Abstract
Background
Advanced lung adenocarcinoma patients often develop resistance to EGFR tyrosine kinase inhibitors (EGFR-TKIs), leaving uncertainties regarding subsequent treatment strategies. Although personalized therapy targeting individual acquired resistances (ARs) shows promise, its efficacy has not been systematically compared with platinum-containing doublet chemotherapy, a widely accepted treatment after EGFR-TKIs failure.
Methods
A retrospective dual-center study was conducted involving patients with advanced lung adenocarcinoma and EGFR mutations who developed resistance to EGFR-TKIs between January 2017 and December 2022. Eligible patients were adults aged 18 years or older with an Eastern Cooperative Oncology Group score of 0–1, normal organ function, and no prior chemotherapy. Patients were divided into the chemotherapy group (CG) or personalized therapy group (PG) based on the treatment received after disease progression. The primary endpoints were progression-free survival (PFS) and objective response rate (ORR).
Results
Of the 144 patients enrolled, there were 53 patients in the PG and 91 patients in the CG. The PG acquired resistance to EGFR-TKIs through the MET amplification (27, 50%) and small cell lung cancer transformation (16, 30%) and 18% of them reported multiple resistance mechanisms. The ORR of the PG was similar to that of the CG (34% vs. 33%, P = 1.0) and the PFS of the PG patients was not statistically different from that of their CG counterparts [4.2 months (95% CI: 3.6–4.8 months) vs. 5.3 months (95% CI: 4.6–6.0 months), P = 0.77].
Conclusions
These findings suggest that the therapeutic efficacy of chemotherapy approximates to that of personalized therapy, which signifies that chemotherapy is still a reliable choice for patients who develop resistance to EGFR-TKIs and that further research is awaited to explore the benefit of personalized treatment.
Similar content being viewed by others
Introduction
Lung cancer is notorious for its high morbidity and mortality rate worldwide [1], in which non‐small cell lung cancer (NSCLC) accounts for 80%‐85% of the incidences [2]. In the NSCLC population, epidermal growth factor receptor (EGFR) gene is found in 10–20% of Caucasians and at least 50% of Asian NSCLC patients [3]. EGFR tyrosine kinase inhibitors (EGFR-TKIs) have dramatically improved survival outcomes and are the first-line treatment for patients with EGFR-mutants [4].
Unfortunately, patients undergoing tyrosine kinase inhibitor (TKI) treatment eventually develop acquired resistance (AR). The common ARs include: (i) secondary mutations to EGFR, such as T790M mutation, C797S mutation [5]; (ii) activation of alternative pathways, such as MET amplification [6], ERBB2 amplification [7]; (iii) activation of downstream targets, for instance, RAS-MAPK pathway signaling [8], PIK3CA mutations [9]; (iv) histologic transformation, for example, small-cell lung cancer (SCLC) transformation [10]; (v) others: fibroblast growth factor receptor (FGFR) amplification, cell cycle gene alterations [8]. Certain EGFR-mutant NSCLCs may harbor multiple mechanisms of EGFR-TKI resistance [9]. However, the potential mechanisms underlying the AR remains obscured in up to 50% of cases [11].
After the progression with EGFR-TKIs, different options are available, including chemotherapy and personalized therapy that is based on individual ARs. For example, the combined treatment with EGFR and MET-TKIs can inhibit the growth of EGFR-mutated NSCLC coupled with MET amplification [12]. The combination with 3rd and 1st or 2nd EGFR-TKIs is also a reasonable strategy against the AR of T790M-trans-C797S [13]. Except for the 3rd EGFR-TKIs, which is approved for the treatment of NSCLC patients with positive T790M mutation after developing AR to the first-line EGFR-TKIs [14], the efficacy of other personalized therapeutic strategies is largely compromised by small samples and absence of comparison with chemotherapy.
Here we conducted a retrospective multi-center study to explore the efficacy of chemotherapy and personalized therapy. We found that the efficacy of chemotherapy approximated to that of personalized therapy, which indicates that chemotherapy may still serve as a promising option for patients who develop resistance to EGFR-TKIs.
