Abstract
Background
Vitamin D and calcium-rich foods, exposure to sunlight, and physical activities (PA) play a pivotal role in promoting the production of sufficient vitamin D and improving grip strength needed for better bone health among school children.
Purpose
This study aimed to determine the effects of hand grip muscle strength (HGS), vitamin D in addition to diets, and PA on bone health status among 6–12 years old schoolchildren.
Methods
This study was based on a cross-sectional observational design, which was descriptive in nature. A diverse sample of 560 elementary school children aged 6–12 years old were invited to participate in this descriptive cross-sectional study. The Dual-Energy X-Ray Absorptiometry (DXA), QUS technique, and ACTi graph GT1M accelerometer were used respectively as a valid tools to identify BMD, BMC, and other parameters of bone health like c-BUA values and bone stiffness (SI), and physical activity (PA) of all individuals participated in this study. In addition, a hydraulic dynamometer was used to measure hand grip strength among the participants. Moreover, an immunoassay technique was used to measure the serum levels of vitamin 25(OH)D level, and bone metabolism markers; NTX, DPD, Ca, and sBAP in all participants. Bone loss (osteoporosis) was cross-sectionally predicted in 19.64% of the total population, most of whom were girls (14.3% vs. 5.4% for boys; P = 0.01). Compared to boys, the incidence of osteoporosis was higher and significantly correlated in girls with lower HGS, deficient vitamin D, inadequate vitamin D and Ca intake, greater adiposity, poor PA, and lower sun exposure. Also, in girls, lower vitamin 25(OH)D levels, and poor HGS were shown to be significantly associated with lower values of BMD, BMC, SI, and higher values of bone resorption markers; NTX, DPD, and sBAP and lower serum Ca than do in boys. The findings suggested that deficient vitamin D, lower HGS, adiposity, PA, and sun exposure as related risk factors to the pravelence of bone loss among school children, particularly in girls. In addition, these parameters might be considered diagnostic non-invasive predictors of bone health for clinical use in epidemiological contexts; however, more studies are required.
Similar content being viewed by others
Introduction
Skeletal muscles are one of the main forces that can generate a maximum enormous reaction during human activities [1,2,3]. These muscle forces produce a trophic or adaptive effect on bone mass locally during exercise training. Thus, a satisfied skeletal muscle adaptation leads in such a way to an increase in humeral bone mass [1,2,3]. In addition, hand grip strength as a model of muscle adaptation technique showed a positive association with local bone mass at the wrist or forearm among nonathletes [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Previously, grip strength showed a positive significant association with bone mass at other sites, including the hip and/or spine in females however, insignificant association was reported between grip strength and bone mineral density (BMD) among men [17,18,19]. The grip strength is associated with the incidence of osteoporotic fractures, particularly vertebral fractures [20]. In recent studies, grip strength exercises was used as a treatment strategy for the patients with primary subacromial impingement syndrome [21]. Also, it was report in recnt study that grip strength along with the levels of vitamin D might be used as a pridector of liver fibrosis and malnutrition in patients with Chronic Hepatitis C virus (HCV) [22].
During childhood and adolescence, the growth of a healthy peak bone mass is needed for better bone health which might be a key limited factor of bone health and future fracture risk during adulthood [23, 24]. A number of physiological and lifestyle factors such as genetics, hormonal, both calcium and vitamin D intake, physical activity, and nutrition showed to be effective in the bone health of children and adolescents [25, 26]. Hand grip strength was associated with bone density and mineral content in children and adolescents [27,28,29,30]. In addition, other physiological factors such as maximum oxygen consumption and maximum expiratory flow were shown to be significantly associated with bone mineral density in children, adolescents, and adults [27,28,29,30].
Physical activity (PA) with higher levels showed to optimize skeletal development, and significantly aid to prevent age-related bone loss and osteoporotic fractures [31,32,33,34,35]. The correlation between muscle optimization and bone status was suggested to be mechanical in origin, whereas the skeletall system clearly adapts to both stress and mechanical loads on muscles which produce powerful loading forces on the bone [36]. In school-based interventions, high-impact PA was clearly observed to improve both the muscle force and strength acting on the bone [37, 38]. These observations obviously suggested the pivotal role of, muscle strength and muscle mass in bone development during growth [39]. Conversely, children with sedentary life behaviors, such as sitting for a long time in front of screens watching television or playing computer games may have a negative or adverse effect on bone health [31, 40,41,42,43].
Vitamin D also showed to have a prospective role in skeletal muscle structure, function, and bone health [44, 45]. Muscle weakness and poor bone health were shown to be linked with vitamin D deficiency in both children and adults. This significantly supports the indirect benefits role of vitamin D on skeletal health via the regulation of calcium homeostasis [43]. Previous reports confirmed the role of vitamin D in muscle function, calcium absorption, and maintaining homeostasis [44,45,46,47,48,49,50,51,52,53,54,55]. In cases with vitamin D deficiency, poor muscle function appears as a sign of bone disease before the biochemical bone profiles [46, 47].
In schoolchildren, serum 25(OH)D levels showed to be associated with handgrip strength. In these studies, the authors signify the pivotal roles of vitamin D levels, calcium and vitamin D intake, and exposure to sunlight in improving hand grip strength and bone health [48].
Given the potential positive impact of vitamin D status and diets on both muscle strength and bone loss risks, it is important to consider the role of vitamin D and its relationship between muscle strength and bone health among school children [41,42,43,44,45,46,47,48]. Thus, the proposed aim of this study was to evaluate the effects of hand grip muscle strength, vitamin D in addition to diets, sunexposure, and physical activity on bone health status among a diverse sample of schoolchildren aged 6–12 years old.
Materials and methods
Study participants
This descriptive cross-sectional study included a total of five-hundred sixty elementary schoolchildren (boys n = 360 and girls n = 200) aged 6–12 years old who were invited via emails to the respective administrative personnel during September 2018 and May 2019. The required students were Students with physical, genetic, endocrine, and cardiovascular disorders who were prevented from participating in this study. Also, students with chronic diseases, including diabetes, cardiac, pulmonary, and neurological diseases as well as acute infections, or who received medical therapy or diets that may affect on the data of vitamin D and bone were excluded from this study [49].
