Abstract
Background
Ankylosing spondylitis (AS) is an inflammatory autoimmune disease that mostly affects different joints of the body. Macrophages are the predominant cells that mediate disease progression by secreting several pro-inflammatory mediators. Different receptors are involved in macrophages’ function including the adenosine receptors (AR). Our main objective in this study was to assess the effect of applying A2A adenosine receptor agonist (CGS-21,680) on the gene expression of inflammatory mediators including bone morphogenetic proteins (BMP)-2, 4 and matrix metalloproteinases (MMP)-3, 8, 9, and 13 on the macrophages from AS patients compared to healthy macrophages.
Methods
Monocytes were isolated from the whole blood of 28 individuals (AS patients and healthy controls in a 1:1 ratio). Macrophages were differentiated using macrophage colony-stimulating factor (M-CSF), and flow cytometry was performed to confirm surface markers. CGS-21,680 was used to treat cells that had been differentiated. Using SYBR green real-time PCR, relative gene expression was determined.
Results
Activating A2AAR diminished MMP8 expression in healthy macrophages while it cannot reduce MMP8 expression in patients’ macrophages. The effect of A2AAR activation on the expression of BMP2 and MMP9 reached statistical significance neither in healthy macrophages nor in the patients’ group. We also discovered a significant positive connection between MMP8 expression and patient scores on the Bath ankylosing spondylitis functional index (BASFI).
Conclusion
Due to the disability of A2AAR activation in the reduction of MMP8 expression in patients’ macrophages and the correlation of MMP8 expression with BASFI index in patients, these results represent defects and dysregulations in the related signaling pathway in patients’ macrophages.
Similar content being viewed by others
Introduction
Ankylosing spondylitis (AS) is an autoimmune disorder that mostly affects the sacroiliac joints and the spine. People suffering from this disease typically complain about spinal stiffness and back pain as a result of enthesitis and vertebrae fusion. AS usually starts at an early age and it is believed that one in every two hundred people is influenced by this disease, making it an important health issue [1, 2]. The exact etiology by which the disease is started is still unknown; however, The most important contributors to disease creation are supposed to be hereditary and environmental causes [3]. Diverse environmental factors, infectious diseases, and gut dysbiosis in genetically predisposed people, which is defined by possessing certain human leukocyte antigen (HLA) and non-HLA genes including HLAB27, IL23R, ERAP1, and certain TLRs alleles; can cause disease occurrence [1, 4, 5].
Different signaling pathways are involved in autoimmune diseases. One of which is the adenosinergic pathway [6]. To date, available data show that the adenosinergic pathway has a tremendous impact on immunosuppression and aids the body to recover from excessive inflammatory responses. Adenosine is a byproduct of the breakdown of an enzyme cascade, consisting of CD39 (ecto-nucleoside triphosphate diphosphohydrolase 1, E-NTPDase1) breaks down adenosine triphosphate/diphosphate (ATP/ADP) to adenosine monophosphate (AMP) and CD73 (ecto-50-nucleotidase, NT5E) produces adenosine from AMP. Adenosine acts through G-protein-coupled cell-surface receptors which are expressed on a variety of cells, and to date four of them A1AR, A2AAR, A2BAR, and A3AR; are recognized. These receptors are known as type 1 purinergic (P1) receptors [6,7,8]. There is not much information regarding the role of adenosine receptors in AS pathogenesis. However, we have previously reported that macrophages from AS patients expressed elevated levels of A2AAR and diminished levels of A1AR and A2BAR compared to healthy macrophages [9]. Besides, our results demonstrated that A2AAR activation results in a reduction in TNF-α production and an increase in IL23A expression in AS macrophages [4].
Bone morphogenetic proteins (BMPs) are important members of the transforming growth factor superfamily with multiple vital roles such as embryonic development and osteoblastic differentiation [10]. Of these, BMP-2 is an active and important member which can independently or synergistically with other signaling pathways like the Wnt/β-catenin pathway, enhance osteoblast differentiation and bone formation [10, 11]. Data regarding the level of these proteins in AS patients are incompatible; however, most of them show higher levels of these proteins in patients compared to healthy controls [12, 13].
