Abstract
Background
Osteoarthritis (OA) is a prevalent, chronic joint condition that commonly affects the knee and hip causing pain, impaired function, and reduced quality of life. As there is no cure, the main goal of treatment is to alleviate symptoms via ongoing self-management predominantly consisting of exercise and weight loss (if indicated). However, many people with OA do not feel adequately informed about their condition and management options to self-manage effectively. Patient education is recommended by all OA Clinical Practice Guidelines to support appropriate self-management, but little is known about the optimal delivery method and content. Massive Open Online Courses (MOOCs) are free, interactive, e-learning courses. They have been used to deliver patient education in other chronic health conditions but have not been used in OA.
Methods
A two-arm parallel-design, assessor- and participant-blinded superiority randomised controlled trial. People with persistent knee/hip pain consistent with a clinical diagnosis of knee/hip OA (n = 120) are being recruited from the Australia-wide community. Participants are randomly allocated into one of two groups i) electronic information pamphlet (control group) or ii) MOOC (experimental group). Those allocated to the control group receive access to an electronic pamphlet about OA and its recommended management, currently available from a reputable consumer organisation. Those allocated to the MOOC receive access to a 4-week 4-module interactive consumer-facing e-Learning course about OA and its recommended management. Course design was informed by behaviour theory and learning science, and consumer preferences. The two primary outcomes are OA knowledge and pain self-efficacy with a primary endpoint of 5 weeks and a secondary endpoint of 13 weeks. Secondary outcomes include measures of fear of movement, exercise self-efficacy, illness perceptions, OA management and health professional care seeking intentions, physical activity levels, and actual use of physical activity/exercise and weight loss, pain medication, and health professional care seeking to manage joint symptoms. Clinical outcomes and process measures are also collected.
Discussion
Findings will determine whether a comprehensive consumer-facing MOOC improves OA knowledge and confidence to self-manage joint pain compared to a currently available electronic OA information pamphlet.
Trial registration
Prospectively registered (Australian New Zealand Clinical Trials Registry ID: ACTRN12622001490763).
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Osteoarthritis (OA) is a prevalent and chronic joint condition that commonly presents in the knee and hip causing joint pain, impaired function, and reduced quality of life [1]. As there is currently no cure, the main goal of treatment is to alleviate symptoms via ongoing self-management using first-line approaches of exercise and weight loss, if indicated [2]. Joint replacement surgery, which is invasive, costly and can result in serious adverse events [3], should only be considered as a last resort for those with severe symptoms who have not seen improvement from recommended non-surgical approaches. However evidence suggests that less than 1 in 3 of those referred to an orthopaedic surgeon feel informed about how to self-manage [4] and the majority are dissatisfied with the quality and amount of information they received about OA and its recommended management [5]. People with OA want more information about their condition and its management options so that they can ‘take action’ to improve their own health state and quality of life [5].
Although patient education is advocated in all OA Clinical Practice Guidelines as essential to support appropriate self-management [2], the optimal delivery method and content remain uncertain. OA patient education interventions evaluated in trials have been found to lack comprehensiveness, not be based on best evidence, behaviour theory or learning science principles, nor designed in collaboration with people with OA [6]. Massive Open Online Courses (MOOCs) are free, interactive, e-learning courses that are used to deliver university-quality education. They have many benefits including convenience, features that support pedagogical principles to enhance learning, are easily updated and allow unlimited registrants so are infinitely scalable [7]. Although limited, there is evidence suggesting MOOCs may be an effective method of providing patient education in Type 2 diabetes [8] and in dementia risk reduction [9]. Thus, MOOCs could be a scalable and effective method of providing comprehensive education to people with OA about the condition and its recommended management practices.
Our team has developed the first consumer-facing MOOC about OA and its management, for people with OA, which we aim to evaluate in this trial. Our primary hypothesis is that people with hip and/or knee OA who receive the 4-module 4-week MOOC will have greater improvements in OA knowledge and/or self-efficacy for pain at 5 weeks compared to those who receive a typical OA education intervention: an electronic OA information pamphlet available from a reputable consumer organisation. Our secondary hypothesis is that those receiving the MOOC will have more favourable changes in a range of other outcomes including fear of movement, exercise self-efficacy, illness perceptions, OA management and health professional care seeking intentions, physical activity levels, and actual use of physical activity/exercise and weight loss, pain medication, and health professional care seeking to manage joint symptoms.
