Abstract
Background
Excessive use of short-acting β2 agonists (SABA) in patients with asthma continues to be a notable concern due to its link to higher mortality rates. Global relevance of SABA overuse in asthma management cannot be understated, it poses significant health risk to patients with asthma and imposes burden on healthcare systems. This study, as part of global SABINA progamme, aimed to describe the prescribing patterns and clinical outcomes associated with SABA use in the Chinese population.
Methods
Retrospective cohort study was conducted using anonymized electronic healthcare records of Clinical Data Analysis and Reporting System (CDARS) from Hong Kong Hospital Authority (HA). Patients newly diagnosed with asthma between 2011 and 2018 and aged ≥12 years were included, stratified by SABA use (≤2, 3–6, 7–10, or ≥11 canisters/year) during one-year baseline period since asthma diagnosis date. Patients were followed up from one-year post-index until earliest censoring of events: outcome occurrence and end of study period (31 December 2020). Cox proportional regression and negative binomial regression were used to estimate the mortality risk and frequency of hospital admissions associated with SABA use respectively, after adjusting for age, sex, Charlson Comorbidity Index (CCI), and inhaled corticosteroid (ICS) dose. Outcomes include all-cause, asthma-related, and respiratory-related mortality, frequency of hospital admissions for any cause, and frequency of hospital admissions due to asthma.
Results
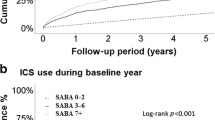
17,782 patients with asthma (mean age 46.7 years, 40.8% male) were included and 59.1% of patients were overusing SABA (≥ 3 canisters per year). Each patient was prescribed a median of 5.61 SABA canisters/year. SABA overuse during baseline period was associated with higher all-cause mortality risk compared to patients with ≤2 canisters/year. Association was dose-dependent, highest risk in those used ≥11 canisters/year (adjusted hazard ratio: 1.42, 95% CI: 1.13, 1.79) and 3–6 canisters/year (adjusted hazard ratio: 1.22, 95% CI: 1.00, 1.50). Higher SABA prescription volume associated with increased frequency of hospital admissions with greatest risk observed in 7–10 canisters/year subgroup (adjusted rate ratio: 4.81, 95% CI: 3.66, 6.37).
Conclusions
SABA overuse is prevalent and is associated with increased all-cause mortality risk and frequency of hospital admissions among the patients with asthma in Hong Kong.
Similar content being viewed by others
Introduction
Asthma is the most common chronic respiratory diseases that affects people of all ages, with a global prevalence of 262 million in 2019 [1]. In Hong Kong, about 68,000 persons were diagnosed with asthma in 2019, accounting for 1% of the total population [2]. Asthma symptoms range from mild coughing and wheezing to life-threatening exacerbation. The goals of asthma treatment are to achieve symptomatic control, minimize the risk of acute exacerbation, and minimize treatment toxicity.
For the last several decades, the use of short-acting β2 agonists (SABA) alone as an intermittent reliever medication (step 1) or with the additional use of a controller medication, low-dose inhaled corticosteroid (ICS) (step 2), have been recommended for the treatment of patients with mild asthma [3, 4]. Due to the minor and occasional nature of symptoms in mild asthma, many patients rely on SABA alone to relieve symptoms, with poor adherence to regular ICS that addresses the underlying inflammatory pathology of asthma [5], leading to an increased risk of severe asthma exacerbations [6]. Evidence shows that excessive use of SABA (≥11 canisters per year), as monotherapy or in combination with ICS, is associated with an increased risk of asthma-related mortality [7, 8]. Since patients with mild asthma account for 50-75% of the asthma population [9], over-reliance on SABA has been a cause for concern. In 2019, to reduce the risk of severe exacerbations in people with mild asthma, the Global Initiative for Asthma (GINA) reported that SABA alone without ICS was no longer recommended. Instead, all adults and adolescents with asthma are recommended to use ICS-containing controller treatment for symptomatic relief (steps 1 to 2) or daily use (steps 2 to 5). This heralds a paradigm shift in asthma management [10].