Matrix metalloproteinases (MMPs) are a family of 23 zinc-dependent enzymes produced by a variety of cells especially immune cells in the event of an inflammatory situation. Inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and IL-6 are produced in this environment [14, 15]. These proteins are mostly involved in the degradation and remodeling of extracellular matrices in humans [16]. High levels of MMPs have been seen in inflammatory autoimmune diseases like rheumatoid arthritis (RA). Different studies showed the relation of high levels of MMP-3 and its certain single nucleotide polymorphisms (SNPs) with AS occurrence [16, 17]. Along with the studies showing the relation of MMP-3 and AS, the correlation of bath AS disease activity index (BASDAI) with a group of clustered biomarkers comprising MMP-8, MMP-9, chemokine (C-X-C motif) ligand (CXCL)-8, and hepatocyte growth factor was found [18]. MMP-8 expression is related to inflammatory cytokines and it can have cartilage destructive activities during spondyloarthropathies. One of the SNPs of this enzyme has also been found to be associated with the risk of AS. MMP-9, also called gelatinase B, along with MMP-2 (gelatinase A), are mediators of joint destruction [16, 19].
It is known from previous studies that Inflammatory lesions and the overlaying synovium of spondyloarthritis (SpA) are dominated by macrophages, which are mostly responsible for the breakdown of fibrocartilage [4, 20, 21]. The inflammatory activities of macrophages can be regulated by P1 receptors through binding to adenosine [4]. The expression of proteins responsible for the pathogenesis of AS in macrophages is an important issue to consider for the treatment and control of the disease progression, so we aimed to evaluate the expression level of MMP3, 8, 9, 13, and BMP2, and 4 in macrophages of AS patients in an untreated situation and after treatment with A2AAR agonist (CGS-21,680) as no study has done this up until now.
Materials and methods
Study population
With a male/female ratio of 3.6/1 and an average age of 32 ± 10 years, 14 AS patients were chosen. Patients were recruited from the Rheumatology Research Center’s outpatient AS clinic at Shariati Hospital at Tehran University of Medical Sciences, and they all met the modified New York categorization criteria [22]. Patients who had not undergone any disease-modifying drugs or methotrexate were identified for this investigation because certain therapies, such as methotrexate, have a considerable impact on adenosine receptor expression [23]. Simultaneously, the study recruited 14 age and sex-matched healthy persons with a gender and sex distribution similar to AS patients with an average age of 32 ± 96 years. There was no personal or familial history of rheumatic disorders, inflammatory diseases, or psoriasis in the control group. All participants signed a written informed consent form. The Tehran University of Medical Sciences ethical committee accepted this work. (IR.TUMS.DDRI.REC.1399.047).
Monocyte isolation and macrophage generation
Twenty milliliters of participants’ peripheral blood samples were collected and inserted in tubes containing ethylenediaminetetraacetic acid (EDTA). Within five hours of the time of collection, samples were diluted at 1:2 in phosphate-buffered saline (GIBCO Invitrogen) at a pH of 7.2. Ficoll (Lymphodex, Inno-Train) density gradient centrifugation was used to extract peripheral blood mononuclear cells (PBMCs), which were then washed in PBS. Cell sorter columns were used to separate monocytes after they were treated with MACS CD14 microbeads to undergo positive CD14 selection (all from Miltenyi Biotec). Immunofluorescence labeling of the separated monocytes was done with a phycoerythrin (PE)-conjugated anti-CD14 antibody (BD bioscience) and flow cytometry results revealed a purity of 92–95% [20]. Following that, the isolated CD14 positive monocytes were cultured in the Roswell Park Memorial Institute (RPMI) at a concentration of 500,000 cells per well in 24-well plates. The media contained 2 mM L-glutamine (Biosera), 10% fetal bovine serum (FBS; Gibco BRL), 0.1 mg/ml streptomycin, and 100 U/ml penicillin (Sigma). For seven days, 0.05 µg/ml recombinant macrophage-colony stimulating factor (M-CSF; eBioscience) was added to culture media to convert monocytes into macrophages [24].
Flow cytometry analysis of macrophage surface markers
The macrophage-specific markers of generated macrophages were examined by flow cytometry using a CyFlow ML flow cytometer (Partec, GmbH, Munster, Germany) and FlowJo software after one weak of monocytes treatment with M-CSF (Tree Star, Ashland, OR, USA) [25]. Cells incubation with fluorescein isothiocyanate (FITC)-conjugated anti-human CD163 and PE-conjugated anti-human CD206, (BD bioscience) or their relative isotype controls was done for half an hour without any light exposure. Analysis of FASC data revealed that 97 and 95% of differentiated macrophages had scavenger receptor CD163 and mannose receptor CD206, respectively [20].
