Abstract
Background
Obesity influences the development of osteoarthritis via low-grade inflammation. Progression of local inflammation (= synovitis) increased with weight gain in overweight and obese women compared to stable weight. Synovitis could be associated with subcutaneous fat (SCF) around the knee. Purpose of the study was to investigate the effect of weight loss on synovitis progression and to assess whether SCF around the knee mediates the relationship between weight loss and synovitis progression.
Methods
We included 234 overweight and obese participants (body mass index [BMI] ≥ 25 kg/m2) from the Osteoarthritis Initiative (OAI) with > 10% weight loss (n = 117) or stable overweight (< ± 3% change, n = 117) over 48 months matched for age and sex. In magnetic resonance imaging (MRI) at baseline and 48 months, effusion-synovitis and Hoffa-synovitis using the MRI Osteoarthritis Knee Score (MOAKS) and average joint-adjacent SCF (ajSCF) were assessed. Odds-ratios (ORs) for synovitis progression over 48 months (≥ 1 score increase) were calculated in logistic regression models adjusting for age, sex, baseline BMI, Physical Activity Scale for the Elderly (PASE), and baseline SCF measurements. Mediation of the effect of weight loss on synovitis progression by local SCF change was assessed.
Results
Odds for effusion-synovitis progression decreased with weight loss and ajSCF decrease (odds ratio [OR] = 0.61 and 0.56 per standard deviation [SD] change, 95% confidence interval [CI] 0.44, 0.83 and 0.40, 0.79, p = 0.002 and 0.001, respectively), whereas odds for Hoffa-synovitis progression increased with weight loss and ajSCF decrease (OR = 1.47 and 1.48, CI 1.05, 2.04 and 1.02, 2.13, p = 0.024 and 0.038, respectively). AjSCF decrease mediated 39% of the effect of weight loss on effusion-synovitis progression.
Conclusions
Effusion-synovitis progression was slowed by weight loss and decrease in local subcutaneous fat. Hoffa-synovitis characterized by fluid in the infrapatellar fat pad increased at the same time, suggesting a decreasing fat pad rather than active synovitis. Decrease in local subcutaneous fat partially mediated the systemic effect of weight loss on synovitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Symptomatic knee osteoarthritis (OA) occurs in an estimated 14 million people in the US [1] and prevalence substantially increased over the last 20 years of the twentieth century, most likely independent of demographic changes in age and BMI [2]. Obesity is a modifiable risk factor for OA. Previous studies showed the effect of weight change on knee OA: Weight gain promoted [3] and weight loss slowed cartilage degeneration [4] in subjects with OA risk factors.
The factors mediating the systemic and local effects of adipose tissue on OA are not well understood. Historically, obesity has been implicated in the pathogenesis of OA due to biomechanical reasons, but current research highlights its effect on OA via altered metabolism and inflammation [5]. Paracrine signaling from fat, rather than body weight was identified as a mediator of joint degeneration in mice [6]. A clinical phenotype of OA related to the metabolic syndrome has been described that is characterized by obesity and chronic low-grade inflammation [7]. Investigations focus on inflammation in synovial tissue (= synovitis) because inflamed synovium produces excess proteolytic enzymes that cause cartilage breakdown and, in turn, amplify inflammation potentially leading to a vicious circle [8].
Non-contrast enhanced MRI is a method to evaluate synovitis in OA that has been used in clinical trials and observational studies [9]. Previous imaging studies using unenhanced MRI investigated the relationship between synovitis, obesity, and OA. Irrespective of excess weight, progression of knee degenerative changes were higher in subjects with sustained synovitis assessed by semi-quantitative MRI [10]. Imaging markers of synovitis were higher in obese and overweight compared to normal weight subjects and higher in cases of severe OA defined by imaging and pain scores [11]. Weight gain was associated with increased synovitis imaging markers in overweight and obese women [12]. Beyond systemic effects of obesity, local SCF around the knee and the infrapatellar fat pad (IPFP) located in the joint capsule is of interest as being involved in the pathophysiology of knee OA [13]. A previous study found that joint-adjacent SCF played a role in the development and progression of knee OA [14]. However, the effect of local SCF on synovitis is unknown.
The purpose of this study was to investigate how weight loss and changes in local SCF around the knee impact knee joint synovitis development over 48 months in obese and overweight participants of the OAI. We also investigated these effects in separate analyses for women and men. We hypothesized that local SCF mediates the effect of weight loss on synovitis development.