Methods
Patients
A retrospective study was conducted at two centers, Fujian Cancer Hospital and Hunan Cancer Hospital, to collect data of patients with advanced NSCLC from January 1, 2017, to December 31, 2022, in China. The inclusion criteria for eligible patients were as follows: (i) aged 18 years or older; (ii) histologically-confirmed lung adenocarcinoma; (iii) Stage IV according to American Joint Committee on Cancer (AJCC) (8th edition); (iv) an Eastern Cooperative Oncology Group (ECOG) score of 0–1 and normal organ functions; (v) EGFR sensitive mutations (deletion of exon 19 or the L858R mutation); (vi) patients who developed resistance after the treatment with the first- or second-generation EGFR-TKIs and were negative for T790M or patients who were positive for T790M after the treatment with the first- or second-generation EGFR-TKIs and developed resistance after the administration of the third-generation EGFR-TKI or those who developed resistance after initial treatment with the third-generation EGFR-TKI; (vii) available resistance mechanism confirmed by the next-generation sequencing (NGS) or Fluorescence in situ hybridization (FISH) after progression.
The eligible patients were divided into the Chemotherapy group (CG) or Personalized group (PG) according to the subsequent therapies after resistance to EGFR-TKIs. The CG patients received platinum-containing doublet chemotherapy, which was combined with or without antiangiogenic therapy and immunotherapy. Genetic testing post-EGFR TKI resistance was not deemed essential in this context. The therapies in the PG were prompted according to the genetic testing or histologic transformation. Data regarding the demographic information, tumor histology and molecular pathology, clinical treatments and outcomes were collected for the further analysis.
The NGS was performed in Geneplus-Beijing Institute or Fujian Cancer Hospital, which covered genomic regions of 1,021 cancer-related genes. The sample included the peripheral blood or frozen tissue [15]. The FISH test was conducted in Fujian Cancer Hospital.
Response evaluation
The tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) in terms of partial response (PR), stable disease (SD), progressive disease (PD) or complete response (CR). The objective response rate (ORR) was defined as the percentage of patients with PR and CR. The disease control rate (DCR) was designated as the percentage of CR, PR and SD. The progression-free survival (PFS) was termed as the time from the initiation of treatment to disease progression or death from any cause. Treatment was considered censored if no evidence of progression was found at the last follow-up and time was recorded from start of treatment to the last follow-up.
Statistical analysis
All data were analyzed using the SPSS 24.0 Software. Unadjusted PFS was estimated by the Kaplan–Meier product-limit method. The clinical and biological characteristics and ORR between two groups were analyzed by the chi-square test. The two-sided significance level was set at P < 0.05.
Results
The baseline characteristics were balanced between CG and PG
A total of 144 patients were enrolled, with a median age of 57 years, of whom, 86 patients reported a deletion of EGFR exon 19. The enrolled patients were further categorized into PG (53 patients) and CG (91patients). The baseline characteristics, including the incidence of EGFR exon 19 deletions and L858R mutations, were balanced between the two groups, with no significant statistical differences observed (all P > 0.05) (Table 1).
The efficacy was no significant difference between CG and PG
Among the 53 PG patients, PR was reported in 18 patients and SD in 21 patients. In the CG, PR was found in 30 patients and SD in 65 patients. No significant differences were observed in ORR (34.0% vs. 33.0%, P = 1.0) and DCR (73.6% vs. 81.3%, P = 0.30) between two groups (Fig. 1).
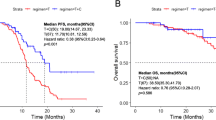
With the cutoff date set on March 15, 2023, the median follow-up time was 26.9 months. The disease progression was reported in 89 (97.8%) CG patients and 46 (86.8%) PG counterparts. The median PFS was 5.3 months (95% CI, 4.6–6.0 months) in CG, and 4.2 months (95% CI, 3.6–4.8 months) in PG (Fig. 2A), demonstrating no significant difference between two groups (HR, 0.95; P = 0.77).