Real-time quantitative PCR analysis of MMP and BMP gene expressions
Monocyte-generated macrophages (M2-like) were seeded on RPMI, and half of the specimens were treated with 10 M CGS-21,680 (Sigma), a well-known A2AAR agonist [26]. The High Pure RNA Isolation Kit was utilized to extract total RNA after 24 h (Roche). The complementary DNA (cDNA) was made from the same amount of total RNA using the CellAmp direct RNA prep kit for real-time PCR (Takara bio). StepOnePlus Real-Time PCR equipment (Applied Biosystems) and SYBR green master mix were employed to evaluate the relative expression levels of MMP3, 8, 9, 13, BMP2, and 4 genes (Ampliqon). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control gene. The primer sequences used for BMP2 and 4 were from the Harvard Primer Bank. The site https://primer3.ut.ee/ and https://genome.ucsc.edu/ were used to design the MMP13 primers. The others were selected from previous research. The specific primer sequences and the references were shown in Table 1. For accuracy and specificity, primers were checked using the Basic Local Alignment Search Tool on the US National Center for Biotechnology Information website (HTTP://www.ncbi.nlm.nih.gov/tools/primer-blast/) and https://genome.ucsc.edu/. The mRNA expression level was compared between A2AAR agonist (CGS-21,680) treated cells and the untreated group using the comparative CT method (2 −ΔΔCT). It’s worth noting that the cytotoxic potential of CGS-21,680 was tested before the results were interpreted. In this study, the MTT test was utilized to determine the cytotoxicity of CGS-21,680. CGS-21,680 had no harmful effects in this assay, and cell viability was identical to that of untreated cells.
Statistical analysis
The Shapiro-Wilk test was used to ensure that all of the variables were normal. To compare non-normally distributed variables, non-parametric methods such as the Mann-Whitney U test were utilized. To compare the levels of mRNA expression in CGS-21,680 treated and untreated cells, the paired sample t-test and Wilcoxon test were performed. The relationship between relative mRNA expression of BMP2, MMP8, and MMP9 genes and patient clinical symptoms was investigated using Spearman’s correlation test. Statistical significance was defined as a P value of less than 0.05. SPSS version 22 was used to conduct all statistical analyses, and GraphPad Prism 6 was used to create the graphs.
Results
Demographic and clinical characteristics
Table 2 represents the demographic characteristics of enrolled patients and the healthy ones. Clinical scores showing disease severity and activity were also displayed for AS group. According to the report, none of the included patients were on biological or methotrexate therapy.
Selected genes mRNA expression
Among selected genes, MMP3, MMP13, and BMP4 were not expressed in macrophages from patients and healthy individuals and were excluded from the analysis. M2- like macrophages only expressed BMP2, MMP8, and MMP9.
BMP2 mRNA expression in M2-like macrophages of AS patients following the A2AAR activation
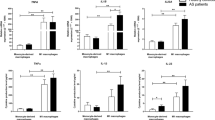
The effect of the A2AAR agonist (CGS-21,680) on BMP2 mRNA expression was determined before and after treating M2-like macrophages of AS patients and healthy controls. Untreated healthy macrophages expressed more BMP2 than AS macrophages (0.47-fold; P = 0.001, Table 3). CGS-21,680 raised BMP2 mRNA expression in AS patients’ macrophages by 1.62-fold, which was not statistically significant (P = 0.167), and it had no effect on BMP2 mRNA expression in healthy people’s macrophages (Fig. 1).
MMP8 mRNA expression in AS patients’ M2-like macrophages after Aa2A AR activation
The expression of MMP8 did not differ between untreated healthy macrophages and AS ones (Table 3). The CGS-21,680 decreased the mRNA expression level of MMP8 in monocyte-derived macrophages of healthy individuals by 0.26-fold (P = 0.002). On the other hand, the decrease noticed in AS patients was not statistically significant (0.78-fold; P = 0.122) (Fig. 1; Table 4).
MMP9 mRNA expression in AS patients’ M2-like macrophages after Aa2A AR activation
We did not find any significant differences in the expression of MMP9 between AS and healthy macrophages (Table 3). Besides, the A2AAR agonist couldn’t significantly change MMP9 mRNA expression level in macrophages from AS patients and healthy persons. (Fig. 1; Table 4).