Methods
Participant selection
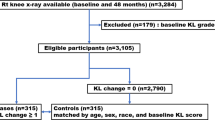
The OAI is a multi-center, longitudinal, observational study investigating biomarkers that relate to the onset and progression of OA in a cohort of 4796 subjects (https://nda.nih.gov/oai/). The OAI recruited two primary subcohorts, one with and one without symptomatic knee OA at baseline. Symptomatic knee OA was defined, similar to the American College of Radiology (ACR) classification for clinical knee OA [15], by having both of the following criteria in at least one native knee at baseline: Frequent knee symptoms (pain, aching, or stiffness in or around the knee on most days) in the past 12 months for at least one month during the past 12 months and radiographic tibiofemoral knee OA, defined as definite tibiofemoral osteophytes (equivalent to Kellgren and Lawrence [KL] grade 2) on the fixed flexion radiograph. Subject for this study were selected irrespective of membership in any cohort.
Overweight and obese participants (BMI ≥ 25 kg/m2) were selected from the OAI for an exposure-matched cohort study (Fig. 1). Participants without BMI data at more than one timepoint, with rheumatoid arthritis, and with advanced OA with a KL grade > 3 (representing end-stage OA) were excluded. Only participants who completed MRI at baseline and 48 months were included. Weight change over 48 months was calculated with linear regression models using annual BMI data in this period. Unexposed participants (stable overweight defined as < ± 3% weight change) were individually matched to exposed participants (> 10% weight loss) by age (± 5 years) and sex to prevent association between exposure and the matching factors among participants at the start of follow-up. The PASE, a brief and reliable instrument for the assessment of physical activity, was collected for all selected participants at baseline [16].
MR imaging
MR images were acquired on four identical 3 T scanners (Magnetom Trio; Siemens Healthineers, Erlangen, Germany) using quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA). Three MRI acquisition sequences included in the OAI imaging protocol for the knee were used in this study [17]. Sagittal 3D dual-echo in steady state (DESS; TR/TE = 16.3/4.7 ms, flip angle = 25°) and coronal T1-weighted 3D fast low-angle shot (FLASH) water excitation (WE) sequences were used for subcutaneous fat measurements. Sagittal intermediate-weighted (IW) 2D turbo-spin echo (TSE) fat suppression (FS) and axial multiplanar reformation of 3D DESS sequences were used for synovitis readings.
Semi-quantitative assessment of synovitis
MR images at baseline and 48 months were analyzed by three radiologists with 4 (MTL), 11 (CN), and 27 years of experience (TML). Images were read separately by two readers (MTL and CN). Final scores were adjudicated by the senior radiologist (TML) in case of mismatch. Images were reviewed in sequential order, unblinded to the time point of imaging visit, to increase sensitivity to change [18].
Two features of the MRI OsteoArthritis Knee Score (MOAKS) that are related to synovitis were semi-quantitatively assessed [19]. The MOAKS was employed in numerous previous studies [11, 12, 20,21,22] and is reliable and sensitive to change [23]. “Effusion-synovitis” represents a composite of synovial thickening and intraarticular joint fluid, as these cannot be distinguished on standard T2/IW/PD (proton density)-weighted images [24]. The amount of effusion-synovitis was evaluated in axial views of the 3D DESS sequence and graded as 0 = physiologic amount, 1 = small amount (fluid continuous in the retropatellar space), 2 = medium amount (with slight convexity of the suprapatellar bursa), and 3 = large amount (with signs of capsular distention). “Hoffa-synovitis” refers to diffuse hyperintense signal changes in T2/IW/PD fat-saturated images of the IPFP that are non-specific, but have been related to mild chronic synovitis [25]. The degree of hyperintensity in the IPFP was evaluated in mid-sagittal slices of sagittal T2 TSE FS. We added a reference to cartilage signal intensity to the original definitions of grades [19] as 0 = normal, 1 = mild (lower than cartilage signal), 2 = moderate (equal to cartilage signal), and 3 = severe hyperintensity (higher than cartilage or equal to fluid signal). Almost perfect inter-rater and intra-rater reliability for effusion-synovitis assessment (with weighted Cohen’s κ of 0.90 and 0.84, respectively) and almost perfect inter-rater and substantial intra-rater reliability for Hoffa-synovitis assessment (with weighted Cohen’s κ 0.93 and 0.76, respectively) were previously reported for different readers trained by the same expert radiologist as in this study [11].
Subcutaneous fat measurements
SCF was measured anterior, medial, and lateral to the knee joint capsule in MRI at baseline and 48 months by two medical students who were trained by an experienced musculoskeletal radiologist. Medial and lateral measurements were performed in coronal 3D FLASH WE sequences (Fig. 2). Anterior measurements were performed perpendicularly anterior to the patellar tendon at half of its length in sagittal views of 3D DESS sequences [14]. The lateral and medial tibial spine served as anatomical landmarks to choose coronal and sagittal cross-sections, respectively, for reproducible measurements. The arithmetic mean was calculated as average joint-adjacent SCF (ajSCF). Good inter-rater and intra-rater reliability for SCF measurements were previously reported with coefficients of variation (CV) of 2.72% and 2.01%, respectively [14].