Study settings
The sample size of 560 was selected from the list of students in 10 public schools in a large geographical area of Riyadh province to give an estimated power of 96% and a significance level of 0.05 with an expected frequency of 5.4%. The study was performed at CAMS, King Saud University, and supervised by an expert physiotherapist with more than 10 years of experience. Blood samples were collected from all subjects using a heparinized syringe, and plasma samples were obtained from whole blood following centrifugation for 1 min at 1400 rpm. The samples were frozen at 20° C until use [49]. Demographic and clinical data of the participants are in Table 1.
Ethical consideration
Based on the ethical guidelines of the 1975 Declaration of Helsinki, the study protocol was reviewed and approved by the ethics Sub-Committee of King Saud University, Saudi Arabia. Assignment of a signed written informed consent was obtained from the parents of all participating schoolchildren before collecting data and blood samples of each participant.
Anthropometric measurements
Standardized procedures such as a tape measure and calibrated Salter Electronic Scales (Digital Pearson Scale; ADAM Equipment Inc., Columbia, MD, USA) were used to measure the height and weight of all participants, respectively. Adiposity parameters such as BMI and Waist-to-height ratio (WHtR) were calculated according to previously validated universal cutoff values [45,46,47,48]. These universal WHtR cut-off values were based on data obtained from schoolchildren internationally and were significantly appreciated to identify severe complications, particularly early cardiovascular risk in both children and adolescents [50,51,52,53].
Assessment of bone structure and body composition
All participants were subjected to a total body scan by using “the Dual-Energy X-Ray Absorptiometry (DXA) (Lunar Prodigy; General Electric, Fairfield, CT) as previously reported in the literature [54]. According to the manufacturer’s instructions, all measurements were evaluated by a well well-trained technician. For each participant, both the BMD (g/cm2) and the BMC (g) were measured in addition to other body composition variables, such as the percentage of body fat (% F), muscle mass (kg), bone mass (kg), and fat mass (kg) respectively. To estimate the reliability of the scan, the measurements were repeated directly on the same day to obtain lower technical error of measurement (TEM; < 1..5%) as previously estimated [54, 55]. T-scores are a commonly used method for assessing bone health in both children and adults. T-scores are calculated by comparing an individual’s bone mineral density (BMD) to the average BMD of a healthy young adult of the same sex. This comparison is made using a statistical measure known as a standard deviation (SD), which expresses how much an individual’s BMD differs from the average young adult BMD [56, 57]. In children, T-scores are often used to identify bone health because they provide a way to compare a child’s BMD to that of a healthy young adult, which is considered the “gold standard” for bone health [56,57,58]. This is important because children’s bones are still growing and developing, and their BMD can vary widely depending on their age, sex, and other factors such as nutrition and physical activity. Thus, in terms of accuracy, T-scores are generally considered the most reliable measure of bone health in children and adults. So, according to the T-score obtained from the DXA scan measurements, osteoporosis was diagnosed among participants as normal (T-score ≥ −1), osteopenia (low bone density; T-score; −1 to −2.5), osteoporosis (T-score ≤ −2.5), and severe or established osteoporosis (T-score; ≤ − 2.5 with fracture [58].
A commercially used Achilles ultrasound densitometer (Lunar Corporation, Madison, WI) QUS technique was used to measure bone health and stiffness (SI) of all participants, as reported previously [57, 58]. As previously reported, both c-BUA values and bone stiffness (SI) were significantly related to bone health in young individuals, [59, 60]. Based on broadband ultrasound attenuation (dB/MHz) and speed of sound (m/s) parameters, both c-BUA values and bone stiffness (SI) were identified in brief detail in young individuals on the left and right calcaneus as previously reported [61,62,63,64]. QUS measurements are correlated with DXA measurements and are used as a valid tool for indicating the risk of osteoporotic fractures in children [61, 62]. In addition, it shows a good reflection on bone changes during growth and recorded the strongest association with DXA measures of bone mass in children [65, 66].
In children, however, the clinical usefulness of QUS has not yet been investigated, and comparison studies showed inconsistent correlations with DXA [59, 60]. In clinical practice, poor bone health was considered among participants when c-BUA values were recorded below or equal to QUS- Z-score cutoff of ≤ −1.5 as previously mentioned [67], and that c-BUA values were coefficiently varied from 0.69% to 1.8% within-day of measurements as mentioned in the literature [68].
Assessment of hand grip strength
For each participant, a manual hydraulic dynamometer labeled JAMAR (Hydraulic Hand Dynamometer® Model PC-5030 J1, Fred Sammons, Inc., Burr Ridge, IL: USA) was used to measure hand grip strength with 0.1 lbf accuracy of both the right and left hands as previously reported in the literature [55, 62]. First, in the standard position, each student was seated in a straight-backed chair. Then, he was asked to squeeze the dynamometer two times with each hand. For each hand, approximately 2-min rest lapsed between trials to control for the effects of fatigue on each hand alternated. The best value of two attempts was recorded. The inter-rater Technical Error of Measurement was less than 2.5% for both hands [55, 69, 70]. Based on grip strength scores (HGS), children were classified into three groups; Low (n = 100; HGS; 0–230mmHg), normal (n = 300; HGS ≥ 300mmHg), and moderate (n = 160; HGS;231–299mmHg) respectively [71,72,73].
Diet information and physical activity
All schoolchildren were instructed not to change their normal eating habits during the study period. Parents were asked to record accurately the amount, type of food, and fluid consumed using food diaries. For each participant, dietary information was extensively referred according to reference dietary intakes for physically active people [74, 75].