Correlation between the relative mRNA expression with clinical manifestations of AS patients
The link between the expression level of studied genes with clinical manifestations of AS patients was also evaluated. A significant positive correlation between MMP8 and BASFI level (Bath Ankylosing Spondylitis Functional Index) was also seen in AS patients (P = 0.034) (Table 5).
Discussion
Regulating inflammation in autoimmune diseases like SpA is very important in ameliorating disease signs. Different inflammatory molecules take part in disease progression like matrix metalloproteinases which contribute to bone and joint degeneration to a great extent [15].
Matrix metalloproteinases are one of many inflammatory mediators in AS patients. They are mostly involved in bone and cartilage degeneration; and are considered a good therapeutic target, especially in a group of patients that don’t respond to TNF therapies who are called non-responders to TNF inhibitor therapies [15, 27]. We investigated the gene expression levels of four matrix metalloproteinases: MMP-3, MMP-8, MMP-9, and MMP-13, which have been linked to pro-inflammatory roles in disease pathogenesis in earlier investigations. We did not detect MMP3 and MMP13 expression in isolated monocyte-derived macrophages. Sames as our results, Huang et al. did not detect MMP13 expression in unstimulated differentiated macrophages and MMP3 was expressed at a low level in them [28]. It is also demonstrated that MMP3 expression is induced in primary macrophages only after LPS stimulation [29].
MMP-8 which is predominantly produced by activated neutrophils and therefore called neutrophil collagenase, besides AS plays important pathogenic roles in other inflammatory autoimmune diseases like multiple sclerosis (MS) and RA [30, 31]. The relation between certain SNPs of MMP8 and AS occurrence has also been shown in previous research [19]. In addition, the serum level of MMP-8 has been shown to be higher in AS patients and associated with disease activity [18, 32]. Here, in this study, although we didn’t see a significant difference in MMP8 gene expression between macrophages from AS patients and healthy controls, we saw a positive correlation between MMP8 expression level and BASFI index in patients. This finding is showing that higher levels of MMP8 expression in macrophages may contribute to the inflammatory process in patients and could influence patients’ ability to cope with everyday life [18]. Applying A2AAR agonist, as an anti-inflammatory agent, on macrophages significantly reduced MMP8 expression in healthy controls but didn’t have this significant reduction in AS patients. Previously we have reported that AS patients’ macrophages express more A2AAR than healthy people’s macrophages [19]. The fact that activation of A2AAR cannot diminish the MMP8 expression in patients’ macrophages may be due to defects or dysregulations in the related signaling pathway in AS patients.
Just like MMP-8, MMP-9 is another important enzyme that plays a part in multiple autoimmune diseases [33, 34]. In AS specifically, MMP-9 has cartilage destructive activities through degrading type IV collagen in extracellular matrices and facilitating lymphocytes’ entrance into sites of inflammation [34]. Higher amounts of this enzyme have been detected in the serum of AS patients along with MMP-8 and CXCL-8 and their relevance to disease activity status has been shown too [18]. Here, we didn’t see any difference in MMP9 gene expression between macrophages from healthy subjects and AS patients. Previously it was demonstrated that activation of the A2AAR receptor can diminish MMP9 expression in neutrophils [35]. Besides Chen et al. found that A2BAR activation reduces MMP9 expression in macrophages depending on TNF-α [36]. Previously we reported that A2AAR agonist can reduce TNF-α production in macrophages from AS patients [4]. It has been also demonstrated that TNF-α upregulates MMP9 expression [37, 38]. however, here we saw that activation of A2AAR receptor couldn’t affect the baseline expression level of MMP9 in macrophages from both healthy and AS groups. Nevertheless, we did not detect MMP-9 protein expression level and further study is required.