The importance of addressing SABA overuse in asthma is significant on a global scale. The SABINA (SABA use IN Asthma) Program is a global research program that aims to describe and understand the treatment pattern of asthma medications, the extent of SABA inhaler use and the associations between SABA use and different clinical outcomes in different parts of the world [11]. SABINA Europe reported excessive use of SABA and poor adherence to ICS among patients with asthma. Overuse of SABA was found to range from 9% in Italy to 38% in the United Kingdom [12]. The SABINA Sweden cohort study with 365,324 patients showed that the risk of exacerbation and mortality rose with increased SABA use. Patients who used ≥11 canisters per year had a two-fold risk of death compared to those who received ≤2 canisters per year [13]. Although there are quite a few studies on the topic, data from an Asian population is scarce. A population-based study in Korea showed that the rate of SABA overuse was about 2–4% among patients with asthma [14]. Except for a recent SABINA study in Taiwan that showed a prevalence rate of 15.9% of SABA overuse and an association between SABA overuse and increased risk of severe exacerbation and all-cause mortality [15]. Studies conducted in various countries have demonstrated a link between SABA overuse and adverse outcomes in patients with asthma. However, data on the treatment pattern of asthma and the clinical outcomes associated with SABA use in the Chinese population are limited. This study, as part of the global SABINA progamme, aimed to describe the prescribing patterns and clinical outcomes associated with SABA use in the Hong Kong population.
Methodology
Data source
This was a retrospective population-based cohort study using anonymized electronic healthcare records of the Clinical Data Analysis and Reporting System (CDARS) from the Hong Kong Hospital Authority (HA). The HA serves all residents in Hong Kong (over 7 million), covering approximately 80% of all hospital admissions and providing ongoing medical treatment for 76% of patients with chronic health conditions through 43 hospitals and institutions [16, 17], 49 specialist outpatient clinics, and 73 general outpatient clinics. Several high-quality pharmaco-epidemiological studies have used CDARS data in the past [16,17,18,19,20]. Data validity and reliability of the database are reflected by the high coding accuracy for clinical outcomes as reported in previous studies with high positive and negative predictive values of more than 90% [16, 18, 20]. Therefore, CDARS is a nationwide source of medical records covering outpatient and inpatient healthcare records as well as mortality data, representative of the population in Hong Kong.
Study design
Patients diagnosed with asthma and aged ≥12 years between January 1, 2011 and December 31, 2018 were identified using the International Classification of Diseases–9th Edition (ICD-9) code 493.x. Patients with a history of chronic obstructive pulmonary disease or a chronic respiratory disease other than asthma on or before the date of first asthma diagnosis, those who received long-acting β2 agonists (LABA) and/or ICS prescription before first asthma diagnosis, or those who died on or within one year from the date of study entry were excluded. The index date was defined as the date of first asthma diagnosis. The baseline period starts from the index date up to one-year post-index, during which patients were categorized based on SABA use (≤2, 3–6, 7–10, or ≥11 canisters per year). Patients were followed up from one-year post-index until the earliest censoring of events: occurrences of outcome(s), end of the study period December 31,2020) or death (Fig. 1).
Flowchart of cohort identification
a Date of admission would be considered as the index date if the index diagnosis is an inpatient episode
b Removal of patients who died within 1 year from the date of first asthma diagnosis due to insufficient baseline period to ascertain SABA use
c COPD = chronic obstructive pulmonary disease
d LABA = long acting ß2 agonist
e ICS = inhaled corticosteroid
Outcomes, other variables, and covariates
Outcomes include all-cause mortality, asthma-related mortality (defined as the cause of death with ICD-10 code J45), respiratory-related mortality (defined as the cause of death with ICD-10 code J00-J99), frequency of hospital admissions for any cause, and frequency of hospital admissions due to asthma (defined as hospital admissions with a primary diagnosis of ICD-9 code 493.x). Covariates, including patient demographics (age, sex, year of first asthma diagnosis) at index date, health status (Charlson Comorbidity Index [CCI], hospitalization one year before the index date), pre-existing comorbidities (allergic rhinitis, gastroesophageal reflux disease, coronary artery disease, hypertension, diabetes, congestive heart failure, atrial fibrillation, stroke, renal disease, and cancer) before the index date, were described. Prescribing patterns and choice of asthma treatment during the baseline period (including the use of SABA, LABA, ICS, long-acting muscarinic antagonists [LAMA], leukotriene receptor antagonists [LTRA], anti-IgE / anti-IL5/5R / anti-IL4R, and oral corticosteroid [OCS] use) were reported. Asthma severity was assessed by the dose of ICS used during the baseline period, categorized into none, low, medium, or high with reference to the Global Strategy for Asthma Management and Prevention issued by GINA [10], and adjusted in the analyses.