Subcutaneous fat (SCF) measurements and effusion-synovitis development. This 53-year-old woman had a baseline BMI of 35.2 kg/m.2 and lost 27.5% weight over 48 months. Medial and lateral SCF decreased by 13.5 mm and 8.5 mm, respectively, from baseline (a) to 48-months follow-up (a’). Effusion-synovitis (*) regressed from grade 2 at baseline (b) to grade 1 at 48-months follow-up (b’). Medial and lateral measurements were performed in coronal 3D FLASH sequences (a, a’) at two levels (at the tip of the medial tibial spine and at the level of the medial joint space) and averaged. Anterior measurements were performed in sagittal reformations (not shown). Semi-quantitative readings of effusion-synovitis by MOAKS were performed in non-enhanced axial reformations of 3D DESS sequences (b, b’)
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 28 (IBM, Armonk, NY) with the extension PROCESS v4.2 for mediation analysis [26]. Level of statistical significance was defined as p < 0.05. Means of numeric variables (i.e., age, BMI, PASE, SCF measurements, synovitis scores) and proportions of categorical variables (i.e., sex, KL score) were compared between men and women using Student’s t-tests and chi-square tests, respectively. Means were compared using Welch’s instead of Student’s t-test when Levene’s test (p < 0.05) indicated that equality of variances could not be assumed. BMI, SCF measurements, and synovitis grades were compared between baseline and 48 months for groups with stable overweight and weight loss using paired t-tests.
To analyze associations between weight, SCF and synovitis change exposure-matched groups (stable overweight and weight loss) were coalesced into a single stratum for better efficiency. Change in synovitis scores between baseline and 48-month follow-up was dichotomized to reflect progression (change ≥ 1) or no progression (change < 1). First, we analyzed associations between baseline measures of BMI and ajSCF (= predictors) and effusion- and Hoffa-synovitis progression (= outcomes) in logistic regression models adjusting for age, sex, baseline PASE, and BMI as applicable. Then, we analyzed associations between weight loss and ajSCF change over 48 months (= predictors) and effusion- and Hoffa-synovitis progression (= outcomes) in logistic regression models adjusting for age, sex, baseline PASE, BMI, and SCF measurements. Odds ratios (ORs) and 95% confidence intervals (CIs) for synovitis progression were calculated. No formal correction for multiple testing was applied. Weight loss in percent BMI decrease and SCF change in mm decrease were transformed into SD as the unit of analysis for better comparability.
Mediation analysis assessed whether the effect of weight loss on synovitis progression was mediated by local SCF change when assuming that natural direct effect (path c’) and natural indirect effect (path ab) point in the same direction (Fig. 3). Outcomes for path ab and c’ are binary as in original analyses. Logistic regression models estimated the indirect effect of weight loss on synovitis progression through local SCF change and the direct effect of weight loss on synovitis through a process other than local SCF change adjusting for the same covariates as above. Percentile bootstrap confidence intervals were calculated for the indirect effect using 5,000 bootstrap samples [27]. The total effect (path c) was calculated as the sum of the direct and indirect effects log-odds ratios (= β coefficients). The proportion mediated was calculated as the indirect effect divided by the total effect (\(ab/{(c}{\prime}+ab)\)).
Direct acyclic graphs of mediation models. Beta coefficients (95% confidence intervals) are given for indirect and direct effects of weight loss on change of effusion-synovitis (A) and Hoffa-synovitis (B). All models are adjusted for age, sex, baseline BMI, PASE, and average joint-adjacent subcutaneous fat (ajSCF). * calculated as percentiles bootstrap confidence intervals
Results
Baseline characteristics
Baseline characteristics of both groups, weight loss and stable overweight, are shown in Table 1. The study cohort included 117 subjects with > 10% weight loss and 117 subjects with stable overweight over 48 months. Overall, there were 162 women and 72 men in the study cohort. Except for PASE, SCF measurements and KL grades there were no significant differences between men and women in baseline characteristics (all p > 0.05). PASE was significantly higher in men compared to women (170 ± 79 vs. 147 ± 73, p = 0.028). AjSCF, anterior, medial, and lateral SCF measurements were significantly higher in women compared to men (ajSCF: 14.6 ± 4.0 mm vs. 8.6 ± 2.6 mm, p < 0.001; anterior SCF: 7.4 ± 3.2 mm vs. 6.7 ± 1.6 mm, p = 0.027; medial SCF: 23.9 ± 7.3 mm vs. 13.0 ± 5.9 mm, p < 0.001; lateral SCF: 12.7 ± 4.8 mm vs. 5.9 ± 2.2 mm; p < 0.001). Distribution of KL grades was significantly different between men and women (p = 0.012) with significantly more KL grade 1 knees in men compared to women (33.3% vs. 19.8%; p = 0.025). There was no significant difference of synovitis grades between men and women (p = 0.960 for effusion-synovitis and p = 0.874 for Hoffa-synovitis).
