colorectal cancer metastatic to the ovary. Furthermore, 8 (6%) patients were diagnosed with bowel infiltration at screening colonoscopy [18]. Preoperative computed tomography dedicated to examining the bowel may be a viable alternative to colonoscopy, but not completely [8]. Given that colon cancer is by far more frequent than ovarian carcinoma, screening colonoscopy should be considered necessary in every case of giant ovarian tumor before treatment.
This case series demonstrates that screening colonoscopy should be considered routinely before treatment for cases of giant ovarian tumor, and multidisciplinary approach is important in order to make the right diagnosis and offer the best treatment.
Availability of data and materials
All data related are included in the manuscript.
Abbreviations
- CT:
-
Computed tomography
- PET:
-
Positron emission tomography
- US:
-
Ultrasonography
- MRI:
-
Magnetic resonance imaging
- CK7:
-
Cytokeratin 7
- CK20:
-
Cytokeratin 20
- CDX2:
-
Caudal-related homeobox transcription factor 2
- CEA:
-
Carcinoembryonic antigen
- CA19-9:
-
Carbohydrate antigen 19-9
- CA125:
-
Carbohydrate antigen 125
References
Ayhan A, Tuncer ZS, Bükülmez O. Malignant tumors metastatic to the ovaries. J Surg Oncol. 1995;60(4):268–76.
Xu KY, Gao H, Lian ZJ, Ding L, Li M, Gu J. Clinical analysis of Krukenberg tumours in patients with colorectal cancer-a review of 57 cases. World J Surg Oncol. 2017;15(1):25.
Renaud MC, Plante M, Roy M. Metastatic gastrointestinal tract cancer presenting as ovarian carcinoma. J Obstet Gynaecol Can. 2003;25(10):819–24.
Mason MH 3rd, Kovalcik PJ. Ovarian metastases from colon carcinoma. J Surg Oncol. 1981;17(1):33–8.
Lee KC, Lin H, ChangChien CC, Fu HC, Tsai CC, Wu CH, et al. Difficulty in diagnosis and different prognoses between colorectal cancer with ovarian metastasis and advanced ovarian cancer: an empirical study of different surgical adoptions. Taiwan J Obstet Gynecol. 2017;56(1):62–7.
Zogbi L, Isaías A, Machado PA, Neutzling A, Juliano C. Krukenberg’s tumour unilateral giant metachronous of colonic origin—case report. Int J Surg Case Rep. 2017;41:184–7.
Saltzman AK, Carter JR, Fowler JM, Carlson JW, Hartenbach EM, Julian SE, et al. The utility of preoperative screening colonoscopy in gynecologic oncology. Gynecol Oncol. 1995;56(2):181–6.
Raś R, Barnaś E, Magierło JS, Drozdzowska A, Bartosiewicz E, Sobolewski M, et al. Preoperative colonoscopy in patients with a supposed primary ovarian cancer. Medicine (Baltimore). 2019;98(12): e14929.
Akhras LN, Akhras LN, Faroog S, AlSebay L. A 27-kg giant ovarian mucinous cystadenoma in a 72-year-old postmenopausal patient: a case report. Am J Case Rep. 2019;20:1601–6.
Ng ZQ, Pradhan S, Wijesuriya R. Dilemma of the giant abdominal cyst. BMJ Case Rep. 2019;12(1): bcr-2018-227255.
Shimazaki J, Tabuchi T, Nishida K, Takemura A, Motohashi G, Kajiyama H, et al. Synchronous ovarian metastasis from colorectal cancer: a report of two cases. Oncol Lett. 2016;12(1):257–61.
Ganesh K, Shah RH, Vakiani E, Nash GM, Skottowe HP, Yaeger R, et al. Clinical and genetic determinants of ovarian metastases from colorectal cancer. Cancer. 2017;123(7):1134–43.
Kiemtoré S, Zamané H, Sawadogo YA, Sib RS, Komboigo E, Ouédraogo A, et al. Diagnosis and management of a giant ovarian cyst in the gravid-puerperium period: a case report. BMC Pregnancy Childbirth. 2019;19(1):523.
Cho KC, Gold BM. Computed tomography of Krukenberg tumors. AJR Am J Roentgenol. 1985;145(2):285–8.
Gasimli K, Braicu EI, Nassir M, Richter R, Babayeva A, Chekerov R, et al. Lymph node involvement pattern and survival differences of FIGO IIIC and FIGO IIIA1 ovarian cancer patients after primary complete tumor debulking surgery: a 10-year retrospective analysis of the Tumor Bank Ovarian Cancer Network. Ann Surg Oncol. 2016;23(4):1279–86.
Fujiwara A, Noura S, Ohue M, Shingai T, Yamada T, Miyashiro I, et al. Significance of the resection of ovarian metastasis from colorectal cancers. J Surg Oncol. 2010;102(6):582–7.
Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(28):3460–73.
Ravizza D, Fiori G, Trovato C, Maisonneuve P, Bocciolone L, Crosta C. Is colonoscopy a suitable investigation in the preoperative staging of ovarian cancer patients? Dig Liver Dis. 2005;37(1):57–61.
Acknowledgements
We are grateful for the sincere help from all paramedical staffs in Hokkaido Cancer Center.
Funding
No funding was received for this case series.
Author information
Authors and Affiliations
Contributions
YM drafted the manuscript. NM, TK, HS and KY participated in treating the patients. SM, YT, HK and TS participated in the surgery and postoperative management. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Clearance was obtained from Research and Ethics Review Committee, Hokkaido Cancer Center for the publication of this report.
Consent for publication
Written informed consent was obtained from all the patients for publication.
Competing interests
The authors declare that they have no competing interests.
Limitations
This study was a report of only three cases in a single center, therefore, was not able to show the proportion of metastatic or primary cancer among the giant ovarian tumor.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maeda, Y., Minagawa, N., Shoji, H. et al. Giant ovarian tumor with colorectal cancer: suggestion concerning the need for colonoscopy screening in cases with large ovarian tumor—a report of three cases. BMC Surg 22, 111 (2022). https://doi.org/10.1186/s12893-022-01565-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01565-4