Abstract
Background
Retroperitoneal non-pancreatic or idiopathic pseudocysts are very rare lesions. This case report aimed to present our patient and to check all the available literature on this kind of rare disease.
Case presentation
Our patient was a 67-year-old Iranian man admitted with mild abdominal discomfort for three months. Ultrasonography and CT scan revealed a huge cystic structure within the retroperitoneal space. The lesion was excised through midline laparotomy and opening of the retro-peritoneum. The histopathology of the cyst wall revealed a benign cystic lesion with no epithelial lining. A histologic diagnosis of non-neoplastic retroperitoneal pseudocyst was made.
Conclusion
The primary non-pancreatic retroperitoneal pseudocysts are rare lesions and have to be distinguished from other differential diagnoses of retroperitoneal lesions, and a surgeon should be aware of the possible occurrence of these lesions with unknown origin. Surgical excision is the only way to exclude malignancy and confirm the diagnosis.
Similar content being viewed by others
Background
The retro-peritoneum is a space situated behind the parietal peritoneum and in front of the transversalis fascia [1]. The retroperitoneum consists of three parts: the anterior pararenal space, the perirenal space, and the posterior pararenal space [1, 2]. The anterior pararenal space contains pancreas, 2nd to 4th parts of the duodenum, and the ascending and descending colon. The perirenal space contains the kidneys, proximal ureters, adrenal glands, and perirenal fat. The posterior pararenal space contains fat tissue and join inferiorly to the pelvic extraperitoneal space [2].
Most of the retroperitoneal masses originate from the retroperitoneal organs and are not considered as the primary retroperitoneal masses. A primary retroperitoneal mass is diagnosed once the location is inside the retroperitoneal space and after exclusion of the originity from an organ [2]. Primary retroperitoneal masses can be divided into solid and cystic groups and these two groups can be classified as neoplastic and non-neoplastic subgroups. Table 1 shows the differential diagnosis of the primary retroperitoneal masses [2, 3].
Primary retroperitoneal cysts are structures not originating from any retroperitoneal organs and are very rare. They reach large sizes before causing any symptom and are often discovered accidentally [4,5,6]. The exact pathogenesis is unknown, but many possible pathologic mechanisms have been proposed and are divided into urogenital, mesocolic, teratomatous, parasitic, traumatic, and lymphatic types [5]. In the urogenital hypothesis, these tumors are originated from the remnants of the embryonal urogenital system, which include tissues of both epithelial and mesothelial origin [4, 6].
Though vague abdominal pain and distension are present in half of the cases, there are no clinical signs of retroperitoneal cysts in half of the patients, and they are diagnosed accidentally [6]. They may occasionally present with acute abdominal pain if they become hemorrhagic or infected [3,4,5,6]. Diagnosis is made with the use of ultrasonography and computed tomography (CT) scans. The walls of pseudocysts consist of dense fibrous tissues (the mesothelium) or mesonephric tissue with no epithelial lining [3,4,5].
Most retroperitoneal pseudocysts are originating from the pancrease [5]. Pancreatic pseudocyst contain pancreatic fluid, which is a complication of acute pancreatitis [2, 3]. Non-pancreatic pseudocysts have some charactersitics: a thick, fibrous wall or capsule, containing blood, pus, or serous fluid, and the cystic fluid is not associated with high levels of amylase or lipase [3, 6]. The characteristics of non-pancreatic pseudocysts are displayed on CT scans as unilocular or multilocular fluid-filled structures with the thick walls [5].
Non-pancreatic or idiopathic pseudocysts are rare lesions. This case report aimed to present our patient and to check all the available literature on this kind of rare disease.
Case presentation
A 67-year-old Iranian man was admitted to Shahid Beheshti hospital affiliated with Yasuj University of Medical Sciences with mild lower abdominal discomfort (constant, dull abdominal aching) with a three-month duration. His bowel habits were normal, and he had no urgency, hesitancy, weak stream, or burning sensation when urinating. There was no fever, body weight loss, or recent history of trauma, surgery and chronic pancreatitis, or other diseases of the pancreas. Before presenting to the hospital, the patient visited other healthcare facilities and clinics and had received some unspecific oral medications like pantoprazole and hyoscine hydrobromide. His vitals were within normal range. A proper abdominal physical examination including inspection, auscultation, palpation, and percussion was done; the abdomen was soft, non-distended, without a palpable mass. The digital rectal exam was normal.