Weight loss, subcutaneous fat change, and synovitis change over 48 months
BMI, SCF measurements, and synovitis grades at baseline and 48 months for both groups are shown in Table 2. Mean BMI significantly decreased by -4.6 ± 1.9 kg/m2 in subjects with weight loss (p < 0.001), whereas it slightly increased by 0.1 ± 0.6 kg/m2 in subjects with stable overweight over 48 months. At the same time, AjSCF significantly decreased by -1.7 ± 2.4 mm (12.6%) in subjects with weight loss (p < 0.001), whereas it significantly increased by 0.3 ± 1.7 mm (2.7%) in subject with stable overweight (p = 0.041; Fig. 2). Mean effusion-synovitis scores significantly increased by 0.2 ± 0.8 only in subjects with stable overweight (p = 0.002). In contrast, mean Hoffa-synovitis scores significantly decreased by 0.2 ± 0.5 only in subject with weight loss (p < 0.001).
Effusion-synovitis relatively progressed from grade 1 to grade 2 in subjects with stable overweight from baseline to 48 months (grade 1/grade 2 frequency: 44/37% to 34/47%). Vice versa, effusion-synovitis relatively regressed from grade 2 to grade 1 in subjects with weight loss from baseline to 48 months (grade 1/grade 2 frequency: 42/42% to 52/31%). At the same time, Hoffa-synovitis showed a trend of increasing frequency of grade 3 in subjects with weight loss (18% to 29%).
Dichotomized change in synovitis-scores over 48 months showed similar differences between groups. Effusion-synovitis progressed significantly more frequently in subjects with stable overweight compared to weight loss (40.2 vs. 25.6%; p = 0.018). At the same time, Hoffa-synovitis progressed significantly less frequently in subjects with stable overweight compared to weight loss (12.0 vs. 25.9%; p = 0.007). Differences in frequencies remained significant after adjusting for age, sex, BMI, and PASE with 16.3% higher frequency for effusion-synovitis progression in subjects with stable overweight (CI: 4.2, 28.5%; p = 0.009) and -14.7% lower frequency for Hoffa-synovitis progression in subjects with weight loss (CI: -24.8, -4.5%; p = 0.005).
Prior to separate analyses of women and men we tested for sex-related interactions. There was no significant interaction of sex and weight loss on the effect of effusion-synovitis (p = 0.223) and Hoffa-synovitis progression (p = 0.758), respectively, or of sex and SCF decrease on the effect of effusion-synovitis (p = 0.191) and Hoffa-synovitis progression (p = 0.079), respectively. AjSCF significantly decreased in men by -1.4 ± 1.6 mm and in women by -1.8 ± 2.7 mm with weight loss. AjSCF significantly increased in men by 0.6 ± 0.9 mm, but not in women (0.2 ± 2.0 mm, p = 0.316) with stable overweight. In adjusted models, Hoffa-synovitis progressed significantly less frequently in women (-13.0%; CI: -25.7, -0.2%; p = 0.046) and men (-18.4%; CI: -35.4, -1.4%; p = 0.034) with stable overweight compared to weight loss.
Associations between BMI or subcutaneous fat at baseline and synovitis progression
The odds of effusion-synovitis progression or Hoffa-synovitis progression over 48 months (dichotomized) did not significantly change for change in baseline BMI or baseline ajSCF, respectively (Table 3).
Associations between weight loss or subcutaneous fat change and synovitis progression
The odds of effusion-synovitis progression significantly decreased, whereas the odds of Hoffa-synovitis progression significantly increased with weight loss (Table 4). Analyzing women and men separately, the association between weight loss and effusion-synovitis progression remained statistically significant in both sexes, but with larger effect size in men compared to women (Table 4). Weight loss was significantly associated with Hoffa-synovitis progression only in men (OR = 2.08; CI: 1.01, 4.29; p = 0.048), but not in women (OR = 1.33; CI: 0.90, 1.95; p = 0.149).
The odds of effusion-synovitis progression significantly decreased, whereas the odds of Hoffa-synovitis progression significantly increased with decrease in ajSCF (Table 4). Analyzing women and men separately, the association between ajSCF decrease and effusion-synovitis progression remained statistically significant in both sexes. In contrast, decrease in ajSCF was not significantly associated with Hoffa-synovitis progression in women (OR = 1.47; CI: 0.99, 2.19; p = 0.059) and men (OR = 1.80; CI: 0.64, 5.12; p = 0.267). Representative cases of the associations between weight loss or SCF decrease and synovitis change are shown in Figs. 2 and 4.
