Abstract
Background
Currently no optimal localization technique has been established for localization of ground glass opacity (GGO). We aimed to introduce a localization technique using geometric localization for peripheral GGO.
Methods
We delineated the location of pulmonary GGO using geometric method which was similar with localization of a point in a spatial coordinate system. The localization technique was based on the anatomical landmarkers (ribs or intercostal spaces, capitulum costae and sternocostal joints). The geometric parameters were measured on preoperative CT images and the targeted GGO could be identified intraoperatively according to the parameters. We retrospectively collected the data of the patients with peripheral GGOs which were localized using this method and were wedge resected between June 2019 and July 2020. The efficacy and feasibility of the localization technique were assessed.
Results
There were 93 patients (male 34, median = 55 years) with 108 peripheral GGOs in the study. All the targeted GGOs were successfully wedge resected in the operative field with negative surgical margin at the first attempt. For each GGO, the localization parameters could be measured in 2–4 min (median = 3 min) on CT images before operation, and surgical resection could be completed in 5–10 min (median = 7 min). A total of 106 (98.15%) GGOs achieved sufficient resection margin. No complications and deaths occurred related to the localization and surgical procedure.
Conclusions
The localization technique can achieve satisfactory localization success rate and good safety profile. It can provide an easy-to-use alternative to localize peripheral GGO.
Similar content being viewed by others
Introduction
For surgical treatment of cancer, lobectomy is the standard procedure. However, some studies have showed that sublobar resection (wedge resection and segmentectomy) can achieve comparable oncological outcomes with lobectomy for the patients with GGO-dominant clinical stage IA adenocarcinomas [1]. Although anatomical resection like lobectomy or segmentectomy is still the best approach for the treatment of lung cancer, wedge resection is a commonly used operative procedure for surgical treatment of peripheral GGOs with good oncological outcomes [1, 2]. Segmentectomy can achieve adequate surgical margin for the cT1N0M0 non-small cell carcinoma located in the central area of a segment, nevertheless it remains a great challenge to identify targeted segmental bronchus, intrasegmental and intersegmental veins and arteries [3]. During wedge resection, GGOs are usually very difficult to be identified intraoperatively. Failure of the identification of the target GGO intraoperatively may lead to conversion to thoracotomy or lobectomy. Many kinds of localization techniques have been developed to facilitate the identification of the targeted small pulmonary nodules, including CT-guided percutaneous marking and bronchoscopic marking [4, 5]. The popularity and application of electromagnetic navigation bronchoscopy (ENB) has also been growing [6].
There are strengths and drawbacks in each localization technique. And complications related to the preoperative invasive procedures should not be ignored. Notably, it seems that the current localization techniques make the localization process a little bit complicated and necessitate professional skills and special instruments. Hence, it is very necessary to find an alternative to simplify the localization process and surgeons in some institutions without the availability of special instruments could also easily identify the targeted GGO.
Inspired by the coordinate system to specify a location, we considered a possibility to delineate the location of peripheral GGO using three-dimensional parameters based on the anatomical landmarkers of the thorax. And a localization technique was thus developed. After more than 1 year clinical practice, we considered it was helpful to identify the lesions. In this article, we will introduce this localization technique and assess its efficacy and feasibility to localize peripheral GGO nodules for wedge resection.
Methods
Patients
The study was approved by the medical ethics committee of the first affiliated hospital of Chongqing Medical University. Written informed consent was obtained from the patients for the surgical procedure and for the medical data to be used in this study. There was no financial support received for this study. No conflicts of interest are declared.
The study included the patients with GGOs: (1) located at the outer third of the lung parenchyma; (2) suspicious-appearing malignancy and no lymph nodes metastasis was indicated by CT (cN0); (3) biopsy proven malignancies; and (4) receiving wedge resection. The surgery indication of GGO at our center was lesion size over 8 mm [7]. For some GGOs suspicious for malignant lesions with the size of smaller than 8 mm, we also sometimes considered performing surgery for those patients in extremely anxiety influencing normal life. For multiple lesions, the GGOs < 8 mm in size were resected synchronously with the main lesion if malignancies were suspected.