Statistical analysis
Patient characteristics, including covariates at baseline and choice of asthma treatment during the baseline period, were reported descriptively as frequencies (percentages) for categorical variables and mean (SD) for continuous variables. The proportion of patients receiving a prescription for SABA and the number of canisters prescribed per year during the baseline period were estimated. Patients were stratified by SABA use (≤2, 3–6, 7–10, or ≥11 canisters per year). SABA overuse was defined as patients who were prescribed ≥ 3 SABA canisters per year. Baseline characteristics for each subgroup were reported. The dose of ICS (low, medium, or high) and the use of other asthma treatments during the baseline period were described. The trend in SABA use (number of canisters per year per patient) in each calendar year during the study period was reported. Incidence rates of all outcomes were reported. Risks of all-cause mortality, respiratory-related deaths, and asthma-related deaths associated with SABA use were estimated using Cox proportional hazards regression, after adjusting for age, sex, CCI, and ICS dose. ICS dose was adjusted as a covariate in the analysis since asthma severity is associated with the risk of mortality. Hazard ratios with their 95% confidence intervals were reported. The frequency of hospital admissions associated with SABA use was estimated using negative binomial regression, after adjusting for age, sex, CCI, and ICS dose. Rate ratios and their 95% confidence intervals were reported. A p-value less than 0.05 was considered statistically significant in all analyzes. All analysis was performed using R 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) and cross-checked by two independent investigators (LF and VY).
Results
After applying the exclusion criteria, we included a total of 17,782 patients with a diagnosis of asthma between January 1, 2011 to December 31, 2018 (Fig. 1). The mean age was 46.7 years and 40.8% were men. (Table 1). The majority of patients had no comorbidities (81.9%) or a very mild comorbidity score (15.1%). Major comorbidities among the study cohort were hypertension (9.5%) and diabetes (4.3%) respectively. Patients receiving more SABA canisters per year during the baseline period were generally older, had more comorbidities, and were more likely to have severe asthma as reflected by the ICS dose and the use of LAMA, LTRA, and ICS.
Prescription pattern of SABA and other asthma medications
Among the study cohort, 59.1% of patients were overusing SABA (≥ 3 canisters per year), of which 3,276 (18.4%) patients were prescribed 3–6 canisters, 1,846 (10.4%) patients were prescribed 7–10 canisters, and 5,394 (30.3%) patients were prescribed ≥11 canisters during the one-year baseline period. Throughout the study period, the median SABA canisters prescribed to each patient with asthma per year was 5.61 canisters (Table 2). The overall prescription rate of ICS and LABA was only 43.4% and 17.3%, respectively. Patients who were prescribed a higher number of SABA canisters had higher number of ICS and LABA prescriptions (Table 1). The highest prescription volume of OCS was found in the ≥11 canisters/year subgroup followed by ≤2 canisters/year subgroup. The overall use of other asthma medications (LAMA and LTRA) was relatively low among patients with asthma at 2.2% and 4.8% respectively. Patients prescribed a higher number of SABA canisters also had a high proportion of prescribed LAMA and LTRA.
Risk of mortality associated with SABA use
After adjusting for age, sex, CCI and ICS dose, patients who were overusing SABA (≥ 3 canisters/year) during the baseline period had a higher risk of all-cause mortality compared to patients with appropriate use (≤ 2 canisters/year). The association was dose-dependent, with the highest risk in those who used ≥ 11 canisters/year (adjusted HR: 1.84, 95% CI: 1.55, 2.19) followed by patients who used 7–10 canisters/year (adjusted HR: 1.42, 95% CI: 1.13, 1.79) and 3–6 canisters/year (adjusted HR: 1.22, 95% CI: 1.00, 1.50) (Table 3). Despite a similar association observed in the risk of respiratory-related (Table 4) and asthma-related mortality (Table 5), the associations were not statistically significant. Only patients who used ≥ 11 SABA canisters/year showed a statistically significant increased risk of respiratory-related mortality (adjusted HR: 1.86, 95% CI: 2.09, 17.86) and asthma-related mortality (adjusted HR: 19.1, 95% CI: 1.95, 187.3) respectively.