Physical activity for each participant was evaluated for 7 consecutive days using ACTi graph GT1M accelerometer (model WAM 7164; Fort Walton Beach, FL). The average intensity of PA was calculated from the total number of minutes each child participated in sports activities with different intensities. This intensity is based mainly on count thresholds and daily activity counts per minute. Children with fewer accelerometer counts (≤ 100 counts/min) were characterized by a sedentary lifestyle [76, 77]. According to energy expenditure, the PA of all participants was classified as low or sedentary ( thresholds are less than 4 metabolic equivalents [METs], moderate activity (thresholds of 4 metabolic equivalents [METs]), and vigorous activity (thresholds of 7 METs), respectively as previously mentioned, whereas 1 MET refers to either energy expenditure of 1 kcal/kg/h or oxygen uptake in 3.5 mL/kg/min during a quiet sitting position [69, 78].
Assessement of 25-hydroxyvitamin D and bone metabolism
From freshly separated serum samples of each student, serum vitamin 25(OH)D level, NTX, DPD, Ca and sBAP concentrations were estimated as outcome measures of bone health as previously reported [79,80,81,82,83]. Colorimetric and immunoenzymometic assays along with immunoassay kits such as (IDS, Tyne & Wear, UK) for vitamin 25(OH)D, (Hoffmann-La Roche Ltd., Basel, Switzerland) for Ca levels, and (Quidel Corporation, San Diego, CA, USA) for sBAP concentrations (U/L) were significantly used to measure Vitamin 25(OH)D levels, Ca levels, and sBAP concentrations (U/L in serum samples of the participating students as mentioned previously in the literature [79,80,81,82,83]. Also, ELISA kits (Osteomark, Ostex International, Seattle, WA, USA) for NTX and enzyme immunoassay kits (Metra Biosystems, Mountain View, CA, USA) for DPD were used to estimate the levels of both NTX and DPD respectively in urine samples of the participating students using enzyme immunoassay techniques [81].
Due to the importance of sunlight exposure is an important source of vitamin D synthesis which contributes to the bone mineralization process [84]. Thus, all schoolchildren’s daily exposure to sun during the previous month was estimated as the average number of hours per day the students were exposed to the sun [85, 86]. The mean daylight duration of exposure was adjusted to be (±0.1h) as previously calculated using astronomical tables [60].
Statistical analysis
In this study, for the analysis of the data, the statistical software SPSS version 18 was used. The results obtained were expressed as Mean and standard deviation Among groups, Kruskal–Wallis one-way ANOVA, and post-hoc (Tukey HSD) test were used to compare the mean values of the studied variables [54]. The relationship between various study parameters were performed by spearman rank correlation analysis. Linear regression analysis was performed in steps for hand grip strength, bone markers, vitamin D, Ca intake, and lifiestyle paramters like physical activity (PA), sunexposure, and adiposity as the independent variables and BMD, BMC,BSI,and osteoprosis as dependent variables. The data obtained were deemed significant at P < 0.05 [54].
Results
A total of 560 school children aged 6–12 years old were recruited in this descriptive cross-sectional study to evaluate the effect of hand grip strength (HGs), vitamin D, and dites on bone health. Based on hand grip strength measurements, children were classified into three groups; Low (n = 100; HGS; 0–230mmHg), normal (n = 300; HGS ≥ 300mmHg), and moderate (n = 160; HGS;231–299mmHg) respectively. All studied variables were described statistically as shown in Table 1.
In relation to children with normal HGs, children with lower and moderate HGs showed a greater percentage of fat, body fat, adiposity markers; BMI, WHtR, and lower percentage of lean mass, total body BMD, total body BMC, bone stiffness index (BSI), and isometric grip strength (right and left) respectively (p < 0.001) as shown in Table 1 and Fig. 1D. Also, lower diet scores, inadequate vitamin D and calcium intake, lower sun exposure, and lower physical activity were significantly reported in children with lower and moderate HGs compared to subjects with normal HGs (Table 1).
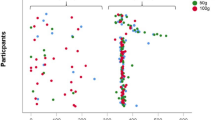
BMD, BMC, vitamin D, and realated bone marker values; NTX, DPD, s-Ca, s-BAP for school childern based on hand grip strength expressed in categories (low, normal and moderate). A Significant difference in vitamin D status in school children with low (p = 0.01) and moderate (p = 0.001) grip strength compared to those with normal HGS, B significant decerease (p = 0.0001) in serum Ca, and increase in s-BAP (p = 0.01) was estimated in subjects with lower to moderate HGS, C significant increase (p = 0.001; P = 0.01) in the levels of bone markers; NTX and DPD in subjects with lower HGS, D significant decrease in total BMD, BMC, and BSI index was estimated in all school chikldern with lower (p = 0.001) to moderate (p = 0.05) HGS respectively
In this study, serum vitamin 25(OH)D level, NTX, DPD, Ca, and sBAP concentrations were estimated as outcome measures of bone health among studied children (Fig. 1). A significant decline in 25(OH)D levels was estimated in school children with low (p = 0.01) and moderate (p = 0.001) grip strength compared to those with normal HGS (Fig. 1A). Also, a significant decrease (p = 0.0001) in serum Ca, and an increase in s-BAP (p = 0.01) concentrations were estimated in subjects with lower to moderate HGS (Fig. 1B). Similarly, NTX and DPD as markers of bone resorption were significantly increased in children with lower (p = 0.001) and moderate HGs (P = 0.01) respectively in comparison with those of control subjects (Fig. 1C).
Figures 2 and 3 illustrate the significant differences in BMD, BMC, BSI, and the percentage of bone loss or osteoporosis based on the categories of hand grip strength and vitamin D status. In both genders, differences occurred between the three categories of hand grip strength (HGS). Moreover, lower BMD, BMC, and BSI, (Fig. 2A, B, C) were significantly estimated (p = 0.01) in girls when compared to the boys in all the categories of HGS.