Methods/design
Trial design
This is a two arm, parallel group, superiority randomised controlled trial (RCT) reported according to SPIRIT guidelines [10] and TIDieR [11].
Participants
People with persistent knee/hip pain consistent with a clinical diagnosis of knee/hip OA [12] are currently being recruited from the Australian-wide community via advertisements (e.g., print/radio/social media) and our volunteer database. Eligibility criteria are presented in Table 1. Informed consent is obtained prior to baseline questionnaires from all participants via online forms using REDCap™ (Research Electronic Data Capture) hosted at the University of Melbourne [13, 14]. Ethics approval was obtained.
Procedures
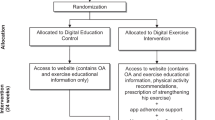
Figure 1 outlines trial phases. Participants first complete eligibility screening via an online survey (REDCap™). The survey outlines trial details and participant requirements and contains questions to assess inclusion criteria. Potentially eligible people are telephoned by a researcher who provides a verbal description of the study and reviews and confirms inclusion and exclusion criteria. Eligible participants are then sent a link to an online consent form (including the trial’s plain language statement) via email. Those who complete the consent form receive a link to the online baseline questionnaire. In cases where multiple joints are equally symptomatic, the participant is asked to select one hip/knee as the focus of assessment.
Randomisation and blinding
Participants are enrolled into the study on completion of baseline questionnaires and are randomised (1:1 ratio) into one of two groups i) electronic OA information pamphlet (control group) or ii) MOOC (experimental group), using randomly permuted blocks of varying sizes, stratified by eligible joint (hip or knee). The randomisation list was computer-generated by an independent statistician and is managed by a researcher centrally to ensure concealment. Participants are blinded to group allocation using limited disclosure (told that the study is investigating two different types of information material about OA but without explicit detail about either intervention nor the study hypotheses given). As primary outcomes are participant-reported, and participants are blinded, by default the assessors of these outcomes are blinded. Statistical analyses will be performed while blinded.
Interventions
Electronic OA information pamphlet (control group)
Participants in this group receive access to an electronic information pamphlet about OA and its management, currently available online from a reputable consumer organisation, Musculoskeletal Australia. Immediately after randomisation, participants receive an email from the research team containing a link to a PDF version of the pamphlet (mskweb.msk.org.au/$web/2022/07/Osteoarthritis.pdf) and are asked to read the pamphlet within the coming 5 weeks. On completion of their involvement in the study (after 13-week assessment is completed), they are provided access to the experimental group intervention (MOOC).
Consumer-facing Massive Open Online Course (experimental group)
Participants in this group receive access to the 4-week 4-module consumer MOOC which is housed on FutureLearn, a web-based platform for university-quality online education courses. Immediately after randomisation, participants receive an email from the research team containing a link to the MOOC registration page (futurelearn.com/courses/taking-control-hip-and-knee-osteoarthritis). They are instructed to register for the free version of the course and complete Module 1 within 7 days. Thereafter, they are encouraged to complete the remaining three modules following the course schedule (modules are ‘unlocked’ week-by-week).
Figure 2 presents the course learning outcomes per module. Content is presented over four modules: (1) Learning about OA; (2) Physical activity and exercise for OA; (3) Body weight and OA; (4) Additional management strategies and conclusion. Each module includes a range of learning activities including non-moderated discussion boards for learner interaction, poll questions, quizzes aligned with learning outcomes and downloadable documents to facilitate turning learnings into action (e.g., physical activity logbooks, meal portion size guide, self-management action plan). Each module also contains a ‘finding out more’ section which includes links to external credible resources, information about a range of healthcare professionals who may be able to provide further assistance, and references to scientific papers that support content. The time required to complete all four modules and learning activities is approximately four hours (about one hour per module/week), although total duration depends on the user’s interaction with the provided resources, external links, and references.
The course was designed by the research team (RKN, KLB, RSH) and a consumer MOOC review team (5 people with hip and/or knee OA). In addition, the course was evaluated by FutureLearn’s Editorial Team to ensure that the course met quality assurance standards for accessibility and pedagogy-informed design. Course design was underpinned by four key pillars that are recommended for the development of high-quality OA education [6]:
Pillar 1. Based on previous OA research
Course content was based on evidence-based Clinical Practice Guidelines [2, 12]; key patient messages about OA and its management [15]; and core capabilities for the delivery of optimal OA care [16].