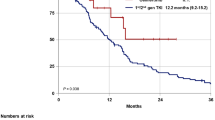
The PFS of different groups. A The PFS between CG and PG. B The PFS between four subgroups in CG. C Tumor response rates between AIC and PG. D The PFS between four subgroups in CG. PG, Personalized group; CG, Chemotherapy group; C, chemotherapy alone subgroup in CG; AC, anti-angiogenesis plus chemotherapy subgroup in CG; IC, immune checkpoint inhibitors plus chemotherapy subgroup in CG; AIC, a combination of immune checkpoint inhibitors, anti-angiogenesis and chemotherapy subgroup in CG. ORR, objective response rate; DCR, disease control rate
In terms of EGFR 19 deletion, a cohort of 8 PG and 19 CG patients were PR, with ORR 26.7% (8/30), PFS 3.9 months (95% CI, 2.9–4.9 months) in PG and ORR 33.9% (19/56), PFS 4.9 months (95% CI, 3.4–6.4 months) in CG. With EGFR L858R, there were 10 PG and 11 CG patients were PR, with ORR 43.5% (10/23), PFS 4.5 months (95% CI, 2.4–6.6 months) in PG and ORR 31.4% (11/35), PFS 5.5 months (95% CI, 4.8–6.2 months) in CG. There were no statistic differences in ORR (P = 0.9) and PFS (P = 0.92) between PG and CG patients in terms of exons 19 and 21.
A cohort of 27 (50.9%) PG patients with MET amplification reported an ORR of 40.7% and a PFS of 4.2 m. Similar outcome was present in the SCLC transformation cohort (16, 30.2%), with an ORR of 37.5% and a PFS of 3.9 months (Table 2). Ten PG patients showed multiple resistance mechanisms, with an ORR of 20% and a PFS of 4.0 months.
The CG patients were further divided into the following subgroups on basis of the received treatment scheme: chemotherapy alone subgroup (C), anti-angiogenesis plus chemotherapy subgroup (AC), immune checkpoint inhibitors plus chemotherapy subgroup (IC) or a combination of immune checkpoint inhibitors, anti-angiogenesis and chemotherapy subgroup (AIC). The analysis revealed that the AIC subgroup with 15 (16.5%) patients reported significant difference in PFS (6.4 m vs. 3.4 m, P = 0.004) and ORR (53.3% vs. 20.7%, P = 0.022) when compared the C subgroup, but no statistical difference when in comparison with the IC and AC subgroups (Table 3, Fig. 2B).
Continuing, we compared the AIC and PG groups. The analysis revealed a trend towards better outcomes in the AIC group, with a median PFS (6.4 m vs. 4.2 m, P = 0.24; Fig. 2C) and ORR (53.3% vs. 23.0%, P = 0.17; Fig. 2D) suggesting a potential advantage. Furthermore, the advantage in DCR reached statistical significance (100% vs. 73.6%, P = 0.032; Fig. 2D).
The resistance mechanisms were complex after failure to EGFR-TKI
Among the 144 patients, 67 patients reported a definite resistance mechanism, with TP53 as the most frequent co-occurring mutation (36/67, 53.7%) and MET amplification as the main resistance mechanism (29/67, 43.2%) (Fig. 3A).
The resistance gene spectrum of EGFR mutation in NSCLC patients. A The gene change frequency after progression to EGFR-TKIs in NSCLC patients. B The main resistance mechanism in PG. C The gene change in SCLC transformation subgroup. D The main resistance mechanism in CG. E The resistance mechanism after failure to 1st or 2nd generation EGFR-TKIs. F The resistance mechanism to the 3rd generation EGFR-TKIs. G The co-occurring resistance genes in the research. PG, Personalized group; CG, Chemotherapy group
In the PG, 38 cases were detected by NGS and 9 were by FISH. The results showed that MET amplification (27/53, 50.9%) and SCLC (16/53, 30.2%) transformation were the most common AR types, which frequently received a personalized therapy (Fig. 3B). In the SCLC transformation samples, TP53, Rb1 and PIK3CA were the most common mutations (Fig. 3C). Of the 91 CG patients, 20 underwent NGS testing, with 18 reporting a detailed type of AR. Cell cycle gene alterations and EGFR amplification were the most common AR types (Fig. 3D).