BMD, BMC, BSI, and bone loss values for school boys and girls based on hand grip strength expressed in categories (normal, moderate and low HGs). Significant decrease in BMD (A), BMC (B), and BSI (C) were estimated in school girls compared to boys. Also, bone loss or osteoporosis was significantly estimated in girls (p = 0.01) than in boys (D)
BMD, BMC, BSI, and realated bone marker values; NTX, DPD for school boys and girls based on vitamin D status expressed in categories (normal, insufficient, and deficient). Significant decrease in BMD (A), BMC (B), BSI (C) along with an increase in the levels of bone resorption markers NTX and DPD (D) were estimated in school girls compared to boys
Also, in this study bone loss (osteopenia/ osteoporosis) was significantly predicted in 19.64% of the total populations most of them were girls (14.3% vs 5.4% for boys; P = 0.01). The incidence of osteoporosis was higher and significantly correlated in girls with lower and moderate HGS than in boys of the same category as shown in Fig. 2D. The data also showed that increases in obesity, lower HGS, deficient vitamin D status, diets containing inadequate vitamin D and Ca values, sedentary lifestyle or lower PA, and inadequate exposure to sunlight, in addition to higher expression of bone resorption markers were significantly considered as related risk factors to the prevalence of bone loss among school children particularly in girls (Table 2).
The correlation between vitamin D status and bone health was evaluated in this study. A significant decrease in BMD, BMC, and BSI (Fig. 3A, B, C) along with an increase in the levels of bone resorption markers NTX and DPD (Fig. 3D) was estimated in school girls who showed a deficient (P = 0.001) or insufficient (P = 0.01) vitamin D status compared to boys of the same category.
In this study, linear regression analysis in step multiples revealed that left and right hand grip strength, vitamin 25(OH)D status, bone markers; NTX, DPD, Ca,s-BAP, diet scores, adiposity, PA, and sun exposure variables were associated with the bone health (BMD, BMC, and BSI), and incidence rates of osteoporosis among school children of both genders. The percentage of explained variation was greater in boys than girls as shown in Table 3. For example; left and right hand grip for boys showed R = 0.76 R2 = 0.48 with percentage of 76% and 48% with higher estimate of 10% than for girls R = 0.58, R2 = 0.29 with EE = 6% compared to boys. Thus, in our Table 3, the results are more than zero with a ratio percentage higher than estimated for girls and were concluded that the selected cofounders could predict the health of bones among the selected schoolchildren.
Discussion
The results of our current research significantly illustrate that the HGS, vitamin D status, physical activity, diets, adiposity, and exposure to sunlight are associated moderately with BMD, BSI, BMC, and osteoporosis in school children of both genders.
These results refer to a positive association between bone formation and hand grip strength in the arms. Therefore, in boys, the HGS explains around 48% of the BMD, 69% of the BMC, 64% of the BSI, and 21% of the bone loss compared to girls who showed 29% of the BMD and 26% of the BMC, 23% of the BSI, and 31% of the bone loss respectively. Also, osteoporosis was significantly predicted in 19.64% of the total population most of them were girls (14.3% vs 5.4% for boys; P = 0.01). The incidence of osteoporosis was higher and significantly correlated in girls with lower and moderate HGS than in boys of the same category. Several studies showed positive correlations between parameters of bone health and HGS in physically active and non-active subjects [14, 60, 87,88,89,90]. These results revealed that the HGS independently could predict the bone health of boys and girls. These systematic associations suggest that elevated levels of HGS help in promoting better bone health by producing a mediating effect over all the musculoskeletal and respiratory systems [14, 90]. HGS showed to be associated with bone mineral density in adolescent students. Lower HGS values significantly correlated with poor scores of the total body BMD and BMC respectively [54]. The defects in hand grip strength were diagnosed as bone fragility in the total body that could be associated with the loss of physical function and a negative impact on recovering health, particularly after an illness or surgery [91, 92]. Our findings, could explain that hand grip isometric exercise provides a promising adaptation to bones via static and dynamic forces created by muscular contractions [93]. Also, bone density and bone minerals content showed to be associated with hand grip strength in children and adolescents [25,26,27,28]. Hand grip strength in association with other physiological factors such as maximum oxygen consumption, and maximum expiratory flow showed to be linked with bone mineral density in children, adolescents, and adults [25,26,27,28].
In this study, there was adifference in isometric strength between both genders. This could be explained by a greater physical performance level in boys compared to girls. During the same stage of isometric strength, boys are liable to have more performance levels than girls [3]. Additionally, girls may be at a greater risk of evolving bone fragility during adulthood as compared to boys of the same category [94].
In this current study, vitamin D status, and diets containing Ca and vitamin D were shown to be the most promising outcome measures significantly associated with both the scores of HGS and bone health among school children. Lower vitamin 25(OH)D status, and inadequate Ca and vitamin D intake were shown to be significantly associated with lower HGS, bone density markers; BMD, BMC, and BSI, in addition to higher expression values of NTX, DPD, s-BAP, and decline in serum calcium, esecially in girls than boys. This was confirmed by the higher incidence of osteoporosis (19.64%) of the total population studied, most of them were girls (14.3% vs 5.4% for boys; P = 0.01).
Our findings are in line with other studies which confirmed the role of calcium and vitamin D intake and vitamin D deficiency, along with other important factors such as genetics, hormonal, physical activity, and adiposity on bone health among children, and adolescents [23, 24].
In children and adults, muscle weakness and “poor bone health were shown to be linked with vitamin D deficiency, this established the indirect benifits role of vitamin D on skeletal health via regulation of calcium homeostasis [43]. The role of vitamin D was clearly observed in muscle function [44], calcium absorption, and maintaining homeostasis [45], and any deficiency in vitamin D produces poor muscle function which appears before the biochemical signs of bone disease [46, 47]. Recently, in schoolchildren, a significant positive association was reported between serum 25(OH)D levels and handgrip strength.
The data of this study clearly imply the importance of vitamin D-rich foods along with calcium intake, and exposure to sunlight in the production of sufficient vitamin D and improving grip strength and bone health among school children [48]. The data also showed that obesity, a sedentary lifestyle or lower PA, and inadequate exposure to sunlight, were significantly considered as related risk factors to bone loss among school children particularly in girls.