Development of Hoffa- and effusion-synovitis with weight loss. This 53-year-old women had a baseline BMI of 33.3 kg/m2 and lost 10.6% weight over 48 months. Medial and lateral subcutaneous fat (SCF) measurements decreased each by 5 mm from baseline to 48-months follow-up. Hyperintense signal changes in Hoffa’s fat pad anterior to trochlear cartilage increased from grade 1 at baseline (a) to grade 2 at 48-months follow-up (a’). Effusion-synovitis remained stable at grade 1 at baseline (b) and 48-months follow-up (b’) with MOAKS definitions not able to capture a slight decrease
Mediation of the effect of weight loss on synovitis progression
Weight loss did not interact with the mediator, i.e. ajSCF change, in its effect on synovitis progression (p = 0.873). Natural direct effects and indirect effects of weight loss on progression of effusion- and Hoffa-synovitis by a pathway through ajSCF decrease are shown in Fig. 3. Weight loss had a significant negative indirect effect on effusion-synovitis progression mediated by ajSCF decrease (β = -0.189; CI: -0.423, -0.016). The positive indirect effect of weight loss on Hoffa-synovitis progression was not statistically significant (β = 0.114; CI: -0.096, 0.341). A mediation proportion of 39% was calculated for the indirect effects of weight loss through ajSCF decrease on effusion-synovitis progression.
Discussion
Effusion- and Hoffa-synovitis represent two parameters to semi-quantitatively assess synovitis in non-enhanced knee MRIs [19, 28]. We found that weight loss and decrease in local SCF around the knee was associated with a decrease in effusion-synovitis, but with an increase in Hoffa-synovitis. This result is surprising given that both scores are supposed to evaluate synovial inflammation but may be explained by a shrinking Hoffa fat pad due to decrease in fat around the knee. Investigating the mediating effect of local SCF we found that 39% of the relationship between effusion-synovitis progression and weight loss was mediated by local SCF.
Obesity is a well-established risk factor for OA that affects joints beyond mechanical loading [29]. Although cartilage composition or thickness evaluated in MRI may be improved by weight loss in early knee OA, a recent systematic review concluded that current evidence is inconsistent in regard to specific structural effects of weight loss on imaging outcomes in overweight and obese persons [30].
Most risk factors for OA (age, obesity, trauma, and excess mechanical loading) are likely to influence OA pathogenesis by a pathway through synovial inflammation [31]. Synovitis might be present in early and advanced stage OA and involved in its development [8, 32,33,34]. In particular, effusion-synovitis and Hoffa-synovitis are strong predictors for the development of incident radiographic knee OA [35] and for pain progression [23]. Contrast-enhanced MRI is the reference for non-invasive assessment of synovitis in the knee joint [24] and showed moderate correlation with histologic macroscopic and microscopic synovial scores [36]. Nonenhanced MRI allows evaluation of effusion-synovitis [19, 37], but synovial proliferation cannot be distinguished from effusion since both appear hyperintense on fluid-sensitive sequences [24]. Furthermore, signal intensity alterations in the IPFP (= Hoffa-synovitis) in nonenhanced MRI are a sensitive but rather nonspecific finding that can represent synovial proliferation [28].
Interestingly, we found inverse associations of weight loss and SCF decrease with effusion- and Hoffa-synovitis progression, respectively. Previous studies described differences for relationships with effusion- and Hoffa-synovitis, too, but none of them specifically investigated individuals with weight loss. The presence of effusion-synovitis, but not Hoffa-synovitis (each grade ≥ 2) predicted cartilage lesions (semi-quantitative Whole-Organ MRI Score [WORMS]) at 30 months follow-up [33]. Comparing subgroups from the OAI incidence cohort (at least one knee with KL grade ≥ 2 at 48 months follow-up) with metabolic OA and OA related to physical activity Hoffa-synovitis was the only parameter that was significantly more prevalent in the active lean group that had baseline BMI < 25 kg/m2 [22]. Unfortunately, no information about weight change was provided in this study, but stable weight to moderate weight loss can be assumed in the active lean group given that they were required to have a PASE score ≥ 2 for strenuous sport/recreation activities. Furthermore, overweight significantly increased the odds of having effusion-synovitis – but not of having Hoffa-synovitis – two years prior to incident radiographic knee OA compared to normal weight [38]. A mediation study found that the effect of BMI on knee OA progression was mediated by effusion-synovitis progression, but not by Hoffa-synovitis progression [20].