The T790M (81 of 124 patients, 65.3%) was the most frequent AR for patients who were irresponsive to the 1st or 2nd generation EGFR-TKIs (Fig. 3E). The resistance mechanisms were complex and the multiple resistance genes more often appeared after the progression to the 3rd generation EGFR-TKIs (Fig. 3F). The alterations of cell cycle genes and EGFR amplification were the most common co-occurring resistance genes (Fig. 3G).
Discussion
For patients with disease progression after EGFR-TKI failure, the optimal treatment strategy remains controversial and no consensus has been reached. Our retrospective study found that the therapeutic efficacy of chemotherapy was comparable to that of personalized therapy. Interestingly, we found that when chemotherapy was supplemented with immune checkpoint inhibitors (ICIs) and anti-angiogenic drugs, there was a trend towards providing additional clinical benefits compared to personalized therapy. This highlights the enduring clinical significance of chemotherapy for patients experiencing relapse after prior EGFR-TKI therapy. Moreover, in some cases, combination therapy based on chemotherapy may provide incremental benefits. This emphasizes that chemotherapy is a promising option for treating patients with EGFR-TKI resistance.
PFS with traditional chemotherapy offers a length of 4.4–5.4 months for previously EGFR-TKI treated patients [16]. The data from clinical trials have not shown substantial survival benefits of single-agent ICI [17]. The combination therapy of chemotherapy and ICI or chemotherapy with anti-angiogenesis reported an efficacy with an ORR of 30–60% and a PFS of 5.0–7.0 m [18,19,20]. ICIs combined with chemotherapy and anti‐angiogenic drugs showed excellent benefits for patients receiving prior EGFR-TKI treatment, with an ORR of 73.5% and a PFS of 10.2 m in the IMpower150 subgroup, and an ORR of 43.9% and a PFS of 6.9 m in the ORIENT-31 trail [21,22,23]. In our study, the efficacy of the combined modality was superior to the chemotherapy alone, particularly in the “quad” model. The patients with EGFR mutations reported a high Treg infiltration, reduced CD8 + T-cell number and decreased tumor mutation burden (TMB) [24,25,26], which induced a poor clinical efficacy of ICI. EGFR-TKIs can remodel the tumor microenvironment (TME) by increasing CD8+ T cell infiltration and the presentation of MHC class I and II molecules, reducing the infiltration and function of Tregs [27]. So immunotherapy is applied after EGFR-TKIs failure. However, the efficacy and responsiveness of ICI monotherapy are far from satisfactory. Anti‐angiogenic drugs can reduce hypoxia, increase the delivery and efficacy of cytotoxic agents, and reduce immunosuppression through preventing angiogenesis and normalizing the tumor vasculature [28]. Consistent with the previous study, the current study revealed a satisfactory outcome when anti-angiogenics was combined with chemotherapy and immunotherapy. It would be beneficial to conduct further large-scale and prospective studies to confirm these results.
To date, many efforts have been invested in the search for effective treatment strategies to overcome EGFR-TKI resistance according genetic testing and histologic transformation. In case of EGFR C797S mutation, the follow-up treatment depends on the allelic relationship with T790M: T790M-trans-C797S is sensitive to the combination of first and third-generation of EGFR-TKIs [29], and a combination of brigatinb with cetuximab can achieve a favorable outcome with a PFS of 14 months and an ORR of 60%, in patients with T790M-cis-C797S [30]. The concomitant treatment with Trastuzumab and EGFR-TKIs has been demonstrated to possibly overcome the resistance to EGFR-TKIs as result of ERBB2 amplification [31]. SCLC transformation is responsive to platinum etoposide regimens with a PFS of 3.0–4.0 m [10, 32]. A retrospective trial found it is invalid to ICIs [10]. But another retrospective trial discovered that immunochemotherapy significantly prolonged the OS than chemotherapy. Positive PD-L1 status was associated with PFS benefit. And the expression of SFTPA1 in RNA sequencing predicted the durable clinical benefit. Large sample and prospective studies were needed to explore the efficacy of ICIs in SCLC transformation patients [33]. In our study, the patients reported a PFS of 4.2 m and an ORR of 34% in the PG, which is no better than the data in the CG and the data of previous studies, demonstrating that chemotherapy is an acceptable choice after the EGFR-TKIs failure. The above-mentioned findings also suggest that the evidence from personalized treatment is insufficient, for most of the above data are derived from retrospective research, preclinical studies, or small sample trials.