In girls, lower values of total bone density parameters; BMD, BMC, BSI, and abnormal expression of biomarkers of bone metabolism; NTC, DPD, Ca, and s-BAP were significantly associated with poor PA, obesity, and inadequate sun exposure. These parameters confer an additional confirmation on the relative higher osteoporosis among girls. Our results are matched with those who reported that physical activity (PA) with higher intensities significantly optimizes skeletal development and significantly aids in preventing age-related bone loss and osteoporotic fractures during childhood [30,31,32,33]. This correlation obtained between muscle optimization and bone status was suggested to be mechanical in origin, whereas the skeletal system clearly adapts to both stress and mechanical loads on muscles, producing powerful loading forces on the bone [34]. Also, in school-based interventions, high-impact PA was clearly observed to improve both the muscle force and strength acting on the bone [35, 36]. These observations obviously suggested the pivotal role of muscle strength and muscle mass in bone development during growth [37]. Conversely, children with sedentary life behaviors, such as sitting for a long time in front of screens, watching television, or playing computer games, may have a negative or adverse effect on bone health [31, 38,39,40,41].
In general, hand grip strength, vitamin D status, adiposity, PA, sun exposure, and diets containing adequate amounts of Ca and Vitamin D are associated with a certain adverse effects on bones or could predict bone health in school children in both genders [30,31,32,33, 48, 87, 95,96,97,98,99,100].
Conclusions
The HGS, vitamin D status, PA, diets, adiposity, and sun exposure are positively associated with the bone health of school children aged 6–12 years old of both genders. The students with the lowest hand grip strength and vitamin D values showed lower BMD, BMC, and BSI values. Moreover, it is important to point out that the influence of vitamin D values, PA, diets, adiposity, and sun exposure is greater with regard to bone health in correlation with isometric grip strength in both arms of children. These results strengthen the enclosure of physical exercise, diets, vitamin D, and sunlight exposure to improve skeletal muscle strength and bone health in schoolchildren. In addition, these outcome measures might be considered diagnostic non-invasive predictors of bone health for clinical use in epidemiological contexts; however, more studies are required.
Availability of data and materials
All data generated or analyzed during this study are presented in the manuscript. Please contact the corresponding author for access to the data presented in this study.
References
Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020;2(9):817–28.
Joanisse S, Lim C, McKendry J, Mcleod JC, Stokes T, Phillips SM. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. F1000Res. 2020;9:F1000 Faculty Rev-141.
Beverly MC, Rider TA, Evans MJ, Smith R. Local bone mineral response to brief exercise that stresses the skeleton. Br Med J. 1989;299:233–5.
Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. 2020;1(94):155–218.
Chen F, Su Q, Tu Y, Zhang J, Chen X, Zhao T, Huang Y, Xu G. Maximal muscle strength and body composition are associated with bone mineral density in chinese adult males. Medicine. 2020;99(6):e19050.
Kapuš O, Gába A, Lehnert M. Relationships between bone mineral density, body composition, and isokinetic strength in postmenopausal women. Bone Rep. 2020;1(12):100255.
Ravi S, Kujala UM, Tammelin TH, Hirvensalo M, Kovanen V, Valtonen M, Waller B, Aukee P, Sipilä S, Laakkonen EK. Adolescent sport participation and age at menarche in relation to midlife body composition, bone mineral density, fitness, and physical activity. J Clin Med. 2020;9(12):3797.
Sinaki M, Wahner HW, Offord KP. Relationship between grip strength and related regional bone mineral content. Arch Phys Med Rehabil. 1989;70:823–6.
Luo Y, Jiang K, He M. Association between grip strength and bone mineral density in general US population of NHANES 2013–2014. Arch Osteoporos. 2020;15:1–9.
Torres-Costoso A, López-Muñoz P, Martínez-Vizcaíno V, Álvarez-Bueno C, Cavero-Redondo I. Association between muscular strength and bone health from children to young adults: a systematic review and meta-analysis. Sports Med. 2020;50:1163–90.
Chan J, Lu YC, Yao MM, Kosik RO. Correlation between hand grip strength and regional muscle mass in older Asian adults: an observational study. BMC Geriatr. 2022;22(1):1–9.
Pratt J, De Vito G, Narici M, Segurado R, Dolan J, Conroy J, Boreham C. Grip strength performance from 9431 participants of the GenoFit study: normative data and associated factors. Geroscience. 2021;43(5):2533–46.
Snow-Harter C, Bouxsein M, Lewis B, Charette S, Weinstein P, Marcus R. Muscle strength as a predictor of bone mineral density in young women. J Bone Miner Res. 1990;5:589–95.
Tsuji S, Tsunoda N, Yata H, Katsukawa F, Onishi S, Yamazaki H. Relation between grip strength and radial bone mineral density in young athletes. Arch Phys Med Rehabil. 1995;76:234–8.
Osei-Hyiaman D, Ueji M, Toyokawa H, Takahashi H, Kano K. Influence of grip strength on metacarpal bone mineral density in postmenopausal Japanese women: a cross sectional study. Calcif Tiss Int. 1999;64:263–6.
Di Monaco M, Di Monaco R, Manca M, Cavanna A. Handgrip strength is an independent predictor of distal radius bone mineral density in postmenopausal women. Clin Rheumatol. 2000;19:473–6.
Foley KT, Owings TM, Pavol MJ, Grabiner MD. Maximum grip strength is not related to bone mineral density of the proximal femur in older adults. Calcif Tiss Int. 1999;64:291–4.
Van Pottelbergh I, Goemaere S, Nuytinck L, De Paepe A, Kaufman JM. Association of the type I collagen alpha1 Sp1 polymorphism, bone density and upper limb muscle strength in community dwelling elderly men. Osteoporos Int. 2001;12:895–901.
Taafe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the health, aging and body composition study. J Bone Miner Res. 2001;16:1343–52.