Inconsistent with the slowing effect of weight loss on the progression of effusion-synovitis we found that weight loss promoted the progression of Hoffa-synovitis. Signal alterations in Hoffa’s fat pad (= IPFP) in unenhanced MRI are known to be a non-ideal surrogate for synovitis [28], due to its low specificity [21] and many diagnostic pitfalls (clefts, recesses, ganglion cysts, vessels) [39]. Moreover, the Hoffa-synovitis score showed lower reliability [23] and virtually no progression in weight gain [12] compared to effusion-synovitis. Hoffa-synovitis evaluates the IPFP, an intracapsular but extra-synovial structure that is enclosed by bone at two surfaces (femur and tibia). In healthy individuals, the volume of the IPFP increased with BMI showing a ceiling effect for those with BMI above 30 kg/m2 [40]. We should expect a negative linear correlation between weight loss and IPFP volume, as the mean baseline BMI of our study population was approximately at this level. Given the rigid, bony structures enclosing part of the IPFP we speculate that volume decreases of the IPFP due to weight loss could create spaces, eg in small fissures and clefts. These void spaces are filled by additional fluid increasing the T2/IW signal intensity. Another explanation could be that the decrease in fat in the region has reduced the cushioning effect of the fat and resulted in greater pressure on other soft tissue structures during ambulation such as synovium. Consistent with this hypothesis we found a longitudinal increase in signal intensity in the IPFP in individuals with weight loss. Consequently, we can speculate that our readings of Hoffa-synovitis progression were largely non-specific for synovial inflammation. This could add to the understanding insofar that Hoffa-synovitis readings are particularly non-specific in the context of weight loss, where signal increases are due to increasing fluid in a shrinking IPFP [40].
Previous studies have investigated the specific effect of adipose tissue on synovitis and knee OA assessed in unenhanced MRI. Progression of synovitis defined as the composite score of effusion- and Hoffa-synovitis had odds of 2.84 in women with weight gain compared to stable weight loss, but there were no significant association of synovitis progression with weight loss [12]. ORs for individual effusion- and Hoffa-synovitis score progression were not calculated. However, they reported progression rates of 6% and 9% for effusion-synovitis and of 1% and 0% for Hoffa-synovitis for the stable weight and weight loss groups, respectively. These rates are considerably lower than in our study likely due to a virtual absence of radiographic knee OA (93% vs. 46% with KL-grade 0 or 1) in their relatively healthy and young women population and a shorter follow-up interval (2.5 vs. 4 years). Furthermore, overweight and obese persons had a higher prevalence of effusion- and Hoffa-synovitis that correlated with cartilage composition and most features of semi-quantitative WORMS [11]. However, a recent study found no significant association between BMI categories and prevalence of effusion-synovitis or size of Hoffa-synovitis [20].
More than one third of the effect of weight loss on effusion-synovitis – but not on Hoffa-synovitis – was mediated by local SCF. In contrast to systemic low-grade inflammation caused by obesity and its effects on OA, the local effects of adipose tissue are largely studied in the context of the IPFP [5]. Only a few studies investigated SCF at the thigh, for example, in association with incident radiographic knee OA [41]. We evaluated local SCF adjacent to the knee joint. A previous study found significant cross-sectional and longitudinal association between joint-adjacent SCF (mostly lateral) and semi-quantitative and quantitative imaging markers of knee OA [14]. How local adipose tissue around the knee – other than the IPFP – affects synovial inflammation on a cellular and cytokine level and how this is different to systemic effects of adiposity could be a focus of future investigations. This study indicates partial mediation. The indirect effect on effusion-synovitis progression is statistically significant, yet the direct effect, despite being non-zero, has a confidence interval that overlaps with zero, which limits our capacity to establish complete mediation.
In sex-stratified analyses we found larger effect sizes for the association between weight loss or ajSCF decrease and effusion-synovitis progression in men compared to women and a significant association between weight loss or ajSCF decrease and Hoffa-synovitis progression only in men. Likely due to gynoid fat distribution, thickness of SCF around the knee at baseline was larger in women compared to men of the same mean BMI. However, absolute changes in local SCF did not significantly differ between men and women. Therefore, we can assume a disproportionate larger decrease in SCF in men compared to women that could have increased the effect on effusion-synovitis progression. Moreover, this could indicate that we observe a significant association with Hoffa-synovitis progression only in men due to relatively stronger volume decrease of the IPFP.
This study has limitations. First, the gold standard for identifying synovitis in imaging is contrast-enhanced MRI. Non-enhanced MRI cannot distinguish between effusion and synovial proliferation, though their occurrence is usually associated. Second, we considered this study exploratory and hypothesis forming. Third, our results are limited to overweight and obese persons (BMI > = 25 kg/m2). Fourth, we did not include individuals with weight gain limiting the generalizability of the findings. Fifth, readers of MOAKS gradings were not blinded to timepoint. Sixth, we used a logistic regression analysis despite a rather small sample size. To address this issue we conducted a sensitivity analysis using Poisson regression to estimate risk ratios. This analysis confirmed the robustness of our primary logistic regression results except for the association between ajSCF decrease and Hoffa-synovitis that did not reach statistical significance but showed the same directionality. This consistency supports the reliability of our findings.