MET amplification has been implicated as one of the bypass resistance mechanisms to EGFR-TKI therapy. Numerous case reports and a growing number of clinical studies have documented the efficacy of the combinatorial regimen of EGFR-TKI and MET-TKI in simultaneously inhibiting both EGFR and MET signaling pathways to overcome EGFR-TKI resistance [34]. The combination of Tepotinib and Gefitinib has reported an amazing efficacy, with an ORR of 66.7% and a PFS of 16.6 m in the INSIGHT study [12]. Osimertinib plus savolitinib demonstrates a strong anti-tumor activity, with an ORR of 52% and a median duration of response (DOR) of 7.1 months in the TATTON Phase Ib expansion cohort [35]. In some trials and the real-world study, however, other MET inhibitors combined with EGFR-TKIs report a PFS of only 5-6 m [34, 36,37,38]. Amivantamab (EGFR-MET bispecific antibody) with lazertinib has shown anti-tumor activity with ORR 36% and PFS 4.9 m in patients of disease progression upon EGFR-TKI [39]. But emibetuzumab (monoclonal bivalent MET antibody) plus erlotinib could not reverse the AR to EGFR-TKI [40]. In our research, the PFS was 4.2 m and ORR was 40.7% in patients with MET amplification in the PG. Therefore, the “quad” model chemotherapy may be a favorable choice to these patients and further studies are awaited to explore the beneficiary group of a personalized therapy.
The analysis of circulating tumor DNA from NSCLC patients reveals that 46% of patients treated with EGFR-TKIs may have multiple resistance mechanisms [41]. Alterations to cell cycle gene and the PI3K pathway were the most common co-occurring resistance mechanism [42]. The multiple resistance mechanisms pose a challenge to personalized therapy. Our study found that 13 patients had the multiple resistance mechanisms; cell cycle gene alterations and EGFR amplification were the most common co-occurring resistance mechanisms; 10 PG patients displayed multiple resistance mechanisms, with an ORR of 20% and a PFS of 4.0 m, which indicated no impact on the efficacy of the therapy.
Some limitations remain in this retrospective study. First, the data of overall survival and adverse events were not available, which may affect the benefit elucidation between the CG and PG. Second, only a small number of patients received ICI plus chemotherapy with or without anti‐angiogenic drugs, which might limit the interpretation to determine the optimal therapeutic strategy. Third, the data regarding PD-L1 expression were insufficient to determine whether PD-L1 expression was balanced across groups or to analyze the correlation between PD-L1 expression and ICI efficacy. There is not ongoing study to compare OS between two therapy modes, a prospective study in these patients can be conducted.
Conclusion
This retrospective study demonstrates that chemotherapy, especially combined with antiangiogenic therapy and immunotherapy, may serve as the standard treatment strategy for patients who experience disease progression after EGFR-TKIs failure.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- EGFR:
-
Epidermal growth factor receptor
- EGFR-TKIs:
-
EGFR tyrosine kinase inhibitors
- TKI:
-
Tyrosine kinase inhibitor
- AR:
-
Acquired resistance
- SCLC:
-
Small-cell lung cancer
- FGFR:
-
Fibroblast growth factor receptor
- AJCC:
-
American Joint Committee on Cancer
- ECOG:
-
Eastern Cooperative Oncology Group
- NGS:
-
Next-generation sequencing
- FISH:
-
Fluorescence in situ hybridization
- CG:
-
Chemotherapy group
- PG:
-
Personalized group
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- CR:
-
Complete response
- ORR:
-
Objective response rate
- DCR:
-
Disease control rate
- PFS:
-
Progression-free survival
- ICI:
-
Immune checkpoint inhibitors
- TME:
-
Tumor microenvironment
- TMB:
-
Tumor mutation burden
- DOR:
-
Duration of response
References
Huang J, Liu D, Wang Y, et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71(4):734–45.
Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond). 2022;42(10):937–70.
Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167–79.
Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-Mutant Lung Cancers: A Phase 1/2 Single-Group Open-Label Trial. JAMA Oncol. 2020;6(7):1048–54.
Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients With EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527–34.
Nilsson MB, Sun H, Robichaux J, et al. A YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance and increased expression of spindle assembly checkpoint components. Sci Transl Med. 2020;12(559):eaaz4589.
Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17(1):38.
Tan CS, Kumarakulasinghe NB, Huang YQ, et al. Third generation EGFR TKIs: current data and future directions. Mol Cancer. 2018;17(1):29.
Jacobsen K, Bertran-Alamillo J, Molina MA, et al. Convergent Akt activation drives acquired EGFR inhibitor resistance in lung cancer. Nat Commun. 2017;8(1):410.
Marcoux N, Gettinger SN, O’Kane G, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. 2019;37(4):278–85.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–37.
Wu YL, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med. 2020;8(11):1132–43.
Miura S, Koh Y, Azuma K, et al. Afatinib plus osimertinib in the treatment of osimertinib-resistant non-small cell lung carcinoma: a phase I clinical trial. BMC Cancer. 2023;23(1):6.
Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR Mutations: a multicenter, open-label, phase II Trial (KCSG-LU15-09). J Clin Oncol. 2020;38(5):488–95.
Chen Y, Chen G, Li J, et al. Association of Tumor Protein p53 and ataxia-telangiectasia mutated comutation with response to immune checkpoint inhibitors and mortality in patients with non-small cell lung cancer. JAMA Netw Open. 2019;2(9): e1911895.
Hayashi H, Sugawara S, Fukuda Y, et al. A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for EGFR-Mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res. 2022;28(5):893–902.
Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12(2):403–7.
Jiang T, Wang P, Zhang J, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther. 2021;6(1):355.
Ma L, Diao B, Huang Z, Wang B, Yu J, Meng X. The efficacy and possible mechanisms of immune checkpoint inhibitors in treating non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Commun (Lond). 2021;41(12):1314–30.
Wiest N, Majeed U, Seegobin K, Zhao Y, Lou Y, Manochakian R. Role of immune checkpoint inhibitor therapy in advanced EGFR-mutant non-small cell lung cancer. Front Oncol. 2021;11: 751209.
Lam TC, Tsang KC, Choi HC, et al. Combination atezolizumab, bevacizumab, pemetrexed and carboplatin for metastatic EGFR mutated NSCLC after TKI failure. Lung Cancer. 2021;159:18–26.
Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(9):1167–79.
Nogami N, Barlesi F, Socinski MA, et al. IMpower150 final exploratory analyses for atezolizumab plus Bevacizumab and Chemotherapy in Key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. 2022;17(2):309–23.
Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11): e1356145.
Jia Y, Li X, Jiang T, et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: Implications for combination therapies. Int J Cancer. 2019;145(5):1432–44.
Madeddu C, Donisi C, Liscia N, Lai E, Scartozzi M, Macciò A. EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: role of the tumor microenvironment. Int J Mol Sci. 2022;23(12):6489.
Chen S, Tang J, Liu F, et al. Changes of tumor microenvironment in non-small cell lung cancer after TKI treatments. Front Immunol. 2023;14:1094764.
Newport EL, Pedrosa AR, Njegic A, Hodivala-Dilke KM, Muñoz-Félix JM. Improved immunotherapy efficacy by vascular modulation. Cancers (Basel). 2021;13(20):5207.
Dong RF, Zhu ML, Liu MM, et al. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharmacol Res. 2021;167: 105583.