The indications for wedge resection at our center were: (1) the GGO was smaller than 20 mm in size at its greatest dimension and the consolidation/tumor ratio was less than 0.25 [8]; (2) sufficient surgical margin could be achieved estimated on the preoperative CT imaging [9]; or (3) patients could not fit enough to undergo segmentectomy and lobectomy. The surgical strategy for each patient was determined in consulting meeting.
The exclusion criteria included that patients were suffering from severe comorbidities and can’t be tolerable for surgery. From June 2019 to July 2020, a total of 93 patients with 108 peripheral GGOs fulfilled the inclusion criteria and exclusion criteria were enrolled in this study. Figure 1 shows the design of the study.
The power analysis was performed by the PASS V15.0 (Power Analysis and Sample Size, NCSS, LLC. Kaysville, UT). According to the data from literature, the localization success rate was above 98%, and the lower limit of the 95% confidence interval should be higher than 91%2. The type I error was 5%. According to the calculation based on these parameters, the power efficacy was 0.88.
Preoperative measurement
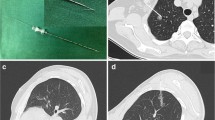
The localization process included preoperative measurement on CT images and intraoperative identification of the targeted GGO according to the preoperative measurement. The measurement was performed by two experienced surgeon respectively on the day before surgery and consensus was reached after discussion when there were discrepancies. There were three geometric parameters measured on CT images to estimate the localization of GGO (Fig. 2). In the transverse section of CT images, we firstly identify the parietal pleural projection of GGO which was defined as the vertical projection point of the center of the lesion to parietal pleura on CT images. Y-axis value referred to the rib or intercostal space corresponding to the parietal pleural projection (Fig. 2A). X-axis value represented the curvilinear distance between the parietal pleural projection and the landmark-capitulum costae (Fig. 2B) or sternocostal joints (Fig. 2C) measured along the pleural surface. Generally, capitulum costa was preferred as the landmark for the GGO in the rear, and sternocostal joint was preferred for the GGO in the front. Values in X and Y axes could describe the estimated position of parietal pleural projection of GGO. Z-axis value was the lesion depth from pleural surface which was used to estimate the depth of resection (Fig. 2D).
The three-dimensional geometric localization parameters of ground glass opacity (GGO) were measured according to CT imaging series before operation. A Y-axis value referred to the rib or intercostal space corresponding to the parietal pleural projection of GGO. B For GGO in the rear, X-axis value represented the measured distance between the parietal pleural projection and capitulum costae. C For GGO in the front, X-axis value represented the measured distance between the parietal pleural projection and sternocostal joint. D Z-axis value was the lesion depth from pleural surface
Surgical procedures
All surgical procedures were performed by three experienced thoracic surgeons in our hospital. Patients were placed in either a left or right lateral decubitus position after dual-lumen endotracheal intubation. The three-port technique for video assistant thoracoscopic surgery (VATS) was preferred. The first port (30-mm) was on the third or fourth intercostal space of the anterior axillary line, as a utility port. The other two ports (20-mm) were all inserted through the seventh intercostal space (midaxillary line and posterior axillary line). Palpation was always the preferred first choice to identify the nodule. Once the nodule was not identified by palpation, this technique will help a lot.