Albrand G, Munoz F, Sornay-Rendu E, DuBoeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone. 2003;32:78–85.
AlAnazi A, Alghadir AH, Gabr SA. Handgrip strength exercises modulate shoulder pain, function, and strength of rotator cuff muscles of patients with primary subacromial impingement syndrome. Biomed Res Int. 2022;30(2022):9151831. https://doi.org/10.1155/2022/9151831.
Gabr SA, Alghadir AH. Handgrip strength and vitamin D as predictors of liver fibrosis and malnutrition in chronic hepatitis C patients. Dis Markers. 2021;2(2021):6665893. https://doi.org/10.1155/2021/6665893.
Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305.
Gordon CM. Evaluation of bone density in children. Curr Opin Endocrinol Diabetes Obes. 2005;12(6):444–51.
Lehtonen-Veromaa M, Mottonen T, Nuotio I, Heinonen OJ, Viikari J. Influence of physical activity on ultrasound an dual-energy x-ray absorptiometry bone measurements in peripubertal girls: a crosssectional study. Calcif Tissue Int. 2000;66:248–54.
Buntain HM, Greer RM, Schluter PJ, Wong JCH, Batch JA, Potter JM, Lewindon PJ, Powell E, Wainwright E, Bel SC. Bone mineral density in Australian children, adolescents and adults with cystic fibrosis: a controlled cross sectional study. Thorax. 2004;59:149–55.
Chan DCC, Lee WTL, Lo DHS, Leung JC, Kwok AWL, Leung PC. Relationship between grip strength and bone mineral density in healthy Hong Kong adolescents. Osteoporos Int. 2008;19:1485–95.
Legroux-Gérot I, Leroy S, Prudhomme C, Perez T, Flipo RM, Wallaert B, et al. Bone loss in adults with cystic fibrosis: prevalence, associated factors, and usefulness of biological markers. Joint Bone Spine. 2012;79:73–7.
Petterson U, Nordstrom P, Lorentzon R. A comparison of bone mineral density and muscle strength in young male adults with different exercise level. Calcif Tissue Int. 1999;64:490–8.
Lekamwasam S, Trivedi DP, Khaw KT. An association between respiratory function and hip bone mineral density in older men: a cross-sectional study. Osteoporos Int. 2005;16:204–7.
Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, et al. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29(10):2161–81. https://doi.org/10.1002/jbmr.2254.
Nilsson M, Sundh D, Ohlsson C, Karlsson M, Mellstrom D, Lorentzon M. Exercise during growth and young adulthood is independently associatedwith cortical bone size and strength in old Swedish men. J Bone Miner Res. 2014;29(8):1795–804. https://doi.org/10.1002/jbmr.2212.
Harvey NC, Cole ZA, Crozier SR, Kim M, Ntani G, Goodfellow L, et al. Physical activity, calcium intake and childhood bone mineral: a population-based cross-sectional study. Osteoporos Int. 2012;23(1):121–30. https://doi.org/10.1007/s00198-011-1641-y.
Cardadeiro G, Baptista F, Ornelas R, Janz KF, Sardinha LB. Sex specific association of physical activity on proximal femur BMD in 9 to 10 year-old children. PLoS One. 2012;7(11):e50657. https://doi.org/10.1371/journal.pone.0050657.
Janz KF, Letuchy EM, Burns TL, Eichenberger Gilmore JM, Torner JC, Levy SM. Objectively measured physical activity trajectories predict adolescent bone strength: Iowa Bone Development Study. Br J Sports Med. 2014;48(13):1032–6. https://doi.org/10.1136/bjsports-2014-093574.
Sanders KM, Scott D, Ebeling PR. Vitamin D deficiency and its role in muscle-bone interactions in the elderly. Curr Osteoporos Rep. 2014;12:74–81.
Herrmann D, Hebestreit A, Ahrens W. Impact of physical activity and exercise on bone health in the life course: a review. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(1):35–54. https://doi.org/10.1007/s00103-011-1393-z.
Meyer U, Romann M, Zahner L, Schindler C, Puder JJ, Kraenzlin M, et al. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone. 2011;48(4):792–7. https://doi.org/10.1016/j.bone.2010.11.018.
Schoenau E, Frost HM. The “muscle-bone unit” in children and adolescents. Calcif Tissue Int. 2002;70(5):405–7. https://doi.org/10.1007/s00223-001-0048-8.
Heidemann M, Molgaard C, Husby S, Schou AJ, Klakk H, Moller NC, et al. The intensity of physical activity influences bone mineral accrual in childhood: the childhood health, activity and motor performance school (the CHAMPS) study. Denmark BMC Pediatr. 2013;13:32. https://doi.org/10.1186/1471-2431-13-32.
De Smet S, Michels N, Polfliet C, D’Haese S, Roggen I, De Henauw S, et al. The influence of dairy consumption and physical activity on ultrasound bone measurements in Flemish children. J Bone Miner Metab. 2014. https://doi.org/10.1007/s00774-014-0577-7.
Alghadir AH, Gabr SA, Iqbal ZA. Television watching, diet and body mass index of school children in Saudi Arabia. Pediatr Int. 2016;58(4):290–4.
Alghadir AH, Gabr SA, Iqbal ZA. Effects of sitting time associated with media consumption on physical activity patterns and daily energy expenditure of Saudi school students. J Phys Ther Sci. 2015;27(9):2807–12.
Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99:4336–45.
Van Der Heyden JJ, Verrips A, Ter Laak HJ, Otten B, Fiselier T. Hypovitaminosis D-related myopathy in immigrant teenagers. Neuropediatrics. 2004;35:290–2.
Visser M, Deeg DJ, Lips P, Longitudinal Aging Study A. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–72.
Lips P. Which circulating level of 25-hydroxyvitamin D is appropriate? J Steroid Biochem Mol Biol. 2004;89–90(1–5):611–4.
Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66(6):419–24.
Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13(3):187–94.