Pillar 2. Informed by behaviour theory
The Common-Sense Model of Self-Regulation [17] guided the approach for addressing illness perceptions that can influence treatment/management choices. The model also informed lesson content addressing maladaptive beliefs about identity, and the causes, consequences, controllability, and prognosis of OA. Social learning and self-efficacy theory [18] informed behaviour change lesson content aimed at empowering participants to make appropriate management choices and enact effective self-management behaviours. Lesson content incorporated the behaviour change techniques of goal setting, problem solving, action planning, self-monitoring, verbal persuasion about capability, instruction on how to perform the behaviour, credible source, and demonstration of behaviours/role modelling [19].
Pillar 3. Informed by learning science
The overall course design was underpinned by a pedagogy of social learning, the approach applied by FutureLearn based on their extensive experience and expertise in delivering online education. Fundamental to this approach is learning through storytelling and interaction with others. In addition, learning science principles were applied to course components, including Bloom's taxonomy, Mayer's principles of multimedia design, and immediate and elaborated feedback [20].
Pillar 4. Incorporates consumer involvement
Consumer involvement was incorporated via online survey completed by 348 people with hip and/or knee OA from our Centre’s consumer Knowledge Translation Network. The survey gauged interest in a consumer-facing online course and collected feedback on the proposed content and learning outcomes. From this, we identified that 99% were interested in the concept and 100% thought the proposed content would be useful for them. Once a prototype was developed, extensive feedback was then collected from our consumer MOOC review team using an iterative think aloud approach [21]. This involved each member of the MOOC review team independently completing each course module while being observed by a member of the research team (RKN) via zoom screen share. Based on feedback, refinements were made to the course after each individual think aloud session, ultimately resulting in the final version of the course. Appendix 1 provides an overview of refinements made.
Outcomes
Table 2 lists all baseline descriptive data and outcome measures. All assessments (baseline, 5-week [primary] and 13-week [secondary]) are completed remotely via online questionnaires (REDCap™). Participants completing both 5- and 13-week questionnaires are given a $AUD50 gift voucher in gratitude for their participation.
Primary outcomes
The two primary outcomes are self-reported measures collected at baseline, 5 and 13 weeks.
OA knowledge
The Knee/Hip Osteoarthritis Knowledge Scale (OAKS) [24] includes 11-items that measure knowledge about hip/knee OA, including causation, diagnosis, symptom interpretation, management principles, treatment and self-care options. Each item is rated using a 5-point Likert scale (response options: False; Possibly False; Unsure; Possibly True; True). Items 1–4, 7, and 11 are scored in reverse. All item scores are added for a total score range of 11 to 55. Higher scores indicate more accurate knowledge. This measure has acceptable psychometric properties when used in mixed cohorts of people with hip and knee OA [25].
Pain self-efficacy
The Pain subscale of the Arthritis Self-Efficacy Scale (ASES pain) [26] includes 5 items that measure one’s self-efficacy for managing their osteoarthritis pain. Each item is rated using a 10-point scale (1 = very uncertain and 10 = very certain). Scores are the mean of all items for a total score range of 1 to 10. Higher scores indicate greater pain self-efficacy. This measure has established validity, reliability and responsiveness in OA [26].
Secondary outcome measures
A range of self-reported secondary outcomes are measured at baseline, 5- and 13-weeks, unless indicated otherwise.
Kinesiophobia via the 6-item Brief Fear of Movement for OA Scale [25]. Each item is rated using a 4-point Likert scale from 1 = Strongly disagree to 4 = Strongly agree. All item scores are added for a total score range of 6 (minimal fear) to 24 (maximal fear).
Exercise self-efficacy via the 9-item Self-efficacy for Exercise (SEE) Scale [27]. Each item is rated on an 11-point scale. Scores range from 0–90 with higher scores indicating higher self-efficacy for exercise.
OA illness perceptions via 8-items of the Brief Illness Perceptions Questionnaire (B-IPQ) [28]. Each item is scored on a NRS (0 to 10). Items 3, 4, and 7 are scored in reverse. All item scores are added for a total score range of 0–80. Higher scores represent a more threatening view of OA.
Management intentions (week 5 only) via four study specific items. Participants are asked about their intentions over the next 2 months to 1) increase amount/intensity of physical activity/exercise; 2) reduce the amount of time spent sedentary; 3) make efforts to lose weight; and 4) have hip/knee joint replacement surgery in the next 2 years. Responses options are Yes/No.