Frequency of hospital admission associated with SABA use
After adjusting for age, sex, CCI, and ICS dose, an increased number of prescribed SABA canisters was associated with an increased frequency of hospital admissions, although a dose-response relationship was not observed (Table 6). The highest risk was observed in the 7–10 canisters/year subgroup (adjusted RR: 4.81, 95% CI: 3.66, 6.37) which was higher than the ≥ 11 canisters/year subgroup (adjusted RR: 3.72, 95% CI: 2.98, 4.66) and 3–6 canisters/year subgroup (adjusted RR: 2.74, 95% CI: 2.16, 3.49). On the other hand, the frequency of asthma-related hospital admission was only found to be statistically significant among ≥ 11 canisters/year subgroup (adjusted RR: 3.62, 95% CI: 2.27, 5.82) but not in the 3–6 canisters/year and 7–10 canisters/year subgroups. (Table 7).
Subgroup analysis
We conducted a subgroup analysis stratified by patients with OCS prescriptions (Supplementary Tables 1–2). Among patients with any OCS prescription during the baseline period, the risk of all-cause mortality was consistent with the main analysis and a dose-response relationship with the increase in SABA use was also observed. Statistically significant relationship was observed for the frequency of hospital admission among patients with OCS prescriptions, but a dose-response relationship was not observed.
Discussion
In this Hong Kong-wide study, SABA overuse was observed in more than half (∼ 60%) of the study population. In particular, more than 30% of patients had 11 or more SABA canisters prescribed within their first year of asthma diagnosis. These results suggest that over-prescription of SABA and potential SABA overuse was considerably more serious in Hong Kong than in other countries such as Taiwan [15], Korea [14], Sweden [13], and other parts of Europe [12] which only had prevalence rates ranging from 16 to 30%.
Consistent with SABINA studies in Europe and Taiwan [12, 13, 15], SABA overuse in Hong Kong was associated with a statistically significant increased risk of all-cause mortality as well as frequency of hospital admissions, after adjusting for age, sex, health status, and asthma severity (in terms of ICS dose). The risk of all-cause mortality increased significantly with SABA overuse, even for those with mild overuse (3–6 canisters/year), and a dose-dependent trend was observed, which further consolidated the statistical association. Since SABA is the reliever medication in asthma treatment and does not possess anti-inflammation effects, high use of SABA for asthma management is merely symptom relieving and does not manage the underlying inflammation, suggesting suboptimal asthma management [21]. Importantly, this leads to progressive worsening of symptoms and other adverse events, which eventually increases the risk of all-cause mortality. The exact biological mechanism between SABA overuse and all-cause mortality is not completely understood, however all-cause mortality is considered as an important indicator for assessing the safety of long-term medications among asthma patients [22, 23]. Among the all-cause mortality events, the majority (40.3%) were respiratory-related, and other common causes (1.2-10.6%) include cancer (lung, liver or unspecified), heart failure, sepsis, acute myocardial infarction and chronic kidney disease.
A dose-dependent trend was not observed, however, for frequency of hospital admissions. Limited by low incidence rates of respiratory- and asthma-related mortality among the study population, no statistically significant association was observed between increased SABA use and risk of respiratory- and asthma-related mortalities, except in patients receiving ≥ 11 canisters per year. Future studies with a larger sample size would be needed to re-assess this potential association.
Despite the change to international guidelines in 2019 that as-needed SABA monotherapy was no longer recommended in patients with mild asthma and that such patients should receive ICS-containing controller treatment to reduce the risk of serious exacerbations and control symptoms [10], our data up to the end of 2020 revealed no evidence of a corresponding change in prescribing practice in Hong Kong. The overall prescription of LABA with ICS, which was the new asthma treatment recommendation, was low among patients with mild asthma. Therefore, physicians might be over-reliant on SABA as a reliever for patients with asthma and this might have contributed to poor symptomatic control and increased risk of adverse outcomes. Furthermore, the possibility of physicians over-prescribing SABA canisters to patients for stockpiling purposes could not be ruled out.