Wang Y, Yang N, Zhang Y, et al. Effective treatment of lung adenocarcinoma harboring EGFR-activating mutation, T790M, and cis-C797S triple mutations by brigatinib and cetuximab combination therapy. J Thorac Oncol. 2020;15(8):1369–75.
La Monica S, Cretella D, Bonelli M, et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J Exp Clin Cancer Res. 2017;36(1):174.
Chen S, He Y, Liu J, et al. Third-generation TKI resistance due to SCLC transformation: a case report and brief review. Onco Targets Ther. 2019;12:11305–11.
Zhang CY, Sun H, Su JW, et al. A potential treatment option for transformed small-cell lung cancer on PD-L1 inhibitor-based combination therapy improved survival. Lung Cancer. 2023;175:68–78.
Wang Y, Tian P, Xia L, et al. The clinical efficacy of combinatorial therapy of EGFR-TKI and crizotinib in overcoming MET amplification-mediated resistance from prior EGFR-TKI therapy. Lung Cancer. 2020;146:165–73.
Combo Therapy Beats Back Relapsed NSCLC. Cancer Discov. 2019;9(6):685.
Wu YL, Zhang L, Kim DW, et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(31):3101–9.
Liu L, Qu J, Heng J, et al. A large real-world study on the effectiveness of the combined inhibition of EGFR and MET in EGFR-mutant non-small-cell lung cancer after development of EGFR-TKI resistance. Front Oncol. 2021;11:722039.
Reckamp KL, Frankel PH, Ruel N, et al. Phase II Trial of cabozantinib plus erlotinib in patients with advanced Epidermal Growth Factor Receptor (EGFR)-mutant non-small cell lung cancer with progressive disease on epidermal growth factor receptor tyrosine kinase inhibitor therapy: a california cancer consortium phase II trial (NCI 9303). Front Oncol. 2019;9:132.
Cho BC, Kim DW, Spira AI, et al. Amivantamab plus lazertinib in osimertinib-relapsed EGFR-mutant advanced non-small cell lung cancer: a phase 1 trial. Nat Med. 2023;29(10):2577–85.
Camidge DR, Moran T, Demedts I, et al. A randomized, open-label phase II study evaluating emibetuzumab plus erlotinib and emibetuzumab monotherapy in MET Immunohistochemistry Positive NSCLC Patients with Acquired Resistance to Erlotinib. Clin Lung Cancer. 2022;23(4):300–10.
Lee S, Jung J, Lee YJ, et al. Targeting HSF1 as a therapeutic strategy for multiple mechanisms of EGFR inhibitor resistance in EGFR mutant non-small-cell lung cancer. Cancers (Basel). 2021;13(12):2987.
Blakely CM, Watkins T, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017;49(12):1693–704.
Acknowledgements
We would like to thank all of the investigators for their involvement in this study.
Funding
This work was supported by Fujian Provincial Natural Science Foundation (grant 2020J011120), National Natural Science Foundation of China (grant 82072565), Bejing Xisike Clinical Oncology Research Foundation (grant Y-2019AZZD-0386) and National Natural Science Foundation of China (grant number 82372954). The funders did not participate in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Gen Lin; (II) Provision of study materials or patients: All authors; (III) Data collection and sorting: All authors; (IV) Data analysis and interpretation: Kan Jiang; (V) Manuscript writing: Kan Jiang, Yiquan Xu, Xinlong Zheng; (VI) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. This study was approved by the Ethics Committee of Fujian Cancer Hospital (SQ2017-015–01). Written informed consent was waived by the Ethics Committee of Fujian Cancer Hospital due to the retrospective nature of the study. Meanwhile, the institutional review boards of the other participating sites waived the ethics requirement, given the role of the research assistants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, K., Wu, L., Zheng, X. et al. Chemotherapy versus personalized therapy for EGFR mutant lung adenocarcinoma resistance to EGFR-tyrosine kinase inhibitors: a retrospective dual-center study. BMC Pulm Med 24, 96 (2024). https://doi.org/10.1186/s12890-024-02905-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-02905-1