Intention to seek care from a health professional (week 5 only) via four study specific items. Participants are asked about their intentions to seek care in the following 2 months for 1) weight loss; 2) an exercise/physical activity program; 3) pain relieving medication; and 4) joint replacement surgery. Responses options are Yes/No. For each item ‘Yes’ is selected, participant will be asked to select which health professionals they intend to see.
Current physical activity (baseline and week 13): via the 10-item Incidental and Planned Exercise Questionnaire, version W (IPEQ-W) [29]. Scores range from 0–128 with higher scores indicating higher levels of activity.
Current exercise/physical activity behaviour (week 13 only): via the question “Over the past 2 weeks, how would you compare your amount of physical activity/exercise to when you started the study?”, rated on a 3 point-Likert with response options less/same/more.
Current weight loss behaviour (week 13 only): via the question “In the past 2 weeks, did you make any effort to lose weight (e.g. diet changes)?” with response options Yes/No.
Current care seeking behaviour (week 13 only): via 4 study specific items. Participants are asked if they have consulted a health professional since they enrolled in the study to discuss 1) weight loss; 2) an exercise/physical activity program; 3) pain relieving medication; and 4) joint replacement surgery. Responses options are Yes/No. For each item ‘Yes’ is selected, participant will be asked to select which health professionals they intend to see.
Oral pain medication usage in the prior month (baseline and week 13) via self-reported use of common oral pain-relieving medications taken at least once a week in the prior month for knee/hip pain. Participants are asked to select Yes/No from options: 1) oral non-steroidal anti-inflammatory drugs; 2) analgesics (paracetamol combinations); 3) oral corticosteroids; and 4) oral opioids.
Other measures
Clinical measures
(Baseline and week 13) 1) average severity of knee pain during walking in the past week is measured via an 11-point NRS (0 = no pain and 10 = worst pain possible); 2) physical function is measured via the 17-item Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function subscale (range 0 to 68, higher scores indicating higher dysfunction) [30]; and 3) body weight (self-reported in kilograms).
Process measures
Several process measures are collected at 5 weeks regarding engagement with and perceived usefulness of each allocated resource (i.e. MOOC or electronic OA information pamphlet). Table 2 lists all process measures.
Data analysis, monitoring and auditing
Sample size calculation
A sample size of 60 participants per arm (120 in total) is required for 90% power to demonstrate that the consumer-facing MOOC is superior to the control with a two-sided 2.5% significance level (accounting for multiple comparisons across the two primary outcomes by using Bonferroni correction) and allowing for a 20% dropout rate. The sample size calculation was based on the following assumptions: a standardised between-group effect size of 0.625 for pain self-efficacy (based on our prior research [31], corresponding to an absolute between-group difference in mean change from baseline to 5 weeks of 1 unit in ASES pain subscale score favouring the MOOC, with within-group standard deviation (SD) of 1.6 units [31], correlation between measures across all three timepoints of 0.5 (i.e., compound symmetry variance–covariance matrix) [31], and using a constrained longitudinal data analysis (cLDA) model [32]. With this sample size, we also have at least 90% power to detect a between-group effect size of 0.8 for OA knowledge (conservative for this type of program [33]), corresponding to an absolute between-group difference in mean change from baseline to 5 weeks of 4.6 units in KOAKS/HOAKS score favouring the MOOC, with within-group SD of 5.8 units [31], and correlation between measures across all three timepoints of 0.2 [31].
Data analysis
The biostatisticians (FM, KEL, ADS) will devise a statistical analysis plan for the study prior to being unblinded to group allocation. It will be published on our Centre’s website. Analyses will include all participants according to their group allocation (intention-to-treat). Each primary outcome will be analysed using a cLDA [32] model. The response will consist of all KOAKS/HOAKS or ASES pain scores (at baseline, 5 and 13 weeks), and the model will include factors for group, time (categorical), and group-by-time interaction, with the restriction of a common baseline mean across treatment groups. The mean change in KOAKS/HOAKS or ASES pain scores from baseline to each follow-up timepoint between the groups will be obtained. The primary hypothesis will be evaluated by obtaining the estimated differences between groups in mean change in KOAKS/HOAKS and ASES pain score from baseline to 5 weeks post randomisation, and multiplicity adjusted two-sided 95% confidence intervals and p-values. These models provide valid inference in the presence of missing data if the data are missing at random (MAR). An analysis will be conducted using the delta-adjustment method under the pattern-mixture modelling framework in the context of multiple imputation to assess sensitivity to missingness not at random. Secondary outcomes: Management intentions and care seeking intentions at 5 weeks, and care seeking behaviour, exercise and weight loss behaviours and pain medication usage (adjusted for baseline usage) at 13 weeks will be analysed using log-binomial regression models. Physical activity levels at 13 weeks will be analysed using a linear regression model adjusted for baseline physical activity. Other continuous outcomes will be analysed the same as the primary outcomes. All analysis models will be adjusted for the stratification factor, eligible joint (hip/knee). Process measures and other baseline measures will be summarised using frequency (proportion) for binary measures and mean (SD)/ median (inter-quartile range) for continuous measures.