Firstly, we marked the parietal pleura projection of GGO using the electrotome cautery according to X and Y axes values. The affected lung was deflated before the pleural space was opened. After entering the thorax, the rib was firstly calculated from the top of chest cavity to the below and then the corresponding rib or intercostal space was identified according to Y-axis value (i.e., 4th rib) (Fig. 3A). In chest cavity, we adopted the sympathetic chain and internal thoracic vessels as the landmarks for the GGO in the rear and the GGO in the front respectively. They are easily recognized during surgery. The sympathetic chain is almost at the same level with the capitulum costae and the internal thoracic vessels are about 0.5 cm away from the sternocostal joint. So we use a thoracic drainage tube with scales and cut it at the length as calculated (= X-axis value for the GGO in the rear, and = X-axis value − 0.5 cm for the GGO in the front). We placed the thoracic drainage tube from the landmark along the identified rib or intercostal space and then the parietal pleural projection of GGO could be determined (Fig. 3B1–B2).
The surgical procedures for wedge resection of GGO. A The corresponding rib or intercostal space was firstly identified according to Y-axis value. B1 We used a thoracic drainage tube at the length of X-axis value for the GGO in the rear. Along the identified rib or intercostal space, we placed the thoracic drainage tube from the sympathetic chain and marked the parietal pleura projection of GGO using electrotome cautery. B2 For the GGO in the front, the length of the drainage tube was calculated as X-axis value—0.5 cm. We placed the thoracic drainage tube from the internal thoracic vessels and marked the parietal pleura projection using electrotome cautery. C Electrotome was placed on the marked parietal pleural projection. And the anesthetist inflated the affected side lung. D When the affected side lung was fully inflated, electrotome cautery marked the visceral pleural surface corresponding to the parietal pleura projection. E Wedge resection was performed using a linear cutting stapler along the identified rib or intercostal space. The depth of the resection was estimated according to the Z-axis value to guarantee the sufficient resection margin
Secondly, we marked the visceral pleura corresponding to the marked parietal pleural projection. Electrocautery was placed on the marked parietal pleural projection and the anesthetist was asked to inflate the affected side lung mimicking the deep respiratory state at the time of CT examination (Fig. 3C). When the affected side lung was completely inflated, a burn was made on the visceral pleura opposite the parietal mark (Fig. 3D). Then, the affected lung was deflated again.
Thirdly, the resected pulmonary area was planned according to the marked visceral pleura and the Z-axis value. Wedge resection was performed using a linear cutting stapler (Fig. 3E). The linear cutting stapler was placed underneath the visceral mark, holding pulmonary tissues of approximately at least 2 cm in diameter centering the mark. After the wedge resection was performed, the operative field was cut open around the marked visceral pleura to find the targeted GGO. Intraoperative frozen section examination was performed for the lesions to determine the pathologic patterns and ensure the negative surgical margin. Sufficient resection margin should be guaranteed, or lager wedge resection should be performed. Pathologic characteristics were described by postoperative paraffin section examination and immunohistochemical analysis.
Data collection
Data were retrospectively collected from the database for demographic characteristics, GGO features like location, size, number; preoperative measurements on CT imaging series; intraoperative data including duration of operation, successful resection of targeted GGO, the distance from nodules to the marked visceral pleura, and sufficient resection margin; complications; and pathological characteristics. Sufficient resection margin was defined as resection margin ≥ 2 cm or the tumor diameter.
Evaluation
The successful localization procedure was defined as a successful localization of the targeted GGO in the operative field and the success rate of localization of targeted GGO was calculated as follows: [(number of successful localization of targeted GGOs − number of misses of GGOs in the operative field)/number of all GGOs localized] * 100. The distance from nodules to the marked visceral pleura was used to assess the accuracy to localize the targeted GGO. The rate of sufficient resection margin, safety, and durations of the preoperative measurement and surgical procedures were also evaluated. The durations of surgical procedures were measured from the start of intraoperative identification of the targeted GGOs to the removal of the lesions. It didn’t include the time of synchronous lobectomy or segmentectomy, and opening and closing of the incision.