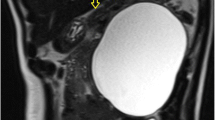
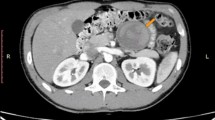
Ultrasonography of the abdomen showed a large retroperitoneal, cystic structure measuring 135 × 88 mm in mid-line position in the level of bifurcation of the aorta, and the urinary bladder was well distended with mildly increased wall thickening (5.5 mm) associated with some trabeculation in the wall without any stone. As the patient had no history of abdominal dysfunction, and there was no suspicion for bowel obstruction, foreign bodies, and urolithiasis, abdominal radiography was not ordered. However, contrast-enhanced CT scan of the abdomen and pelvic revealed a well-defined huge (140 × 120 mm), unilocular, thickened wall cystic structure within the left hemi-pelvic cavity extended from recto-sigmoid junction to the level of upper end of iliac crests and crossing mid-line, as well as with pressure effect over recto-sigmoid, lower rectus muscle, and urinary bladder. The cystic structure showed no solid components or gross internal septations and the content was homogenous. There was no overt connection the cystic structure and the surrounding organs (Fig. 1). The radiologist reported some differential diagnoses for this huge cystic structure as neuro-entric cysts, anterior sacral meningocele, and other cystic structures of the pelvic cavity. Paraclinical and laboratory findings as complete blood count (CBC), plasma concentrations of pancreatitis (amylase, lipase) and neoplasm markers (carcinoembryonic antigen (CEA), alpha fetoprotein (AFP), Cancer antigen (CA) 15–3, CA 19–9, and CA 125), urea, creatinine, bilirubin, aminotransferases, alkaline phosphatase and gamma-glutamyl transferase (GGT) were within normal range.
As this retroperitoneal cystic structure did not cause ureteric obstruction leading to obstructive uropathy, early preoperative ureteric stenting was not indicated according to the preoperational consult to the urologist.
The patient underwent explorative laparotomy, and after opening into the retro-peritoneum, a large thick-walled retroperitoneal cyst, compressing the bladder wall was found (Fig. 2), afterward connections and adhesions of the mentioned structure to the adjacent organs such as recto-sigmoid colon, both ureters, iliac arteries, and the sacrum were released, and the structure was isolated and was removed successfThe mass was incised and contained clear serous fluid (pale yellow). The cytologic findings showed acellular cystic fluid with no malignant or epithelial cells. Cyst fluid culture was negative. One of our differential diagnoses was hydatid cyst which was ruled out by the absence of daughter cysts and normal echinococcal titers.
The histopathology of the cyst wall revealed a benign cystic lesion with no epithelial lining. A histologic diagnosis of non-neoplastic retroperitoneal pseudocyst was made (Fig. 3).
The patient got per os (PO) on the 1st day after the operation, and became pain-free, had passed stool, had acceptable urine output on the second day after the operation, so then was discharged home on day 3 post-operation. The patient was followed up in the clinic with a normal abdominal examination and well-healing wounds after one week, and one month after discharge from the hospital, and there has not been any evidence of recurrence after 3 months of follow-up. He is optimistic about the future and says that this event has helped him to pay more attention to his health afterward.
Discussion
A retroperitoneal non-pancreatic pseudocyst is a rare surgical entity that carries a range of differential diagnoses with the incidence rate of 1 in 5750 to 1 in 250,000 [3]. In this regard, all previously published reports on the cases with idiopathic or primary retroperitoneal pseudocyst were searched and found on the web and only available full-text original articles were met. As a result, thirteen previous cases were elucidated ad after adding our case report to them, a total of fourteen cases were evaluated and summarized in Table 2 according to the originated country, the published year of the case report, patient’s gender, age, chief complaints, physical examinations, ultrasound, CT scan, magnetic resonance imaging (MRI), and other radiological findings (if done), surgery, and pathologic results [3,4,5,6,7,8,9,10,11,12,13,14,15,16].