Besides chronic inflammation, AS is characterized by the bone formation and ankyloses of the sacroiliac joints while BMPs play a pivotal role in this process [39]. BMPs are a group of transforming growth factors and cytokines that can induce bone formation [40]. Inflammatory conditions and pro-inflammatory cytokines can induce BMPs expression. Previously demonstrated that macrophages participate in osteoinduction and early steps of bone formation by producing BMPs molecules [41]. It is demonstrated that M2 type of macrophages that participate in wound healing express an upregulated level of BMP-2 compared to M1 macrophages [41, 42]. Due to the role of macrophages in AS pathogenesis, here we investigated the expression of BMP2 in M2-like macrophages of patients and controls. In a meta-analysis by Yang et al., it is demonstrated that serum BMP-2 level is significantly higher in patients compared to controls [10]. Imbalances between different molecules in the BMP-2 signaling pathway also play a role in inducing bone formation and disease progression and participate in abnormal osteogenic differentiation of mesenchymal stem cells [39, 43]. It has also been shown by different studies that higher BMP-2 levels in AS patients are usually accompanied by higher BASDAI and c-reactive protein (CRP) levels [10, 44]. In the present study, we did not measure the level of BMP-2 in the serum of AS patients, and for the first time, we analyzed BMP2 gene expression in macrophages of patients and healthy people. We saw higher BMP2 expression in the macrophages of healthy subjects than in AS patients. The decreased expression of BMP2 in patients’ macrophages may be due to the negative feedback loop to suppress excessive bone formation in patients. Activation of A2AAR didn’t affect BMP2 levels in macrophages from patients and controls. We also did not see any association between the level of BMP2 gene expression in macrophages and the level of patients’ clinical manifestations.
Conclusion
In conclusion, the present study shows that activating A2AAR on macrophages cannot reduce MMP8 expression in patients’ macrophages while it diminished MMP8 expression in healthy macrophages. These results represent a dysregulation in the related signaling pathway in AS patients. Moreover, we found a significant positive correlation between MMP8 gene expression level and BASFI score in patients, telling us that higher MMP8 expression can be associated with higher BASFI and patients’ incapacitation. The effect of A2AAR activation on the expression of BMP2 and MMP9 did not reach statistical significance.
However, the small sample size and healthy controls as comparators are the limitations of the current study. Besides, other relevant gene expressions, protein levels in the supernatant, and the entire downstream pathway were not assessed in our study. Therefore, further studies with bigger sample sizes on different genes and signaling pathways in macrophages of AS patients can be useful to investigate and determine the exact effect of A2AAR on the pathogenesis of the disease.
Data availability
Data are available on request from the corresponding authors.
Abbreviations
- ADP:
-
Adenosine diphosphate
- AMP:
-
Adenosine monophosphate
- AR:
-
Adenosine receptor
- AS:
-
Ankylosing spondylitis.
- ATP:
-
Adenosine triphosphate
- BASDAI:
-
Bath ankylosing spondylitis activity index
- BASFI:
-
Bath Ankylosing Spondylitis Functional Index
- BMP:
-
Bone morphogenetic protein
- CD39:
-
Ecto-nucleoside triphosphate diphosphohydrolase 1, E-NTPDase1
- CD73:
-
Ecto-50-nucleotidase, NT5E
- CRP:
-
C-reactive protein
- CXCL:
-
Chemokine (C-X-C motif) ligand
- EDTA:
-
Ethylenediaminetetraacetic acid
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- IFN-γ:
-
Interferon-gamma
- M-CSF:
-
Macrophage colony-stimulating factor
- MMP:
-
Matrix metalloproteinases
- MS:
-
Multiple sclerosis
- PBMC:
-
Peripheral blood mononuclear cell
- RA:
-
Rheumatoid arthritis
- RPMI:
-