The findings of this study have clinical implications. Firstly, consistent with studies conducted in other countries, SABA overuse was associated with increased mortality and hospitalization even after accounting for age, sex, health status, and asthma severity. The research findings contribute to the global understanding of SABA overuse in asthma management particularly among the Chinese population, it reinforces the importance of addressing the issue not only in Hong Kong but also in other regions worldwide. Secondly, over-prescription of SABA to patients with asthma in Hong Kong was observed. Despite the change in recommendations to international guidelines, changes in local clinical practice to reduce SABA overuse were not evident in Hong Kong. It is imperative to identify the gaps and develop action plans for updating local clinical guidelines and changing clinical practice. The GINA treatment strategy is one of the main clinical guidelines used by physicians to assess asthma control [24]. Hence, promotion of changes in the GINA treatment strategy (as-needed low dose ICS-formoterol as the preferred controller and reliever option in steps 1–2 and removal of SABA monotherapy as the recommended reliever option) to physicians at clinics frequently attended by patients with mild asthma, such as General Out-patient Clinics (GOPC), Respiratory and Family Medicine Specialist Clinics, would be necessary. Currently, drug choices at GOPC are limited as patients are perceived to have mild disease [25], various ICS and combination medications such as ICS-formoterol are usually prescribed by respiratory specialists according to the Drug Formulary in HA, physicians at GOPC may tend to prescribe SABA as relievers to the patients among the limited asthma medication options, thus contributing to the SABA overuse. Thirdly, study findings have shed light on SABA over-prescription in clinical practice in Hong Kong and high prescription of OCS, indicating the need to critically review the standard drug formulary for treating asthma in primary care and specialty care clinics in local public health care settings, given that these are the contexts in which SABA over-prescription took place. For instance, a critical review of the drug formulary and prescribing practices for treating asthma in primary care and specialty care clinics should be warranted to minimize SABA overuse and adherence to controller medications. Access to ICS in primary care clinics should be considered a priority. Prescribing and dispensing practice should also be monitored over time to assess whether changes in drug availability would lead to the desired results and if additional factors should be considered including physician, patient, and/or systems. The study also highlights for increased awareness and education among healthcare providers regarding the appropriate use of SABA medications in asthma management. This includes understanding the potential risks associated with SABA overuse and the importance of promoting controller medications for long-term asthma control. Further investigations can look into the underlying factors contributing to SABA overuse such as patient preferences and healthcare system barriers which can help to develop a comprehensive understanding of SABA overuse in asthma management. Apart from the promotion of conventional treatment guidelines, the implementation of national or regional asthma programs [26] and encouraging patient involvement in disseminating appropriate treatment information [27] may also be effective measures to improve asthma care in the city. For instance, training primary care providers on the appropriate asthma management approach or enhance the access to specialized care, as well as establishment of robust data collection and surveillance systems to monitor asthma prevalence, control and medication use patterns. This can help identify areas of improvement and guide further adjustments in asthma management strategies. These strategies could also be useful in other regions worldwide.
Limitations
Several limitations deserve attention. Firstly, prescription data were used to estimate SABA inhaler usage, hence reflecting only the number of SABA canisters dispensed to patients. Data on treatment adherence and stockpiling were not available; therefore, the actual consumption trend of patients with asthma may not be adequately reflected by the prescriptions and the actual use of SABA by patients might be overestimated. Secondly, only prescription data and patients treated in hospitals and clinics managed by the HA, the sole public health service provider in Hong Kong, were captured in this study, hence it may not be representative of the private healthcare sector. Lastly, as with any observational studies, the possibility of unmeasured residual confounding, such as socioeconomic status could not be ruled out. Such unmeasured confounding could potentially under- or over-estimate the risks associated with SABA overuse. Nevertheless, essential covariates associated with SABA use have been adjusted for in the main analysis, and the possibility of an unmeasured confounder with sufficient effect size to change our main conclusions is unlikely.