Conclusions
We found that weight loss and reduction in subcutaneous fat around the knee decrease synovitis progression in overweight and obese persons. The systemic effect of weight loss on synovitis was partially mediated by local subcutaneous fat decrease. While effusion-synovitis in unenhanced MRI showed associations with weight loss in line with previous understanding, the imaging biomarker for synovitis of Hoffa’s fat pad should be used with caution in the context of weight loss because it could reflect effects that are non-specific for synovial inflammation.
Availability of data and materials
This article was prepared using a public-use data set of the Osteoarthritis Initiative (OAI) (https://nda.nih.gov/oai/).
Abbreviations
- ACR:
-
American College of Radiology
- AjSCF:
-
Average joint-adjacent SCF
- BMI:
-
Body mass index
- CV:
-
Coefficients of variation
- DESS:
-
Dual-echo in steady state
- FLASH:
-
Fast low-angle shot
- FS:
-
Fat suppression
- IPFP:
-
Infrapatellar fat pad
- IW:
-
Intermediate-weighted
- KL:
-
Kellgren and Lawrence
- MOAKS:
-
MRI Osteoarthritis Knee Score
- MRI:
-
Magnetic resonance imaging
- NIH:
-
National Insittutes of Health
- NIAMS:
-
National Institute of Arthritis and Musculoskeletal and Skin Diseases
- OA:
-
Osteoarthritis
- OAI:
-
Osteoarthritis Initiative
- OR:
-
Odds ratio
- OSMB:
-
Observational Study Monitoring Board
- PASE:
-
Physical Activity Scale for the Elderly
- PD:
-
Proton density
- SCF:
-
Subcutaneous fat
- TSE:
-
Turbo-spin echo
- WE:
-
Water excitation
- WORMS:
-
Whole-Organ MRI Score
References
Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, Age, Sex, and Obesity. Arthritis Care Res. 2016;68:1743–50.
Nguyen USDT, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155:725–32.
Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, et al. Association of cartilage degeneration with four year weight gain–3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23:525–31.
Gersing AS, Schwaiger BJ, Nevitt MC, Zarnowski J, Joseph GB, Feuerriegel G, et al. Weight loss regimen in obese and overweight individuals is associated with reduced cartilage degeneration: 96-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27:863–70.
Urban H, Little CB. The role of fat and inflammation in the pathogenesis and management of osteoarthritis. Rheumatol Oxf Engl. 2018;57 suppl_4:iv10-21.
Collins KH, Lenz KL, Pollitt EN, Ferguson D, Hutson I, Springer LE, et al. Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci U S A. 2021;118:e2021096118.
Courties A, Berenbaum F, Sellam J. The phenotypic approach to osteoarthritis: a look at metabolic syndrome-associated osteoarthritis. Joint Bone Spine. 2019;86:725–30.
Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35.
Hayashi D, Roemer FW, Jarraya M, Guermazi A. Update on recent developments in imaging of inflammation in osteoarthritis: a narrative review. Skeletal Radiol. 2022. https://doi.org/10.1007/s00256-022-04267-3.
Ramezanpour S, Kanthawang T, Lynch J, McCulloch CE, Nevitt MC, Link TM, et al. Impact of Sustained Synovitis on Knee Joint Structural Degeneration: 4-Year MRI Data from the Osteoarthritis Initiative. J Magn Reson Imaging JMRI. 2022. https://doi.org/10.1002/jmri.28223.
Kanthawang T, Bodden J, Joseph GB, Lane NE, Nevitt M, McCulloch CE, et al. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: data from the osteoarthritis initiative. Skeletal Radiol. 2021;50:217–29.
Landsmeer MLA, de Vos BC, van der Plas P, van Middelkoop M, Vroegindeweij D, Bindels PJE, et al. Effect of weight change on progression of knee OA structural features assessed by MRI in overweight and obese women. Osteoarthritis Cartilage. 2018;26:1666–74.
Eymard F, Chevalier X. Inflammation of the infrapatellar fat pad. Joint Bone Spine. 2016;83:389–93.
Bodden J, Ok AH, Joseph GB, Nevitt MC, McCulloch CE, Lane NE, et al. Joint-adjacent adipose tissue by MRI is associated with prevalence and progression of knee degenerative changes: data from the osteoarthritis Initiative. J Magn Reson Imaging JMRI. 2021;54:155–65.
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49.
Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62.
Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41.
Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthritis Rheumatol Hoboken NJ. 2016;68:2422–31.
Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19:990–1002.
Bañuls-Mirete M, Lombardi AF, Posis AIB, Shadyab AH, Chang EY, Lane NE, et al. Effusion-synovitis worsening mediates the association between body mass index and Kellgren-Lawrence progression in obese individuals: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2022;30:1278–86.