Roswell Park Memorial Institute
- SNP:
-
Single nucleotide polymorphism
- SPA:
-
Spondyloarthritis
- TNF-α:
-
Tumor necrosis factor-alpha
References
Gao S, Zhang J, Xu T, Xun C, Cao R, Guo H, Liang W, Sheng W. Associations of toll-like receptor 4 and 2 gene polymorphisms with susceptibility to ankylosing spondylitis: A meta-analysis. Int J Immunogenet. 2021;48(2):219–28. https://doi.org/10.1111/iji.12524
Hwang MC, Ridley L, Reveille JD. Ankylosing spondylitis risk factors: a systematic literature review. Clin Rheumatol. 2021;40(8):3079–93. https://doi.org/10.1007/s10067-021-05679-7
Chen S, Deng L. Risk factors for radiological hip involvement in patients with ankylosing spondylitis. Rev Assoc Med Bras (1992). 2021;67(9):1293-8. https://doi.org/10.1590/1806-9282.20210585
Akhtari M, Vojdanian M, Javinani A, Ashraf-Ganjouei A, Jamshidi A, Mahmoudi M. Activation of adenosine A(2A) receptor induced interleukin-23 mRNA expression in macrophages of ankylosing spondylitis patients. Cytokine. 2020;128:154997. https://doi.org/10.1016/j.cyto.2020.154997
Sharip A, Kunz J. Understanding the Pathogenesis of Spondyloarthritis. Biomolecules. 2020;10(10). https://doi.org/10.3390/biom10101461
Dong K, Gao ZW, Zhang HZ. The role of adenosinergic pathway in human autoimmune diseases. Immunol Res. 2016;64(5–6):1133–41. https://doi.org/10.1007/s12026-016-8870-2
Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–67. https://doi.org/10.1016/j.molmed.2013.03.005
Di Virgilio F, Vuerich M. Purinergic signaling in the immune system. Auton Neurosci. 2015;191:117–23. https://doi.org/10.1016/j.autneu.2015.04.011
Akhtari M, Zargar SJ, Mahmoudi M, Vojdanian M, Rezaeimanesh A, Jamshidi A. Ankylosing spondylitis monocyte-derived macrophages express increased level of A(2A) adenosine receptor and decreased level of ectonucleoside triphosphate diphosphohydrolase-1 (CD39), A(1) and A(2B) adenosine receptors. Clin Rheumatol. 2018;37(6):1589–95. https://doi.org/10.1007/s10067-018-4055-9
Yang J, Xu S, Chen M, Yuan Y, Zhang X, Ma Y, Wu M, Han R, Hu X, Liu R, Deng J, Guan S, Gao X, Pan M, Xu S, Shuai Z, Jiang S, Guan S, Chen L, Pan F. Serum Sclerostin and Bone Morphogenetic Protein-2 Levels in Patients with Ankylosing Spondylitis: A Meta-Analysis. Calcif Tissue Int. 2019;105(1):37–50. https://doi.org/10.1007/s00223-019-00542-z
Joo YB, Bang SY, Kim TH, Shim SC, Lee S, Joo KB, Kim JH, Min HJ, Rahman P, Inman RD. Bone morphogenetic protein 6 polymorphisms are associated with radiographic progression in ankylosing spondylitis. PLoS ONE. 2014;9(8):e104966. https://doi.org/10.1371/journal.pone.0104966
Chen MH, Chen HA, Chen WS, Chen MH, Tsai CY, Chou CT. Upregulation of BMP-2 expression in peripheral blood mononuclear cells by proinflammatory cytokines and radiographic progression in ankylosing spondylitis. Mod Rheumatol. 2015;25(6):913–8. https://doi.org/10.3109/14397595.2015.1029221
Mahmoud A, Fayez D, Gabal MM, Hamza SM, Badr T. Insight on Bone Morphogenetic Protein 7 in Ankylosing Spondylitis and its association with disease activity and radiographic damage. Electron Physician. 2016;8(7):2670–8. https://doi.org/10.19082/2670
Malemud CJ. Matrix Metalloproteinases and Synovial Joint Pathology. Prog Mol Biol Transl Sci. 2017;148:305–25. https://doi.org/10.1016/bs.pmbts.2017.03.003
Moz S, Aita A, Basso D, Ramonda R, Plebani M, Punzi L. Spondyloarthritis: Matrix Metalloproteinasesas Biomarkers of Pathogenesis and Response to Tumor Necrosis Factor (TNF) Inhibitors. Int J Mol Sci. 2017;18(4). https://doi.org/10.3390/ijms18040830
Vandooren B, Kruithof E, Yu DT, Rihl M, Gu J, De Rycke L, Van Den Bosch F, Veys EM, De Keyser F, Baeten D. Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and down-regulation by tumor necrosis factor alpha blockade in spondylarthropathy. Arthritis Rheum. 2004;50(9):2942–53. https://doi.org/10.1002/art.20477
Zhu Y, Li S, Huang Z, Xing W, Li F, Da Y, Xue J, Li M, Sun K, Jia H, Yang X. Association study between matrix metalloproteinase-3 gene (MMP3) polymorphisms and ankylosing spondylitis susceptibility. Mol Genet Genomic Med. 2019;7(7):e00752. https://doi.org/10.1002/mgg3.752