Conclusion
The overuse of SABA remains prevalent among patients with asthma in Hong Kong despite updates in treatment recommendations to international asthma treatment guidelines. Overuse was associated with an increased risk of all-cause mortality and increased risk of hospital admissions for all-cause mortality, which was consistent with findings from our global SABINA studies. Effective physician and patient education and communication on the importance of potential adverse outcomes of SABA overuse and adherence to controller medications are key to improving asthma treatment.
Data availability
The datasets generated and/or analysed during the current study are not publicly available as the data custodians (the Hospital Authority of Hong Kong SAR) has not given permission for sharing due to patient confidentiality and privacy concerns, but are available from the corresponding author on reasonable request and with permission from the Hospital Authority of Hong Kong SAR.
Abbreviations
- CCI:
-
Charlson Comorbidity Index
- CDARS:
-
Clinical Data Analysis and Reporting System
- GINA:
-
Global Initiative for Asthma
- HA:
-
Hospital Authority
- ICD-9:
-
International Classification of Diseases–9th Edition
- ICS:
-
Inhaled corticosteroid
- LABA:
-
Long-acting β2 agonists
- LAMA:
-
Long-acting muscarinic antagonists
- LTRA:
-
Leukotriene receptor antagonists
- OCS:
-
Oral corticosteroid
- SABA:
-
Short-acting β2 agonists
References
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204-22.
Department CaS. Thematic Household Survey Report No.68. Health Status of Hong Kong residents Hong Kong Hong Kong. Hong Kong Special Administrative Region Government; 2019.
Asthma GIf. Global Strategy for Asthma Management and Prevention 2018.
Society BT, Network SIG. British Guideline on the Management of Asthma - A National Clinical Guideline London, UK; 2016.
Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-Needed budesonide–formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877–87.
Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MCJM, Verhamme KMC. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396.
Sadatsafavi M, Tavakoli H, Lynd L, FitzGerald JM. Has Asthma Medication Use Caught up with the evidence? A 12-Year Population-based study of Trends. Chest. 2017;151(3):612–8.
FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of Inappropriate Use of Short Acting Beta agonists in Asthma. Respir Med. 2017;131:135–40.
Dusser D, Chanez DM, de Blic P, Delacourt J, Deschildre C. Mild asthma: an Expert Review on Epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62(6):591–604.
Asthma GIf. Global Strategy for Asthma Managment and Prevention 2019.
β2-agonist use in asthma. European Respiratory Journal. 2020;55(2):1901858.
Janson C, Menzies-Gow A, Nan C, Nuevo J, Papi A, Quint JK, et al. SABINA: an overview of short-acting β2-Agonist use in Asthma in European Countries. Adv Therapy. 2020;37(3):1124–35.
β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. European Respiratory Journal. 2020;55(4):1901872.
Lee H, Ryu J, Chung SJ, Yeo Y, Park TS, Park DW, et al. Short-acting Beta2-Agonist use in Asthma in Korea: a 10-Year Population-based study. Allergy Asthma Immunol Res. 2021;13(6):945–53.
Wang C-Y, Lai C-C, Wang Y-H, Wang H-C. The prevalence and outcome of short-acting β2-agonists overuse in asthma patients in Taiwan. npj Prim Care Respiratory Med. 2021;31(1):19.
Lau WCY, Chan EW, Cheung C-L, Sing CW, Man KKC, Lip GYH, et al. Association between Dabigatran vs Warfarin and Risk of osteoporotic fractures among patients with Nonvalvular Atrial Fibrillation. JAMA. 2017;317(11):1151–8.
Zhao J, Blais JE, Chui CSL, Suh I-H, Chen EYH, Seto W-K et al. Association between Nonvitamin K antagonist oral anticoagulants or Warfarin and Liver Injury: a Cohort Study. Official J Am Coll Gastroenterol | ACG. 2020;115(9).
Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for <em>Helicobacter pylori</em>: a population-based study. Gut. 2018;67(1):28.
Wong AYS, Wong ICK, Chui CSL, Lee EHM, Chang WC, Chen EYH, et al. Association between Acute Neuropsychiatric Events and Helicobacter pylori Therapy containing clarithromycin. JAMA Intern Med. 2016;176(6):828–34.