Wakayo T, Belachew T, Whiting SJ. Serum vitamin D level associates with handgrip muscle strength among Ethiopian schoolchildren: a cross-sectional study. Food Nutr Bull. 2018;39(1):54–64.
Al-Rawaf HA. Circulating microRNAs and adipokines as markers of metabolic syndrome in adolescents with obesity. Clin Nutr. 2018. https://doi.org/10.1016/j.clnu.2018.09.024.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J. 2000;320:1–6.
Ashwel M, Lejeune S, McPherson K. Ratio of waist circumference to height may be better indicator ofneed for weight management. BMJ. 1996;312(7027):377.
Ramírez-Vélez R, Anzola A, Martinez-Torres J, Vivas A, Tordecilla-Sanders A, Prieto-Benavides D, Izquierdo M, Correa-Bautista JE, Garcia-Hermoso A. Metabolic syndrome and associated factors in a population-based sample of schoolchildren in Colombia: the FUPRECOL study. Metab Syndr Relat Disord. 2016;14:455–62.
Siervo M, Prado CM, Mire E, Broyles S, Wells JC, Heymsfield S, Katzmarzyk PT. Body composition indices of a load-capacity model: gender- and BMI-specific reference curves. Public Health Nutr. 2015;18:1245–54.
Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM, International Society for Clinical Densitometry. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225–42. https://doi.org/10.1016/j.jocd.2014.01.003.
Crabtree NJ, Shaw NJ, Bishop NJ, Adams JE, Mughal MZ, Arundel P, Fewtrell MS, Ahmed SF, Treadgold LA, Högler W, Bebbington NA, Ward KA, ALPHABET Study Team. Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults-the ALPHABET study. J Bone Miner Res. 2017;32(1):172–80. https://doi.org/10.1002/jbmr.2935.
Kelly TL, Berger N, Richardson TL. DXA body composition: theory and practice. Appl Radiat Isot. 1998;49:511–3.
Cossio-Bolaños M, Lee-Andruske C, de Arruda M, Luarte-Rocha C, Almonacid-Fierro A, Gómez-Campos R. Hand grip strength and maximum peak expiratory flow: determinants of bone mineral density of adolescent students. BMC Pediatr. 2018;18:96. https://doi.org/10.1186/s12887-018-1015-0.
Alghadir AH, Aly FA, Gabr SA. Effect of moderate aerobic training on bone metabolism indices among adult humans. Pak J Med Sci. 2014;30(4):840–4.
Ramírez-Vélez R, Ojeda-Pardo ML, Correa-Bautista JE, et al. Normative data for calcaneal broadband ultrasound attenuation among children and adolescents from Colombia:the FUPRECOL study. Arch Osteoporos. 2016;11:2. https://doi.org/10.1007/s11657-015-0253-0.
Jaworski M, Lebiedowski M, Lorenc RS, Trempe J. Ultrasound bone measurement in pediatric subjects. Calcif Tissue Int. 1995;56:368–71.
Miura S, Saavedra OL, Yamamoto S. Osteoporosis in urban post-menopausal women of the Philippines: prevalence and risk factors. Arch Osteoporos. 2008;3:17–24.
Baroncelli GI. Quantitative ultrasound methods to assess bone mineral status in children: technical characteristics, performance, and clinical application. Pediatr Res. 2008;63(3):220–8. https://doi.org/10.1203/PDR.0b013e318163a286.
Sioen I, Goemare S, Ahrens W, De Henauw S, De Vriendt T, Kaufman JM, et al. The relationship between paediatric calcaneal quantitative ultrasound measurements and dual energy X-ray absorptiometry (DXA) and DXA with laser (DXL) as well as body composition. Int J Obes (Lond). 2011;35(Suppl 1):S125–30. https://doi.org/10.1038/ijo.2011.44.
Herrmann D, Intemann T, Lauria F, Marild S, Molnár D, Moreno LA, et al. Reference values of bone stiffness index and C-terminal telopeptide in healthy European children. Int J Obes (Lond). 2014;38(Suppl 2):S76-85. https://doi.org/10.1038/ijo.2014.138.
Sioen I, Mouratidou T, Herrmann D, De Henauw S, Kaufman JM, Molnár D, et al. Relationship between markers of body fat and calcaneal bone stiffness differs between preschool and primary school children: results from the IDEFICS baseline survey. Calcif Tissue Int. 2012;91(4):276–85. https://doi.org/10.1007/s00223-012-9640-3.
Van den Bussche K, Michels N, Gracia-Marco L, Herrmann D, Eiben G, De Henauw S, et al. Influence of birth weight on calcaneal bone stiffness in Belgian preadolescent children. Calcif Tissue Int. 2012;91(4):267–75. https://doi.org/10.1007/s00223-012-9636-z.
Wang Q, Nicholson P, Timonen J, et al. Monitoring bone growth using quantitative ultrasound in comparison to DXA and pQCT. J Clin Densitom. 2007;11(2):295–301.
Sani FM, Sarji SA, Bilgen M. Quantitative ultrasound measurement of the calcaneus in Southeast Asian children with thalassemia: comparison with dual energy X-ray absorptiometry. J Ultrasound Med. 2011;30:883–94.
Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–386.
Vignolo M, Brignone A, Mascagni A, et al. Influence of age, sex, and growth variables on phalangeal quantitative ultrasound measures: a study in healthy children and adolescents. Calcif Tissue Int. 2003;72:681–8.
Richards LG, Olson B, Palmiter-Thomas P. How forearm position affects grip strength. Am J Occup Ther. 1996;50(Suppl 2):133–8.
Verroken C, Collet S, Lapauw B, T’Sjoen G. Osteoporosis and bone health in transgender individuals. Calcif Tissue Int. 2022;110(5):615–23. https://doi.org/10.1007/s00223-022-00972-2.
Dixon WG, Lunt M, Pye SR, Reeve J, Felsenberg D, Silman AJ, et al. Low grip strength is associated with bone mineral density and vertebral fracture in women. Rheumatology (Oxford). 2005;44(5):642–6. Epub 2005 Feb 22.