Monitoring
The research team meet fortnightly to review recruitment and monitor trial progress.
Patient and public involvement
As described earlier, 348 people with hip/knee OA were involved in the MOOC design via an online survey to gauge interest in the intervention concept (MOOC) and its proposed content. Additioanlly, a MOOC review team (5 people with hip/knee OA) provided feedback (totalling > 21 h) on versions of the MOOC using an iterative think aloud process. One person with OA (NB) is an investigator on this study. They provided input into the trial design and processes including reviewing participant-facing materials (e.g., plain language statement and consent form). They will assist with interpretation of findings.
Dissemination plans
Findings will be disseminated via conference presentations; journal publications; lay summaries to participants and via our Centre’s social media channels and Knowledge Translation Network. Findings will also be disseminated by the Physiotherapy Research Foundation’s channels.
Discussion
This RCT will determine whether a comprehensive consumer-facing eLearning course (MOOC) about OA and its management improves OA knowledge and/or confidence to self-manage symptoms compared with a typical OA education intervention: an electronic OA information pamphlet available from a reputable consumer organisation.
Availability of data and materials
Not applicable.
Abbreviations
- MOOC:
-
Massive Open Online Course
- RCT:
-
Randomised controlled trial
References
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745.
The Royal Australian College of General Practitioners. Guideline for the management of knee and hip osteoarthritis. East Melbourne. Royal Australian College of General Practitioners. 2018.
Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(17):1597–606.
Ingelsrud LH, Roos EM, Gromov K, Jensen SS, Troelsen A. Patients report inferior quality of care for knee osteoarthritis prior to assessment for knee replacement surgery - a cross-sectional study of 517 patients in Denmark. Acta Orthop. 2020;91(1):82–7.
Wluka A, Chou L, Briggs A, Cicuttini F. Understanding the needs of consumers with musculoskeletal conditions: consumers’ perceived needs of health information, health services and other non-medical services: a systematic scoping review. Melbourne: MOVE muscle, bone & joint health; 2016.
Goff AJ, De Oliveira Silva D, Ezzat AM, Bell EC, Crossley K, O'Halloran P, et al. Knee osteoarthritis education interventions in published trials are typically unclear, not comprehensive enough, and lack robust development: ancillary analysis of a systematic review. J Orthop Sports Phys Ther. 2022;52(5):276-86.
Yuan L, Powell S. MOOCs and open education: implications for higher education. 2013.
Mackenzie SC, Cumming KM, Garrell D, Brodie D, Wilson L, Mehar S, et al. Massive open online course for type 2 diabetes self-management: adapting education in the COVID-19 era. BMJ Innov. 2021;7(1):141–7.
Farrow M, Klekociuk S, Bindoff A, Vickers J. The Preventing Dementia Massive Open Online Course results in behaviour change associated with reduced dementia risk: The My INdex of DEmentia Risk (MINDER) study: Prevention (nonpharmacological) / Multidomain. Alzheimers Dement. 2020;16:6565.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krle AJK, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Rev Rev Panam Salud Publica. 2015;38(6):506–14.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
National Institute for Health and Care Excellence (NICE). Osteoarthritis in over 16s: diagnosis and management. NICE guideline [NG226]. London: National Institute for Health and Care Excellence; 2022.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
French SD, Bennell KL, Nicolson PJA, Hodges PW, Dobson FL, Hinman RS. What do people with knee or hip osteoarthritis need to know? An international consensus list of essential statements for osteoarthritis. Arthritis Care Res. 2015;67(6):809–16.
Hinman RS, Allen KD, Bennell KL, Berenbaum F, Betteridge N, Briggs AM, et al. Development of a core capability framework for qualified health professionals to optimise care for people with osteoarthritis: an OARSI initiative. Osteoarthritis Cartilage. 2020;28(2):154–66.