Wong AYS, Root A, Douglas IJ, Chui CSL, Chan EW, Ghebremichael-Weldeselassie Y et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ [Internet]. 2016 2016/01//; 352:[h6926 p.]. http://europepmc.org/abstract/MED/26768836https://doi.org/10.1136/bmj.h6926.
Ritchie AI, Singanayagam A, Wiater E, Edwards MR, Montminy M, Johnston SL. β(2)-Agonists enhance asthma-relevant Inflammatory mediators in Human Airway Epithelial cells. Am J Respir Cell Mol Biol. 2018;58:128–32.
Bloom CI, Cabrera C, Arnetorp S, Coulton K, Nan C, van der Valk RJP, et al. Asthma-related Health outcomes Associated with short-acting β2-Agonist inhaler use: an observational UK Study as Part of the SABINA Global Program. Adv Therapy. 2020;37(10):4190–208.
Anderson HR, Ayres JG, Sturdy PM, Bland JM, Butland BK, Peckitt C, et al. Bronchodilator treatment and deaths from asthma: case-control study. BMJ. 2005;330(7483):117.
Price D, David-Wang A, Cho S-H, Ho JC-M, Jeong J-W, Liam C-K, et al. Asthma in Asia: physician perspectives on control, inhaler use and patient communications. J Asthma. 2016;53(7):761–9.
Sze-wai Yeung Pang-fei, Chan, Loretta KP, Lai Kai-lim, Chow MMH, Luk, Chao DV. Prevalence of different severities of chronic obstructive pulmonary disease in an out-patient clinic in Hong Kong. Hong Kong Practitioner - J Hong Kong Coll Family Physicians. 2016;38:3–12.
Selroos O, Kupczyk M, Kuna P, Łacwik P, Bousquet J, Brennan D, et al. National and regional asthma programmes in Europe. Eur Respiratory Rev. 2015;24(137):474.
Localio AM, Black HL, Park H, Perez L, Ndicu G, Klusaritz H, et al. Filling the patient-provider knowledge gap: a patient advocate to address asthma care and self-management barriers. J Asthma. 2019;56(10):1027–36.
Acknowledgements
The corresponding author had full access to all data in the study and takes responsibility for the content of the manuscript, integrity of the data, and accuracy of data analysis. We thank Eliza Chan, Jessica Shami and Lisa Lam for proofreading the manuscript.
Funding
This study was sponsored by AstraZeneca (Hong Kong). The sponsor had no role in the study design, data collection, and analysis.
Author information
Authors and Affiliations
Contributions
Concept and design: Fung, Yan, Wong, ChanAcquisition, analysis or interpretation of data: Fung, Yan, ChanDrafting of the manuscript: Fung, Yan, KwanCritical revision of the manuscript for important intellectual content: Kwan, Kwok, Lam, Bloom, McDonald, Wong, Chan Statistical analysis: Fung, YanSupervision: Wong, Chan.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of the University of Hong Kong/Hong Kong West Cluster (UW-20-873). Informed consent has been waived by the Institutional Review Board of the University of Hong Kong/Hong Kong West Cluster for this study because the data involved were de-identified. This study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklists to guide transparent reporting of the cohort study. All the procedures were followed in accordance with the Declaration of Helsinki.
Consent to Publish
Not applicable.
Competing interests
CB receives funding from National Institute for Health and Care Research (NIHR UK) and Asthma and Lung UK. ICKW receives research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, National Institute for Health Research in England, European Commission, and the National Health and Medical Research Council in Australia; has received speaker fees from Janssen and Medice in the previous 3 years; and is an independent non-executive director of Jacobson Medical in Hong Kong. EWC has received grants from Research Grants Council (RGC, Hong Kong), Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Novartis, Amgen, AstraZeneca, Takeda, the RGA Reinsurance Company, Hong Kong, Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region, the National Health and Medical Research Council Australia; consulting fees from AstraZeneca, Pfizer and Novartis, and honorarium from the Hospital Authority Hong Kong, outside the submitted work. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fung, L.W., Yan, V.K., Kwan, C. et al. SABINA + Hong Kong: a territory wide study of prescribing trends and outcomes associated with the use of short-acting β2 agonists in the Chinese population. BMC Pulm Med 24, 232 (2024). https://doi.org/10.1186/s12890-024-03038-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03038-1