Statistical analysis
The quantitative data were presented as mean ± standard deviation or a number, median and range. Categorical variable was described as a number and percentage. Univariate logistic and linear regression analyses were performed to determine the associations of GGO related characteristics with sufficient resection margin and the distance from nodules to the marked visceral pleura. Baseline characteristics for univariate analysis include lesion size, lesion type, lesion location, and geometric parameters for GGO. Statistically significant variables (i.e., P < 0.20) found by univariate regression analysis were included in the multivariate regression analysis. Two-tailed P value < 0.05 was considered to be statistically significant. All statistical analysis was performed using the SPSS 21.0 software for windows (SPSS, Chicago, IL, USA).
Results
The mean age was 54.0 ± 13.0 years, ranging from 20 to 81 years; 34 (36.5%) patients were male. The baseline demographic and clinical characteristics of the patients are summarized in Table 1. Eleven (11.83%) patients received a contralateral pulmonary operation previously, including 1 patient with wedge resection, 4 patients with segmentectomy and 6 patients with lobectomy.
Twelve patients had multiple GGOs, and the maximal number of GGO in one patient was three. GGO was classified as pure GGO and mixed GGO. Table 2 showed the clinical characteristics and pathological outcomes of the GGOs. For each GGO, the localization parameters could be measured in 2–4 min (median = 3 min) on CT images before operation. All GGOs underwent wedge resection in VATS, and surgical resection could be completed in 5–10 min (median = 7 min). No procedures were converted to thoracotomy, lobectomy or segmentectomy. All the targeted GGOs were successfully resected in the operative field with negative surgical margin at the first attempt (Table 3), which were then immediately found and confirmed by pathologists.
The median distance from nodules to the marked visceral pleura was 0.6 cm (0–1.5 cm). A total of 106 (98.15%) GGOs achieved sufficient resection margin. Only 2 pure GGOs had a shortage of resection margin, although they were completely removed with a pathologically negative surgical margin. The lesion depths for the two GGOs were 2.4 cm and 2.6 cm respectively. Enlarged wedge resection was performed to guarantee the sufficient resection margin. No complications and deaths occurred related to the localization and surgical procedure.
In univariate analysis (Table 4), the median distance from nodules to the marked visceral pleura was only significantly associated with X-axis value (Standard coefficient = 0.061, P = 0.000), and sufficient surgical margin was only significantly associated with Z-axis value (OR = 12.204, P = 0.012). Multivariate regression analysis was not performed because other characteristics related to GGOs were not significantly indicated in univariate analysis.
Discussion
This study has shown a high successful localization rate and good safety profile of the localization technique for peripheral GGO in wedge resection. It can be considered as an effective and safe method in localizing the peripheral GGOs. It can provide an easy-to-use alternative to localize peripheral GGO without assistance with the professional skills and special instruments.
Some localization techniques and effective devices have been developed to help identify the targeted GGO with a high successful localization rate, such as hookwire localization, microcoil localization, methylene-blue and ENB [10,11,12]. However, they need the assistance of special devices and professional skills, and they are not available in a lot of institutions. Current localization techniques seem complicate the process of preoperative preparation, resulting in increased medical costs and patients’ waiting time. The new localization method can be an important alternative especially when other localization techniques can’t be available. It can also simplify the localization process for the pulmonary nodules to improve work efficiency. The surgeons can undergo the localization process by themselves without multi-disciplinary collaboration and assistance of professional skills and special devices.
The underlying principle of this technique was to localize the lesion using three-dimensional parameters based on the anatomical landmarkers of the thorax. These landmarkers could not be shifted as position changed. We used only one landmarker in latitudinal line for preoperative measurement-capitulum costae or sternocostal joints, which were easily recognized. And it was very easy to localize the parietal pleural projection according to the X-axis value. As we know, it was much different from the previous reported techniques using the anatomical landmarkers [13, 14]. Our results showed that all GGOs were successfully resected in the operative field. The successful localization rate of the localization technique was similar to the commonly used localization techniques reported. Notably, our localization technique could be accessible to the areas which a CT-guided percutaneous approach cannot reach, including lung apex, and those facing mediastinum, diaphragm and scapula. Moreover, this method could be considered to be safe. Our results showed that this technique was free of any complications related to the localization process. With the application of this method, preoperative localization of the pulmonary nodule could be completed just using the CT images. Patients didn’t have to receive an additional invasive procedure before pulmonary operation, and didn’t have the chest discomfort due to the percutaneous puncture or bronchoscopy; hence better feelings could be experienced.