Crema MD, Felson DT, Roemer FW, Niu J, Marra MD, Zhang Y, et al. Peripatellar synovitis: comparison between non-contrast-enhanced and contrast-enhanced MRI and association with pain. The MOST study. Osteoarthritis Cartilage. 2013;21:413–8.
Roze RH, Bierma-Zeinstra SMA, Agricola R, Oei EHG, Waarsing JH. Differences in MRI features between two different osteoarthritis subpopulations: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24:822–6.
Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort - Methodologic aspects and definition of change. BMC Musculoskelet Disord. 2016;17:466.
Roemer FW, Kassim Javaid M, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18:1269–74.
Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–83.
Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Publications; 2022.
Igartua J-J, Hayes AF. Mediation, moderation, and conditional process analysis: concepts, computations, and some common confusions. Span J Psychol. 2021;24:e49.
Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa’s fat pad: evaluation on unenhanced mr images as a measure of patellofemoral synovitis in osteoarthritis. AJR Am J Roentgenol. 2009;192:1696–700.
Aspden RM. Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol. 2011;7:65–8.
Daugaard CL, Hangaard S, Bartels EM, Gudbergsen H, Christensen R, Bliddal H, et al. The effects of weight loss on imaging outcomes in osteoarthritis of the hip or knee in people who are overweight or obese: a systematic review. Osteoarthritis Cartilage. 2020;28:10–21.
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–75.
Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24:458–64.
Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–9.
Wang Y, Teichtahl AJ, Pelletier J-P, Abram F, Wluka AE, Hussain SM, et al. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatol Oxf Engl. 2019;58:246–53.
Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75:390–5.
Shakoor D, Demehri S, Roemer FW, Loeuille D, Felson DT, Guermazi A. Are contrast-enhanced and non-contrast MRI findings reflecting synovial inflammation in knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2020;28:126–36.
Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage. 2014;22:668–82.
Roemer FW, Guermazi A, Hannon MJ, Fujii T, Omoumi P, Hunter DJ, et al. Presence of Magnetic Resonance Imaging-Defined Inflammation Particularly in Overweight and Obese Women Increases Risk of Radiographic Knee Osteoarthritis: the POMA study. Arthritis Care Res. 2022;74:1391–8.
Roemer FW, Jarraya M, Felson DT, Hayashi D, Crema MD, Loeuille D, et al. Magnetic resonance imaging of Hoffa’s fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthritis Cartilage. 2016;24:383–97.
Burda B, Steidle-Kloc E, Dannhauer T, Wirth W, Ruhdorfer A, Eckstein F. Variance in infra-patellar fat pad volume: Does the body mass index matter?-Data from osteoarthritis initiative participants without symptoms or signs of knee disease. Ann Anat Anat Anz Off Organ Anat Ges. 2017;213:19–24.
Culvenor AG, Felson DT, Wirth W, Dannhauer T, Eckstein F. Is local or central adiposity more strongly associated with incident knee osteoarthritis than the body mass index in men or women? Osteoarthritis Cartilage. 2018;26:1033–7.
Acknowledgements
This manuscript was prepared using an Osteoarthritis Initiative (OAI) public-use data set and does not necessarily reflect the opinions or views of the OAI investigators, the National Institutes of Health (NIH), or the private funding partners. The OAI is a public–private partnership between the NIH (contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR- 2-2261, and N01-AR-2-2262) and private funding partners (Merck Research Laboratories, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer, Inc.), with research conducted by the Osteoarthritis Initiative Study Investigators. The private sector funding for the OAI is managed by the Foundation for the NIH.
Funding
This study was funded by the NIAMS at the NIH (grants R01-AR064771, R01-AR078917, and R01-AG070647). The funding source had no role in the study design, collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: MTL, GBJ, JAL, NEL, MN, CEM, TML. Acquisition of data: MTL, CN, PG, ZA, JAL. Analysis and interpretation of data: MTL, GBJ, JAL, NEL, MN, CEM, TML. Statistical expertise: MTL, GBJ, JAL, NEL, MN, CEM. Drafting of article or revising it critically for important intellectual content: MTL, GBJ, ZA, NEL, MN, CEM, TML. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the National Institutes of Health (NIH) appointed an independent Observational Study Monitoring Board (OSMB) to oversee the OAI study from 2002 to 2014. The OSMB’s responsibilities included protecting the safety of study participants, considering ethical and other external factors that could impact participant safety, and monitoring the progress of the study. This Health Insurance Portability and Accountability Act (HIPAA)-compliant study has been approved by the OSMB and written informed consent was obtained from all study participants. The OSMB was disbanded upon study completion.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Löffler, M.T., Ngarmsrikam, C., Giesler, P. et al. Effect of weight loss on knee joint synovitis over 48 months and mediation by subcutaneous fat around the knee: data from the Osteoarthritis Initiative. BMC Musculoskelet Disord 25, 300 (2024). https://doi.org/10.1186/s12891-024-07397-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07397-y