Of the total 15 reports, 4 reports were for India, and that was amazing. Males were the dominant sex encountered in 10 cases. The mean age was 50.7857 ± 10.977 with a 95% confidence interval. The youngest patient was a 3-year-old girl and the oldest was an 80-year-old woman. Physical examination was not mentioned in one, and in two patients, the abdomen was non-tender, non-distended, and soft to touch, and no definite mass palpated, but in the majority of the cases (10 patients), a palpable mass was detected 9 cases in the abdominal, and one in the rectal examination. Abdominal radiography was used as the only diagnostic modality in one case and showed a grape-fruit-sized mass. Trans-abdominal ultrasound was used in 6 cases and revealed a retroperitoneal cystic/mass lesion in 5 patients and a multi-cystic lesion in one patient (ultrasound was the only diagnostic modality used in this case). Endo-rectal ultrasound was used in one patient and showed a retro-rectal mass. CT scan was the most common modality used in detecting the pathology and was used in 13 patients. MRI was used in two patients and confirmed the ultrasound and CT scan findings in one patient each retrospectively. Barium enema was done in only one case and showed the compression of the rectum by the lesion in this case.
Location of the cystic structure was reported to be in the pelvic cavity in 2 patients, retro-peritoneum in 3, and left upper quadrant of the abdomen within the small bowel mesentery, just mentioned right-sided, right-sided with the extension from the liver to the pelvis, right-sided lumbar and iliac region, pancreatic head and neck with the extension into the sub-hepatic space, in the right hepato-renal space, adjacent to the medial border of the spleen, and the left adrenal gland in one patient each retrospectively. Dislocation or displacement of the viscera was seen in the right kidney and adrenal gland, rectum, and transverse colon in one patient each retrospectively. Pressure effect of the lesion was seen over the urinary bladder in two cases, and recto-sigmoid, and lower rectus muscle in one patient each retrospectively.
Laparoscopic surgery was performed in four cases [3, 4, 12, 15], the other majority of the cases were operated through an open approach with total resection of the lesion [5,6,7,8,9,10,11,12,13,14, 16]. Endoscopic ultrasonographic (EUS) guided cystogastrostomy with the placement of a self-expandable metallic stent (SEMS) was done in one patient [14]. The absence of an epithelial lining was seen in the majority of the patients (13 cases) [3,4,5,6, 8,9,10,11,12,13,14,15,16].
The retroperitoneal pseudocysts are asymptomatic before reaching a large size, and compress over the adjacent structures, and are often diagnosed accidentally [6, 12]. There seem to be two theories for the occurrence of such an enlarged cyst in the retroperitoneal cavity: 1. the retroperitoneal space contains organs originating from the ectoderm and endoderm and are surrounded by a loose network of connective tissue. In this setting, both primary and metastatic tumors grow silently and become symptomatic when they become so large. If they originate from the Wolffian duct, we would see clear fluid, and if they are teratomatous, sebaceous material would be seen [4], 2. Because there is no mesothelial lining within the retroperitoneal space, the extra fluid cannot be reabsorbed and the pseudocysts usually make much volume [6].
Complete excision is a cure for these retroperitoneal cysts. Marsupialization and partial excision are not recommended, because recurrence is common. The surgical methods are conventional laparotomy (intraperitoneal approach), extraperitoneal approach, and transperitoneal flank approach [5], although the laparoscopic approach has been reported either [5,6,7,8,9,10,11,12, 14, 16]. We used the conventional method because the size of the cyst was very large, and the cyst has to be dissected from the other retroperitoneal structures and adhesions without being ruptured.
Conclusion
Primary non-pancreatic retroperitoneal pseudocysts are very rare lesions and have to be distinguished from other differential diagnoses of retroperitoneal lesions, and a surgeon should be aware of the possible occurrence of these lesions with unknown origin. Surgical excision is the only way to exclude malignancy and confirm the diagnosis.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFP:
-
Alpha fetoprotein
- CA:
-
Cancer antigen
- CEA:
-
Carcinoembryonic antigen
- CT:
-
Computed tomography
- CBC:
-
Complete blood count
- EUS:
-
Endoscopic ultrasonographic
- GGT:
-
Gamma-glutamyl transferase
- MRI:
-
Magnetic resonance imaging
- PO:
-
Per os
- SEMS:
-
Self-expandable metallic stent
References
Coffin A, Boulay-Coletta I, Sebbag-Sfez D, Zins M. Radioanatomy of the retroperitoneal space. Diagn Interv Imaging. 2015;96(2):171–86. https://doi.org/10.1016/j.diii.2014.06.015.
Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40(6):1887–903. https://doi.org/10.1007/s00261-014-0311-x.