Ito T, Sugiura H, Ito Y, Noritake K, Ochi N. Relationship between the skeletal muscle mass index and physical activity of Japanese children: a cross-sectional, observational study. PLoS One. 2021;16(5):e0251025.
Gerber M, Ayekoé S, Bonfoh B, Coulibaly JT, Daouda D, Gba BC, Kouassi B, Traoré SG, du Randt R, Nqweniso S, Walter C. Is grip strength linked to body composition and cardiovascular risk markers in primary schoolchildren? Cross-sectional data from three African countries. BMJ Open. 2022;12(6):e052326.
Otten JJ, Hellwig JP, Meyers LD. Dietary reference intakes: the essential guide to nutrient requirements. Washington, DC: National Academy Press; 2006.
Harrell JS, McMurray RG, Baggett CD, et al. Energy costs of physical activities in children and adolescents. Med Sci Sports Exerc. 2005;37:329–36.
Trost SG, Pate RR, Sallis JF, et al. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc. 2002;34:350–5.
Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71:S114–20.
Mäder U, Martin BW, Schutz Y, et al. Validity of four short physical activity questionnaires in middle-aged persons. Med Sci Sports Exerc. 2006;38:1255–66.
Filteau S, Rehman AM, Yousafzai A, Chugh R, Kaur M, Sachdev HP, et al. Associations of vitamin D status, bone health and anthropometry, with gross motor development and performance of school-aged Indian children who were born at term with low birth weight. BMJ Open. 2016;6(1):e009268. https://doi.org/10.1136/bmjopen-2015-009268.
Alghadir AH, Gabr SA, Al-Eisa ES. Mechanical factors and vitamin D deficiency in schoolchildren with low back pain: biochemical and cross-sectional survey analysis. J Pain Res. 2017;10:855–65. https://doi.org/10.2147/JPR.S124859. eCollection 2017.
Alghadir AH, Gabr SA, Al-Eisa ES. Green tea and exercise interventions as nondrug remedies in geriatric patients with rheumatoid arthritis. J Phys Ther Sci. 2016;28(10):2820–9. Epub 2016 Oct 28.
Hassannia T, GhaznaviRad E, Vakili R, Taheri S, Rezaee SA. High prevalence of vitamin D deficiency and associated risk factors among employed women in a sunny industrial city. Int J Vitam Nutr Res. 2015;85(3–4):119–28.
Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;158:1–235.
Mcgrouther DA. Skin burns. In: Mann CV, Russel RCG, Williams NS, editors. Bailey and Love’s short practice of surgery. London: Chapman and Hall; 1995. p. 124–48.
Lotfi A, Abdel-Nasser AM, Hamdy A, Omran AA, El-Rehany MA. Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol. 2007;26(11):1895–901.
Timeanddate.com. http://www.timeanddate.com/. Accessed Mar 2023.
Ducher G, Jaffré C, Arlettaz A, Benhamou CL, Courteix D. Effects of longterm tennis playing on the muscle-bone relationship in the dominant and nondominant forearms. Can J Appl Physiol. 2005;30(1):3–17.
Baptista F, Barrigas C, Vieira F, Santa-Clara H, Homens PM, Fragoso I, Sardinha LB. The role of lean body mass and physical activity in bone health in children. J Bone Miner Metab. 2012;30(1):100–8.
Torres-Costoso A, Gracia-Marco L, Sánchez-López M, García-Prieto J, García-Hermoso A, Díez-Fernández A, Martínez-Vizcaíno V. Lean mass as a total mediator of the influence of muscular fitness on bone health in schoolchildren: a mediation analysis. J Sports Sci. 2014;33(8):817–30. https://doi.org/10.1080/02640414.2014.964750.
Sandler RB, Cauley JA, Sashin D, Scialabba MA, Kriska AM. The effect of grip strength on radial bone in postmenopausal women. J Orthop Res. 1989;7:440–4.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56.
Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30(2):135–42.
Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–101.
Trost SG, Rosencrantz RR, Dzewaltowski D. Physical activity levels among children attending after school programs. Med Sci Sports Exerc. 2008;40:622–9.
Campion JM, Maricic MJ. Osteoporosis in men. Am Fam Physician. 2003;67(7):1521–6.
Alghadir AH, Gabr SA, Rizk AA. Physical fitness, adiposity, and diets as surrogate measures of bone health in schoolchildren: a biochemical and cross-sectional survey analysis. J Clin Densitom. 2018;21(3):406–19. https://doi.org/10.1016/j.jocd.2017.12.006.
Acknowledgements
The authors are grateful to the Researchers Supporting Project number (RSP2023R382), King Saud University, Riyadh, Saudi Arabia for funding this research.
Funding
The study was funded by the Researchers Supporting Project number (RSP2023R382), King Saud University, Riyadh, Saudi Arabia. The funding body played no role in the study design, manuscript writing, or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
G.S.A. A.H.A. and A.I. proposed the study conception and design. G.S.A. completed the practical work. G.S.A. collected data. A.I. and A.H.A. contributed to the data analysis. A.H.A. G.S.A. and A.I. contributed to data interpretation. G.S.A. A.H.A. and A.I. prepared the manuscript’s initial draft. G.S.A. A.H.A. and A.I. critically reviewed and edited the intellectual content of the manuscript. All authors read, understood, reviewed, and approved the manuscript’s final version to be submitted/published and took responsibility for the intellectual content of the same manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed between June and December 2013. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was reviewed and approved by the Ethics Sub-Committee of King Saud University, Saudi Arabia. Before data collection, a signed written informed consent was obtained from each participating patient.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alghadir, A.H., Gabr, S.A. & Iqbal, A. Hand grip strength, vitamin D status, and diets as predictors of bone health in 6–12 years old school children. BMC Musculoskelet Disord 24, 830 (2023). https://doi.org/10.1186/s12891-023-06960-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06960-3