Mattey DL, Packham JC, Nixon NB, Coates L, Creamer P, Hailwood S, Taylor GJ, Bhalla AK. Association of cytokine and matrix metalloproteinase profiles with disease activity and function in ankylosing spondylitis. Arthritis Res Ther. 2012;14(3):R127. https://doi.org/10.1186/ar3857
Meng C, Bai R, Zhao Z, Huang G, Jin T, Feng W, Liu W. MMP-8 single-nucleotide polymorphisms are related to ankylosing spondylitis in Chinese Han population. Med (Baltim). 2018;97(35):e12136. https://doi.org/10.1097/md.0000000000012136
Akhtari M, Zargar SJ, Vojdanian M, Jamshidi A, Mahmoudi M. Monocyte-derived and M1 macrophages from ankylosing spondylitis patients released higher TNF-α and expressed more IL1B in response to BzATP than macrophages from healthy subjects. Sci Rep. 2021;11(1):17842. https://doi.org/10.1038/s41598-021-96262-2
Lari A, Gholami Pourbadie H, Jafari M, Sharifi-Zarchi A, Akhtari M, Nejatbakhsh Samimi L, Jamshidi A, Mahmoudi M. Downregulation of ITM2A Gene Expression in Macrophages of Patients with Ankylosing Spondylitis. Int Arch Allergy Immunol. 2021;182(11):1113–21. https://doi.org/10.1159/000516179
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–8. https://doi.org/10.1002/art.1780270401
Stamp LK, Hazlett J, Roberts RL, Frampton C, Highton J, Hessian PA. Adenosine receptor expression in rheumatoid synovium: a basis for methotrexate action. Arthritis Res Ther. 2012;14(3):R138. https://doi.org/10.1186/ar3871
Mia S, Warnecke A, Zhang XM, Malmström V, Harris RA. An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-β yields a dominant immunosuppressive phenotype. Scand J Immunol. 2014;79(5):305–14. https://doi.org/10.1111/sji.12162
Rőszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. https://doi.org/10.1155/2015/816460
Ravani A, Vincenzi F, Bortoluzzi A, Padovan M, Pasquini S, Gessi S, Merighi S, Borea PA, Govoni M, Varani K. Role and Function of A(2A) and A3 Adenosine Receptors in Patients with Ankylosing Spondylitis, Psoriatic Arthritis and Rheumatoid Arthritis. Int J Mol Sci. 2017;18(4). https://doi.org/10.3390/ijms18040697
Ågren MS. Auf dem Keller U. Matrix Metalloproteinases: How Much Can They Do? Int J Mol Sci. 2020;21(8). https://doi.org/10.3390/ijms21082678
Huang WC, Sala-Newby GB, Susana A, Johnson JL, Newby AC. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS ONE. 2012;7(8):e42507. https://doi.org/10.1371/journal.pone.0042507
Ho HH, Antoniv TT, Ji JD, Ivashkiv LB. Lipopolysaccharide-induced expression of matrix metalloproteinases in human monocytes is suppressed by IFN-gamma via superinduction of ATF-3 and suppression of AP-1. J Immunol. 2008;181(7):5089–97. https://doi.org/10.4049/jimmunol.181.7.5089
Alexander JS, Harris MK, Wells SR, Mills G, Chalamidas K, Ganta VC, McGee J, Jennings MH, Gonzalez-Toledo E, Minagar A. Alterations in serum MMP-8, MMP-9, IL-12p40 and IL-23 in multiple sclerosis patients treated with interferon-beta1b. Mult Scler. 2010;16(7):801–9. https://doi.org/10.1177/1352458510370791
Schmalz G, Davarpanah I, Jäger J, Mausberg RF, Krohn-Grimberghe B, Schmidt J, Haak R, Sack U, Ziebolz D. MMP-8 and TIMP-1 are associated to periodontal inflammation in patients with rheumatoid arthritis under methotrexate immunosuppression - First results of a cross-sectional study. J Microbiol Immunol Infect. 2019;52(3):386–94. https://doi.org/10.1016/j.jmii.2017.07.016
Reveille JD. Biomarkers for diagnosis, monitoring of progression, and treatment responses in ankylosing spondylitis and axial spondyloarthritis. Clin Rheumatol. 2015;34(6):1009–18. https://doi.org/10.1007/s10067-015-2949-3
Faber-Elmann A, Sthoeger Z, Tcherniack A, Dayan M, Mozes E. Activity of matrix metalloproteinase-9 is elevated in sera of patients with systemic lupus erythematosus. Clin Exp Immunol. 2002;127(2):393–8. https://doi.org/10.1046/j.1365-2249.2002.01758.x
Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26(4):299–307. https://doi.org/10.1007/s10875-006-9022-6
Ernens I, Rouy D, Velot E, Devaux Y, Wagner DR. Adenosine Inhibits Matrix Metalloproteinase-9 Secretion By Neutrophils. Circ Res. 2006;99(6):590–7. https://doi.org/10.1161/01.RES.0000241428.82502.d4
Chen H, Koupenova M, Yang D, Sume SS, Trackman PC, Ravid K. Regulation of MMP-9 expression by the A2b adenosine receptor and its dependency on TNF-α signaling. Exp Hematol. 2011;39(5):525–30. https://doi.org/10.1016/j.exphem.2011.02.004
Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31(3):407–15. https://doi.org/10.1016/j.mcn.2005.10.011
Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183(4):2741–6. https://doi.org/10.4049/jimmunol.0803164
Carter S, Braem K, Lories RJ. The role of bone morphogenetic proteins in ankylosing spondylitis. Ther Adv Musculoskelet Dis. 2012;4(4):293–9. https://doi.org/10.1177/1759720x12444175
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–34. https://doi.org/10.1126/science.3201241
Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30(1):26–31. https://doi.org/10.1016/s8756-3282(01)00638-x
Li B, Cao H, Zhao Y, Cheng M, Qin H, Cheng T, Hu Y, Zhang X, Liu X. Retraction Note: In vitro and in vivo responses of macrophages to magnesium-doped titanium. Sci Rep. 2021;11(1):8959. https://doi.org/10.1038/s41598-021-88664-z
Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, Li D, Wu Y, Shen H. Imbalance Between Bone Morphogenetic Protein 2 and Noggin Induces Abnormal Osteogenic Differentiation of Mesenchymal Stem Cells in Ankylosing Spondylitis. Arthritis Rheumatol. 2016;68(2):430–40. https://doi.org/10.1002/art.39433
Kwan YH, Tan JJ, Phang JK, Fong W, Lim KK, Koh HL, Lui NL, Tan CS, Østbye T, Thumboo J, Leung YY. Validity and reliability of the Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in patients with axial spondyloarthritis (axSpA) in Singapore. Int J Rheum Dis. 2019;22(12):2206–12. https://doi.org/10.1111/1756-185x.13735
Lin J, Yu Y, Wang X, Ke Y, Sun C, Yue L, Xu G, Xu B, Xu L, Cao H, Xu D, Olsen N, Chen W. Iguratimod Inhibits the Aggressiveness of Rheumatoid Fibroblast-Like Synoviocytes. J Immunol Res. 2019;2019:6929286. https://doi.org/10.1155/2019/6929286
Toegel S, Wu SQ, Otero M, Goldring MB, Leelapornpisid P, Chiari C, Kolb A, Unger FM, Windhager R, Viernstein H. Caesalpinia sappan extract inhibits IL1β-mediated overexpression of matrix metalloproteinases in human chondrocytes. Genes Nutr. 2012;7(2):307–18. https://doi.org/10.1007/s12263-011-0244-8
Mousavi MJ, Farhadi E, Vodjgani M, Karami J, Tahmasebi MN, Sharafat Vaziri A, Asgari M, Rezaei N, Mostafaei S, Jamshidi A, Mahmoudi M. Role of Fibroblast Activation Protein Alpha in Fibroblast-like Synoviocytes of Rheumatoid Arthritis. Iran J Allergy Asthma Immunol. 2021;20(3):338–49. https://doi.org/10.18502/ijaai.v20i3.6335
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Deputy of Research, Tehran University of Medical Sciences (Grant No. 99-3-96-51351).
Author information
Authors and Affiliations
Contributions
OS, MTE, NA, and MV: Acquisition of data, interpretation of data, drafting the article, and final approval of the article. MA, AJ, EF, and MM: The conception and design of the study, analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the article.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was performed based on the Declaration of Helsinki guidelines and was approved by the ethics committee at the Tehran University of Medical Sciences (Approval ID: (IR.TUMS.DDRI.REC.1399.047). The written informed consent was signed by all patients before enrolling in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sadatpour, O., Ebrahimi, M.T., Akhtari, M. et al. A2A adenosine receptor agonist reduced MMP8 expression in healthy M2-like macrophages but not in macrophages from ankylosing spondylitis patients. BMC Musculoskelet Disord 23, 908 (2022). https://doi.org/10.1186/s12891-022-05846-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-05846-0