Leventhal H, Phillips LA, Burns E. The Common-Sense Model of Self-Regulation (CSM): a dynamic framework for understanding illness self-management. J Behav Med. 2016;39(6):935–46.
Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95.
Gordon DG, Wiltrout ME. A framework for applying the learning sciences to MOOC design. Front Educ. 2021;5:500481.
Van Someren M, Barnard YF, Sandberg J. The think aloud method: a practical approach to modelling cognitive. London: AcademicPress; 1994. p. 11.
Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–63.
Osborne RH, Batterham RW, Elsworth GR, Hawkins M, Buchbinder R. The grounded psychometric development and initial validation of the Health Literacy Questionnaire (HLQ). BMC Public Health. 2013;13:658.
Darlow B, Abbott H, Bennell K, Briggs AM, Brown M, Clark J, et al. Knowledge about osteoarthritis: development of the hip and knee osteoarthritis knowledge scales and protocol for testing their measurement properties. Osteoarthritis Cartilage Open. 2021;3(2):100160.
Darlow B, Krägeloh C, Abbott JH, Bennell K, Briggs AM, Brown M, et al. The osteoarthritis knowledge scale. Musculoskeletal Care. 2022. Epub ahead of print.
Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44.
Resnick B, Jenkins LS. Testing the reliability and validity of the self-efficacy for exercise scale. Nurs Res. 2000;49(3):154–9.
Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–7.
Delbaere K, Hauer K, Lord SR. Evaluation of the incidental and planned activity questionnaire for older people. Br J Sports Med. 2010;44(14):1029–34.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40.
Egerton T, Bennell KL, McManus F, Lamb KE, Hinman RS. Comparative effect of two educational videos on self-efficacy and kinesiophobia in people with knee osteoarthritis: an online randomised controlled trial. Osteoarthritis Cartilage. 2022;30(10):1398–410.
Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhyā. 2000;62(1):134–48.
Fary RE, Slater H, Chua J, Ranelli S, Chan M, Briggs AM. Policy-into-practice for rheumatoid arthritis: randomized controlled trial and cohort study of e-learning targeting improved physiotherapy management. Arthritis Care Res (Hoboken). 2015;67(7):913–22.
Acknowledgements
Not applicable.
Funding
Funding was provided by the Physiotherapy Research Foundation and the National Health & Medical Research Council. Neither are involved in study design, data collection, analysis, interpretation or manuscript preparation. RSH is supported by a National Health & Medical Research Council Fellowship (#1154217). KLB is supported by a National Health & Medical Research Council Fellowship (#1058440).
Author information
Authors and Affiliations
Contributions
RKN, KLB and RSH conceived the idea for the RCT. RKN is leading the study and RKN and KLB obtained funding. RKN, KLB, RSH, TE, NB, ADS, FM, KEL designed the study. ADS formulated and is responsible for the sample size, and statistical analysis plan. RKN drafted the protocol manuscript and all tables and figures. MG is responsible for recruitment and eligibility screening. All authors provided input into manuscript preparation and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Human Ethics Committee approval has been obtained from the University of Melbourne STEMM 3 committee (reference number 2022–24596-34590–4). Informed consent is obtained prior to baseline questionnaires from all participants via online forms using REDCap™ (Research Electronic Data Capture) hosted at the University of Melbourne. All methods will be carried out in accordance with the Australian Code for the Responsible Conduct of Research, 2018.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Summary of course refinements based on feedback from the consumer think aloud evaluations
-
1.
Minor changes to the course including:
-
the reordering of content within modules/weeks 1 and 2;
-
changes to text to improve readability, all modules/weeks;
-
modifications to end of module quiz questions, module/week 1.
-
-
2.
One major change:
-
the redesign of module 3 “Body weight and osteoarthritis”. During review, participants indicated that the original module focused too heavily on the negative impacts of body weight on joint and overall health. It was thought that this could evoked feelings of guilt and judgment and may not be relevant to all people (e.g., those who have low body weight or those with low interest in the topic). Based on participants feedback the module was re-designed to remove content describing the negative impacts of high body weight and instead was written to educate about the positive benefits of healthy weight maintenance and weight loss.
-
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nelligan, R.K., Hinman, R.S., Egerton, T. et al. Effects of a Massive Open Online Course on osteoarthritis knowledge and pain self-efficacy in people with hip and/or knee osteoarthritis: protocol for the MOOC-OA randomised controlled trial. BMC Musculoskelet Disord 24, 381 (2023). https://doi.org/10.1186/s12891-023-06467-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06467-x