Wedge resection is not an anatomically procedure like the lobectomy and segmentectomy, the resection range is usually not clearly defined and tends to be subjective with poor reproducibility. The difficulty in estimating the resection depth also leads to the concern about the sufficient resection margin. The sufficient resection margin should be guaranteed to prevent local recurrence after operation [15]. This localization technique could partly solve these problems based on the measured parameters. We planned the resection line along the direction of the identified rib or intercostal space (Y-axis value) and estimated the resection depth according to the lesion depth from pleura (Z-axis value). Our experience suggested that sufficient resection margin tended to be easily acquired for the nodules located in outer third of lung field, especially for those close to pulmonary surface. As the lesion seated deeper, the acquisition of the sufficient resection margin seemed to be increasingly difficult. It was similar to CT-guided percutaneous marking and virtual-assistant lung mapping. Masahiro Yanagiya et al. reported that the optimal cutoff value of the required resection depth was 3.1 cm [16]. We evaluated the accuracy of localization using the distance from nodules to the marked visceral pleura. Our results were satisfactory. The median distance was 0.6 cm (ranging from 0 to 1.5 cm), and the distance from nodules to the marked visceral pleura was within 1 cm for 88.89% of the GGOs. It was only associated with the X-axis value indicated by the linear regression analysis. Our experience suggested that satisfactory accuracy of localization could be achieved when the distance was within 10 cm between the parietal pleural projection and the specified landmark-capitulum costae or sternocostal joint (X-axis value ≤ 10 cm). For the GGO of which X-axis value was more than 10 cm, the bias was a little bigger but still could be acceptable.
There were several aspects to explain the bias. The obliquity of the direction of ribs led to the measuring bias. The farther the parietal pleural projection was away from specified landmark, the bigger the measurement bias was. As the X-axis value > 10 cm, the difficulty turned to be increased to operate the thoracic drainage tube in the chest cavity and recognized the right rib covered by the intercostal muscle. Additionally, the respiratory state, the respiratory movement and change of the position might also have an influence on the localization accuracy of the targeted GGO.
This technique is easy to operate and does not need a lot of time. For each GGO, the localization parameters could be measured in 2–4 min (median = 3 min) on CT images before operation, and surgical resection could be completed in 5–10 min (median = 7 min). Additionally, there were no additional costs for the patients. We suggest that the patients should be selected carefully so that wedge resection can achieve good oncological outcomes. The GGO should be smaller than 20 mm in size at its greatest dimension and the consolidation/tumor ratio was less than 0.25 [8]. And the lesion should be located in the outer third of lung field in order to guarantee sufficient surgical margin. We suggest that the resection area should be more than 2 cm of lung tissues centering mark on the visceral pleura. For the GGO of which X-axis value was more than 10 cm and that located nearby the free margin of inferior lobe, lager pulmonary areas should be resected due to the lower accuracy of localization.
There were several limitations in this study. Our study was a single-center design, and the sample size was relatively small. We didn’t compare the new localization technique with the currently used localization techniques. We will further improve this technique to make it more accurate, especially for the nodules with X-axis value > 10 cm. We hope to establish a model to promote its application in different institutions.
Conclusions
This localization technique can achieve satisfactory localization success rate and avoid some complications. It can provide an easy-to-use alternative to localize peripheral GGO. Additionally, it can simplify the process of localization for GGO.
Availability of data and materials
The datasets used and/or analyzed in this present study are available from the corresponding author on reasonable request.
Abbreviations
- GGO:
-
Ground glass opacity
- ENB:
-
Electromagnetic navigation bronchoscopy
- VATS:
-
Video assistant thoracoscopic surgery
References
Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. 2014;145(1):66–71.
Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, Okumura M, Doki Y, Endo S, Hirata Y, Kobayashi J, et al. Thoracic and cardiovascular surgery in Japan during 2014: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2016;64(11):665–97.
Nex G, Schiavone M, De Palma A, Quercia R, Brascia D, De Iaco G, et al. How to identify intersegmental planes in performing sublobar anatomical resections. J Thorac Dis. 2020;12(6):3369–75.
Park CH, Han K, Hur J, Lee SM, Lee JW, Hwang SH, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. 2017;151(2):316–28.
Sato M. Precise sublobar lung resection for small pulmonary nodules: localization and beyond. Gen Thorac Cardiovasc Surg. 2020;68(7):684–91.
Hsu PK, Chuang LC, Wu YC. Electromagnetic navigation-guided preoperative localization of small malignant pulmonary tumors. Ann Thorac Surg. 2020;109(5):1566–73.
[Chinese Medical Association guidelines for clinical diagnosis and treatment of lung cancer (Edition 2018)]. Zhonghua zhong liu za zhi [Chin J Oncol]. 2018;40(12):935–64.
Migliore M, Fornito M, Palazzolo M, Criscione A, Gangemi M, Borrata F, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med. 2018;6(5):90.
Aokage K, Saji H, Suzuki K, Mizutani T, Katayama H, Shibata T, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg. 2017;65(5):267–72.
Yang F, Zhao H, Sui X, Zhang X, Sun Z, Zhao X, et al. Comparative study on preoperative localization techniques using microcoil and hookwire by propensity score matching. Thorac Cancer. 2020;11(6):1386–95.
Sun SH, Gao J, Zeng XM, Zhang YF. Computed tomography-guided localization for lung nodules: methyleneblue versus coil localization. Minim Invasive Ther Allied Technol. 2021;30(4):215–20.
Piao Z, Han SJ, Cho HJ, Kang MW. Feasibility of electromagnetic navigation bronchoscopy-guided lung resection for pulmonary ground-glass opacity nodules. J Thorac Dis. 2020;12(5):2467–73.
Mao R, Shi W, Chen D, Kadeer XM, Li M, Fei K, et al. Technique of skeletal navigation for localizing solitary pulmonary nodules. J Surg Oncol. 2017;116(6):763–5.
Kao MW. Intracorporeal direct measurement for localizing peripheral pulmonary nodules during thoracoscopy. J Thorac Dis. 2019;11(10):4119–26.
Moon Y, Park JK, Lee KY, Kim ES. Prognosis after wedge resection in patients with 8(th) edition TNM stage IA1 and IA2 non-small cell lung cancer. J Thorac Dis. 2019;11(6):2361–72.
Yanagiya M, Sato M, Ueda K, Nagayama K, Kawahara T, Kawashima S, et al. Preoperative lung surface localization for pulmonary wedge resection: a single-center experience. J Thorac Dis. 2020;12(5):2129–36.
Acknowledgements
The authors thank all patients who consented to the enrollment in this study. No one else than the authors have participated in the design, data collection and analysis, data interpretation, and preparation of this article.
Funding
None.
Author information
Authors and Affiliations
Contributions
MG and MB planned the study. MB, XZ, MZ and GF collected the data. MB performed the statistical analysis and drafted the manuscript. MB, XZ, MZ, GF and MG convinced of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out following the international ethical recommendations for medical research in humans. Both authors were sure that this study was conducted in accordance with the standards set out in the Declaration of Helsinki. Before starting the study, the Ethical Committee of the first affiliated hospital of Chongqing Medical University approved the study protocol. All participants provide written informed consent before enrollment.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest to declare in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bie, M., Zhao, X., Zhang, M. et al. A novel localization technique for peripheral ground glass opacity using geometric parameters measured on CT images. BMC Surg 21, 345 (2021). https://doi.org/10.1186/s12893-021-01343-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-021-01343-8