Latif E, Musthafa S, Ahmed A, Khanna M. Idiopathic retroperitoneal non-pancreatic pseudocyst in an adult male: radiological images and surgical video of laparoscopic excision. Cureus. 2020;12(3): e7243. https://doi.org/10.7759/cureus.7243.
Palanivelu C, Rangarajan M, Senthilkumar R, Madhankumar MV, Annapoorni S. Laparoscopic excision of an infected “egg-shelled” retroperitoneal pseudocyst. J Gastrointestin Liver Dis. 2008;17(4):465–8.
Geng J-H, Huang C-H, Wen-Jeng Wu, Huang S-P, Chen Y-T. Huge retroperitoneal nonpancreatic pseudocyst. Urol Sci. 2012;23(2):61–3. https://doi.org/10.1016/j.urols.2012.03.002.
Durczyński A, Hogendorf P, Szymański D, Strzelczyk J. First report worldwide of huge retroperitoneal pseudocyst with high fluid concentration of CA 125. Med Sci Tech. 2011;52(1–2):67–9.
Tsukada O, Kawabe K. A case of retroperitoneal pseudocyst filled with necrotic material. Jpn J Urol. 1978;69(12):1667–70. https://doi.org/10.5980/jpnjurol1928.69.12_1667.
Sharma AK, Wakhlu A. Retroperitoneal pseudocyst. Pediatr Surg Int. 1995;10:186–7. https://doi.org/10.1007/BF00171194.
Gallego JC, González JM, Fernández-Virgós A, del Castillo M. Retrorectal mesenteric cyst (non-pancreatic pseudocyst) in adult. Eur J Radiol. 1996;23(2):135–7. https://doi.org/10.1016/0720-048x(96)01024-8.
Alzaraa A, Mousa H, Dickens P, Allen J, Benhamida A. Idiopathic benign retroperitoneal cyst: a case report. J Med Case Rep. 2008;2:43. https://doi.org/10.1186/1752-1947-2-43.
Çizginer S, Tatlı S, Snyder EL, Goldberg JE, Silverman SG. CT And MR imaging features of a non-pancreatic pseudocyst of the mesentery. Eur J Gen Med. 2009;6(1):49–51. https://doi.org/10.29333/ejgm/82637.
Prabhu R, Rodrigues G, Sarma YS, Benakatti R. Non-pancreatic retroperitoneal pseudocyst: a benign disease with non-specific symptoms. BMJ Case Rep. 2013;2013:bcr2013200184. https://doi.org/10.1136/bcr-2013-200184.
Wu Z, Huang H, Meng X, Xu C. Laparoscopic excision of simple giant retroperitoneal pseudocyst: case report. Int J Clin Exp Med. 2016;9(8):16856–9.
Karim L, Larkin D, Sadat M. Examine the patient not the hernia: identification of an asymptomatic giant primary retroperitoneal pseudocyst. A case report and literature review. J Surg Case Rep. 2016;5:rjw092. https://doi.org/10.1093/jscr/rjw092.
Vidyachandra G, Pratik G, Pranav M, Nitin P. Retroperitoneal non-pancreatic pseudocyst: a diagnostic enigma. Int J Med Sci Diagn Res (IJMSDR). 2019;3(7):59–62.
Farag M, Gyomber D, Norden S. Primary retroperitoneal pseudocyst: beware of the hoax solid enhancing adrenal mass. BMJ Case Rep. 2019;12(12): e232852. https://doi.org/10.1136/bcr-2019-232852.
Acknowledgements
We all express our gratitude to the patient who kindly gave consent for this case to be presented in this paper.
Funding
Not received.
Author information
Authors and Affiliations
Contributions
LA evaluated the patient clinically and operated the patient (main surgeon), interpreted of data, and read and revised the paper. RH and SM interpreted of data, and read and revised the paper. SH evaluated the pathological slides, interpreted of data, and read and revised the paper. MJYB evaluated the patient clinically and helped to operate the patient (co-surgeon), prepared the manuscript, and helped in writing the review of literature, interpreted data, and read and revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors of this manuscript declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abedini, L., Hosseinpour, R., Mehrabi, S. et al. An asymptomatic huge primary retroperitoneal pseudocyst: a case report and review of the literature. BMC Surg 22, 58 (2022). https://doi.org/10.1186/s12893-022-01510-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01510-5